Abstract
Breast-conserving surgery (BCS) plus postoperative radiotherapy has become the standard treatment for early-stage breast cancer. The aim of this study was to compare the setup accuracy of optical surface imaging by the Sentinel system with cone-beam computerized tomography (CBCT) imaging currently used in our clinic for patients received BCS. Two optical surface scans were acquired before and immediately after couch movement correction. The correlation between the setup errors as determined by the initial optical surface scan and CBCT was analyzed. The deviation of the second optical surface scan from the reference planning CT was considered an estimate for the residual errors for the new method for patient setup correction. The consequences in terms for necessary planning target volume (PTV) margins for treatment sessions without setup correction applied. We analyzed 145 scans in 27 patients treated for early stage breast cancer. The setup errors of skin marker based patient alignment by optical surface scan and CBCT were correlated, and the residual setup errors as determined by the optical surface scan after couch movement correction were reduced. Optical surface imaging provides a convenient method for improving the setup accuracy for breast cancer patient without unnecessary imaging dose.
Similar content being viewed by others
Introduction
Radiotherapy after early-stage breast-conserving surgery (BCS) can increase the 5-year local control by 19%, and the 15-year overall survival benefit added is about 5%1. Modern treatment techniques such as intensity modulated radiotherapy (IMRT) and volumetric modulated arc radiotherapy (VMAT) can further improve the dosimetry in the target and surrounding healthy tissues in terms of target dose conformity and uniformity, and reduced skin dose. Delivery of highly conformal dose distribution requires accurate patient setup2. Image-guided radiotherapy (IGRT) using electronic portal imaging detector (EPID) or cone-beam computerized tomography (CBCT) can help reduce patient setup errors but inevitably increases the setup time with burdens of added work flow for positioning correction and extra imaging dose to patients3. Three dimensional (3D) optical surface imaging is a fast and non-invasive method for assisting patient setup, which is particularly suitable for patients with breast cancer4. The purpose of this study is to compare the setup accuracy of optical surface imaging by the Sentinel system with CBCT imaging currently used in our clinic for BCS patients.
Results
A total of 145 sets of CE, SE-before and SE-after scans in 27 patients were analyzed. The numbers of treatment sessions for individual patients were from 4 to 7, and the median was 5.
The correlation coefficient r of CE and SE-before errors in every dimension were between 0.541~0.796, indicating significant correlations, and the p-values were less than 0.001 (Table 1 and Fig. 1).
Table 2 shows the systemic error and random errors for CE and SE-before. These errors were comparable between the two alignment methods with the differences in X, Y, and Z directions by 0.13 mm and 0.53 mm, 0.28 mm and 0.34 mm, and 0.34 mm and 0.17 mm, respectively. The rotational errors were also very similar for the two methods with the differences less than 0.2°. Accordingly, the anisotropic PTV margins derived from Sentinel were different from the CBCT data by 0.63 mm, 0.33 mm, and 0.56 mm in X, Y, and Z directions, respectively. The impact of rotational errors to the PTV margins are expected to be included.
The residual errors based on the Sentinel surface imaging scanned after the couch movement are given in Table 3. The PTV margins derived from the data were reduced to 4.49 mm, 4.85 mm, 3.67 mm in X, Y, and Z directions, respectively.
Discussion
BCS plus postoperative radiotherapy has become the standard treatment for early-stage breast cancer. Treatment techniques for BCS have been improved in recent couple of decades, aiming at minimizing the dose to the lung and heart. The use of IMRT not only increases the target dose uniformity but also reduces the dose to surrounding normal tissues, especially the heart and coronary arteries for the left side treatment5. However, the changes in breast shape and size due to variations of patients’ position and tissue inflammatory reaction such as postoperative serum like swelling, along with respiratory motion, may lead to dose delivery errors6. As result the benefit in local control and side effect reduction of IMRT may get nullified7. However, accurate patient positioning could increase the dose coverage to the CTV by 10% while significantly reduced the exposure to the lung and heart8. This means that patient setup accuracy, specifically the positioning of the target volume position, is important for the delivered dose distribution9. Therefore, to ensure the accuracy and reproducibility of the patient positioning is paramount to the safe and effective implementation of radiation therapy for BCS. The use of EPID, CBCT and other image-guided equipment can detect and correct the setup error either offline or online, which greatly improves the accuracy of treatment10. However, X-ray based IGRT process involves additional radiation to patients11. Studies showed that the dose of the single IGRT was about 0.01 mGy~1 mGy, for example, see12. The imaging dose of EPID was the highest, followed by KV- CBCT, and KV portal film the lowest. The dose with EPID was almost 2~10 times bigger than that of CBCT13. Surface imaging techniques can obtain the patients’ contour in three-dimensions with millimeter accuracy, which can be used to assist patient setup conveniently without any radiation.
Semaniak et al.14 measured the setup errors in two groups of 130 patients with radiotherapy after modified radical mastectomy by using EPID. It was found that for the left-right direction Σ were 1.6 mm and 1.7 mm, and δ were 1.6 mm and 1.3 mm, respectively. The resulted PTV margin were 5.1 mm and 5.4 mm for these two groups of patients, respectively. In cranial-caudal direction Σ were 1.5 mm and 1.9 mm, δ were 1.7 mm and 1.3 mm, respectively. The cranial-caudal PTV margins were 4.9 mm and 6.4 mm, respectively. Chung et al.15 studied the setup errors of 147 patients with postoperative radiotherapy for early-stage breast cancer by using MVCT (Megavoltage Computerized Tomography). They found that Σ and δ were 1.98 mm and 1.87 mm in left-right, 2.02 mm and 2.10 mm in cranial-caudal, and 2.99 mm and 2.82 mm in anterior-posterior direction, respectively. According to the Stroom formula16, the PTV margins were 5.27 mm, 5.51 mm and 7.95 mm respectively. In our study, PTV margins based on CBCT were 6.00 mm, 6.64 mm and 7.14 mm in left-right, cranial-caudal, and anterior-posterior direction, and this was similar to the above two studies. Another study by Lakosi et al.17 used CBCT to study the setup errors of 36 cases of breast cancer in the prone position. Their results showed that Σ and δ were about 4.5 mm and 5.4 mm in left-right, 3.9 mm and 3.8 mm in cranial-caudal, 3.3 mm and 2.8 mm in anterior-posterior direction, the PTV margins were 15.0 mm, 12.3 mm and 10.3 mm, respectively. The setup errors were significantly greater than that of Semaniak et al.14, Chung et al.15 and our study. This means that prone treatment could involve larger uncertainties than that in supine treatment.
Walter et al.18 retrospectively analyzed a total of 154 surface imaging (Catalyst) scans in 25 patients with thoracic, abdominal and pelvic tumors. They found that the setup errors of CBCT were 0.0 ± 2.1 mm in left-right, −0.4 ± 2.4 mm in cranial-caudal, and 1.1 ± 2.6 mm in anterior-posterior direction; and the setup errors by Catalyst scan in the three translation directions were −0.1 ± 2.1 mm, −1.8 ± 5.4 mm and 1.4 ± 3.2 mm, respectively. There was no significant difference between the two approaches, which led the authors to believe that Catalyst could be used as at least an effective alternative to CBCT. Crop et al.19 studied the setup error based on laser light, Catalyst and mega voltage computed tomography (MVCT) in postoperative radiotherapy for breast cancer. The results showed that the setup error of the Catalyst was significantly better than that of laser-based positioning and was very close to the MVCT. However, Padilla et al.20 reported negative results in their study of the correlation of surface imaging (AlignRT) and MV portal imaging in postoperative radiotherapy for breast cancer. The AlignRT showed poor correlation with MV portal imaging in cranial-caudal direction (r = 0.14), and only moderate correlations (r = 0.49 and 0.66) in anterior-posterior and left-right direction. Our results showed a significant correlation between CBCT and Sentinel errors (r = 0.541–0.796) and statistical significance with p-values less than 0.001. The differences in setup errors between CBCT and Sentinel, in terms of Σ, δ, and PTV margins, were less than 1 mm and 0.5°. Our result was similar to Walter et al.18 and Crop et al.19, but significantly better than Padilla et al.20. One can argue that using MV images did not take the shape of the breast and deformation in the treatment process into account, while Sentinel and other surface imaging techniques is sensitive to changes in shape and size of breast21. In our study the ROI of Sentinel scan only included the treated breast but excluded the chin, the ipsilateral upper limb, and armpit, which were likely to have an impact on matching. Likewise, the clip box for CBCT matching was limited to the region of treated breast and surrounding tissues. Manual adjustment performed after auto-registration could have improved the accuracy. A study to compare the accuracy of EPID and CBCT in BCS found that using MV images underestimated the setup errors by 20% to 50%22.
The Sentinel scan uses patient’s surface in 3D to match with that reconstructed from planning CT, instead of bony markers distant from the target volume. This makes it especially suitable for breast cancer treatment with the target volume situated close to the body surface. Batin et al.21 used radioopaque metal markers placed on the skin near the tumor bed to replace the bony marker away from the target volume, in order to improve the accuracy of portal imaging assisted setup. The results with portal imaging were unsatisfactory due to the limited area that the metal marker surrogates could identify. Furthermore, there was an uncertainty in placing those metal markers reproducibly for each treatment. On the contrary, the reference for matching with optical surface is the entire target volume.
We detected the residual setup errors after correction of the patient’s position by use of Sentinel. PTV margins were all less than 5 mm after IGRT, which was decreased by 1~2 mm in all directions. The result was similar to the literature report: Batin et al.21 studied the residual errors detected by AlignRT in breast cancer after modified radical mastectomy. PTV margin were required to expand 3.5 mm, 2.4 mm, 4.0 mm in X, Y, and Z directions. Betgen et al.23 also studied the residual errors after correcting positions by AlignRT in breast cancer. They found that PTV margins were required to add 3.2 mm, 4.3 mm, 3.2 mm in three directions. In our center, PTV margins added were 5 mm in three shifting directions without considering rotation, and the PTV-boost margins were 7 mm, which are consistent with the experimental results by this study. After IGRT, PTV margins were all reduced to less than 5 mm. The residual errors are expected to be improved if a smaller slice thickness in the planning CT were used. Hence, in the future work to use Sentinel for assisting patient setup for every treatment session, we can expect to further decrease the margin of the PTV-boost, and that will result in reduced integral dose to patients without compromising the dose to the target. In addition, the staff of our department have strong sense of responsibility and tried their best during the process of fixation and setup of the patients to minimize the errors.
The advantages of Optical Laser 3D Surface imaging make it a certain value in clinical use, Batin et al.21 hold that surface imaging can offered more accurate positioning, shorter setup time and the reduction of imaging dose to patient from the traditional orthogonal X-ray setup technique. They have adopted this technique for all PBS PMRT treatment at their clinic. Studies by Padilla et al.20 have shown that instant feedback on patient position changes is a key benefit of surface imaging, which make this technique better for clinical use. Palotta et al.24 found that in patients with thoracic targets, the use of surface imaging resulted in improved positioning in 50%. Walter et al.18 reported the clinical data on the accuracy of patient positioning using the Catalyst system, suggesting that optical surface scanning by the Catalyst can be used for daily positioning at least to complement complementing conventional imaging modalities.
Materials and Methods
This was a prospective study had approved from the Medical Ethics Committee of the Third Affiliated Hospital of Soochow University, and written informed consent was obtained from the patients before treatment. The methods used in this study were carried out in accordance with the guidelines outlined in the Declaration of Helsinki. This study enrolled 27 female patients with breast cancer who underwent radiotherapy after BCS from November 1, 2016 to April 30, 2017. The median age of the patients was 50 years old (range 35~64 years). The pathologic types of breast cancer are classified into non invasive carcinoma and invasive carcinoma, among which non invasive carcinoma includes ductal carcinoma in situ (DCIS) and Paget disease of the nipple not associated with invasive carcinoma and/or carcinoma in situ (DCIS) in the underlying breast parenchyma; in TNM staging, “Tis” represents non invasive carcinoma, on the other hand, T1 (Tumor ≤20 mm in greatest dimension) and T2 (Tumorå 20 mm but ≤50 mm in greatest dimension) represent invasive carcinoma25. The case number for intraductal carcinoma and invasive carcinoma of the study population were 6 and 21, the case numbers for Tis, T1, and T2 stage were 6, 17, and 4, respectively; the N and M stage for all cases were N0 and M0. The characteristics of the study population are shown in Table 4.
Patients were immobilized using a breast board (Posirest-2 Arm Support, CIVCO, US) in supine position with arms raised above the head. Planning CT scan was performed on a Sensation Open CT scanner (Siemens, Germany). CT images with a slice thickness of 5 mm covered the region from the annular membrane to the lower boarder of liver. Three longitudinal laser lines and one transversal laser line were marked on patient’s skin surface, and their intersecting points were tattoo marked for setup positioning at treatment.
For treatment planning, the clinical target volume (CTV) was delineated to include all breast tissues. The planning target volume (PTV) was defined as the CTV with a 5 mm margin, modified to exclude the volume adjacent to the skin surface within 5 mm. The CTV-boost volume consisted of the tumor bed, as marked by the surgery metal clips, postoperative serum swelling and surgical scars. The PTV-boost was defined as adding 7 mm margin to the CTV-boost and modified to be inside the PTV. Organ as risk (OAR) delineated were the lung, heart, and contralateral breast.
Dose prescription was 57.5 Gy in 25 fractions for PTV-boost and 47.5 Gy in 25 fractions for PTV. The dosimetric objectives were to cover 95% of the target volumes with the prescription dose and limit the OAR dose such that the V5 of ipsilateral lung was less than 50%, the mean dose to the heart less than 6 Gy, and the maximum dose of contralateral breast less than 5 Gy.
Treatment plans were generated using the Monaco treatment planning system (TPS) (Monaco 3.3, Elekta, Sweden). A single 190° arc was used for the beam setup. A 6MV photon beam was used. For the left-side treatment the arc started at gantry angle of 300° and ended at 130°, and for the right-side treatment the gantry angles were from 60° to 230°. The maximum number of control points was 150.
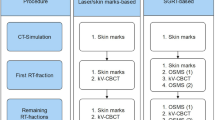
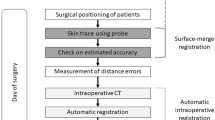
This study was designed for evaluating the patient setup accuracy of using the optical surface imaging in the clinical setting but without deviating from the current CBCT based method for patient positioning correction. The starting point of patient setup was the alignment of treatment room lasers to the tattoo marks on the patient. Patient skin surface was acquired using the Sentinel system (C-RAD, Sweden), and the region of interest (ROI) consisted of the disease side breast and the adjacent chest wall. The upper boundary was the clavicle, and the medial boundary was the midline. The contralateral breast, chin, arm and armpit were excluded. The matching of the acquired surface ROI with the surface reconstructed by the planning CT yielded setup corrections that can be applied by a 6-dimensional (6D) treatment couch (HexaPOD, Elekta, Sweden). The shifts in the X, Y, Z directions and the three rotations about the X, Y, Z axes were recorded as a data set named SE-before (Sentinel errors before the treatment couch correction). Next, the routine CBCT scan was acquired for setup correction (XVI, Elekta, Sweden) using the Chest M20 mode, 120 kV, and F1 Bowtie filter. The ROI for matching the CBCT and planning CT included ipsilateral breast, chest wall, sternum and parts of the lung. Automatic registration was applied first followed by manual adjustment to best match the metal clips and the shape of the breast. The resulted couch movement by the 6D couch was obtained, denoted as CE (CBCT error), and the correction was applied using the automatic couch movement function for the HexaPOD couch. Prior to treatment delivery, the second optical surface imaging scan was performed, and the residual setup error as determined by the C-RAD software was denoted as SE-after (Sentinel error after shifting the treatment couch). The modified procedure to conduct the current study was facilitated by the MOSAIQ (Elekta, Sweden) Oncology Information System. In this workflow, it was the attending physician, Mengjiao Liu and Wendong Gu, and the technician, Xiaobo Wei, analysed the images and data for the first time, and then finished by the same technician, Xiaobo Wei. The images and data of setup corrections by Sentinel and CBCT is shown in Fig. 2, and a step by step procedure is shown in Fig. 3.
The images and data of setup corrections by Sentinel (a) and CBCT (a,b): The green area represents the body surface profile reconstructed by CT scan, the blue area represents the body surface profile reconstructed by Sentinel scan, and the dark blue represents areas that match with errors. The data on the left is the result of matching error.
As the study was limited to assessment of patient setup accuracy with the optical surface imaging, comparison of initial couch setup errors between surface matching and CBCT matching with the planning CT was made. The correlation between the SE-before and CE was analyzed by Pearson correlation analysis using the SPSS 18.0 software. A correlation coefficient (r) ≤ 0.3 was considered with poor correlation, in the range 0.3~0.5 as moderately correlated, 0.5~0.8 as significantly correlated, equal to or greater than 0.8 as highly correlated.
The setup errors measured by SE-before and CBCT for the patient cohort can be used to determine the PTV margins required for treatment without couch movement correction. This is relevant to the margins for the PTV-boost in simultaneous integrated boost radiotherapy and PTV margins for partial breast radiotherapy. The PTV margins derived from SE-before and CBCT data were compared based on a statistical model. The average setup error and standard deviation (SD) of each patient was calculated for multiple treatment sessions. The group mean (M) of the study cohort was the average of the averaged error for each patient. The systematic error (Σ) was the standard deviation of the individual mean for each patient, and the randomize error (δ) was the root mean square of the mean square deviation of each patient26. The PTV margins were calculated by 2Σ + 0.7δ, based on the work by Sroom et al.16. For this study the SE-after was considered the residual error following CBCT based setup correction, and the resulting PTV margins were evaluated.
Conclusion
In summary, optical surface imaging by Sentinel has a significant correlation with CBCT in detecting setup errors in postoperative radiotherapy for breast cancer. The differences of Σ and δ between the two IGRT methods were less than 1 mm. Sentinel can be used to replace CBCT to avoid unnecessary imaging radiation to patients.
References
Clarke, M. et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet (London, England) 366, 2087 (2005).
Donovan, E. et al. Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiotherapy & Oncology 82, 254–264 (2007).
Bujold, A., Craig, T., Jaffray, D. & Dawson, L. A. Image-guided radiotherapy: has it influenced patient outcomes? Seminars in Radiation Oncology 22, 50 (2012).
Gu, W. D. & Gao, L. Q. M. A 002LFC intensity-modulated radiotherapy (IMRT) phantom evalution of SentinelTM system for patient set-up verification in radiotherapy. Chinese Journal of Radiation Oncology 22, 493–496 (2013).
Poortmans, P., Aznar, M. & Bartelink, H. Quality indicators for breast cancer: revisiting historical evidence in the context of technology changes. Seminars in Radiation Oncology 22, 29 (2012).
Djajaputra, D. & Li, S. Real‐time 3D surface‐image‐guided beam setup in radiotherapy of breast cancer. Medical Physics 32, 65–75 (2005).
Jeng, S. C. et al. Mathematical estimation and in vivo dose measurement for cone-beam computed tomography on prostate cancer patients. Radiotherapy & Oncology Journal of the European Society for Therapeutic Radiology & Oncology 92, 57 (2009).
Li, S. et al. Initial Validation and Clinical Experience with 3D Optical-Surface-Guided Whole Breast Irradiation of Breast Cancer. Technology in Cancer Research & Treatment 11, 57 (2012).
Salm, A. V. D., Murrer, L., Steenbakkers, I., Houben, R. & Boersma, L. J. Actual target coverage after setup verification using surgical clips compared to external skin markers in postoperative breast cancer radiotherapy. Practical Radiation Oncology (2017).
Penninkhof, J., Quint, S., Baaijens, M., Heijmen, B. & Dirkx, M. Practical use of the extended no action level (eNAL) correction protocol for breast cancer patients with implanted surgical clips. Int J Radiat Oncol Biol Phys 82, 1031–1037 (2012).
Murphy, M. J. et al. The management of imaging dose during image-guided radiotherapy: Report ofthe AAPM Task Group 75. Medical Physics 34, 4041–4063 (2007).
Schneider, U., Hälg, R. & Besserer, J. Concept for quantifying the dose from image guided radiotherapy. Radiation Oncology 10, 1–6 (2015).
Ding, G. X. & Munro, P. Radiation exposure to patients from image guidance procedures and techniques to reduce the imaging dose. Radiotherapy & Oncology Journal of the European Society for Therapeutic Radiology & Oncology 108, 91 (2013).
Semaniak, A. & Kukołowicz, P. Set-up uncertainty during postmastectomy radiotherapy with Segmented Photon Beams Technique. Rep Pract Oncol Radiother 20, 181–187 (2015).
Mi, J. C. et al. Setup Error and Effectiveness of Weekly Image-Guided Radiation Therapy of TomoDirect for Early Breast Cancer. Cancer Research & Treatment Official Journal of Korean Cancer Association 47, 774–780 (2015).
Stroom, J. C., de Boer, H. C., Huizenga, H. & Visser, A. G. Inclusion of geometrical uncertainties in radiotherapy treatment planning by means of coverage probability. International Journal of Radiation Oncology Biology Physics 43, 905 (1999).
Lakosi, F. et al. Feasibility evaluation of prone breast irradiation with the Sagittilt© system including residual-intrafractional error assessment. Cancer Radiotherapie 20, 776–782 (2016).
Walter, F. et al. Evaluation of daily patient positioning for radiotherapy with a commercial 3D surface-imaging system (Catalyst™). Radiat Oncol 11, 154, https://doi.org/10.1186/s13014-016-0728-1 (2016).
Crop, F. et al. Surface imaging, laser positioning or volumetric imaging for breast cancer with nodal involvement treated by helical TomoTherapy. Journal of Applied Clinical Medical Physics 17, 1–12 (2016).
Padilla, L. et al. Assessment of interfractional variation of the breast surface following conventional patient positioning for whole-breast radiotherapy. Journal of Applied Clinical Medical Physics 15, 177–189 (2014).
Batin, E., Depauw, N., Macdonald, S. & Lu, H. M. Can surface imaging improve the patient setup for proton postmastectomy chest wall irradiation? Pract Radiat Oncol 6, e235–e241, https://doi.org/10.1016/j.prro.2016.02.001 (2016).
Topolnjak, R. et al. Breast patient setup error assessment: comparison of electronic portal image devices and cone-beam computed tomography matching results. Int J Radiat Oncol Biol Phys. 78, 1235–1243 (2010).
Betgen, A. et al. Assessment of set-up variability during deep inspiration breath hold radiotherapy for breast cancer patients by 3D-surface imaging. Radiotherapy & Oncology Journal of the European Society for Therapeutic Radiology & Oncology 106, 225 (2013).
Pallotta, S. et al. Surface imaging, portal imaging, and skin marker set-up vs. CBCT for radiotherapy of the thorax and pelvisOptisches Oberflächenscanning, Portal Imaging und Hautmarkerpositionierung vs. CBCT beim Setup für Thorax- und Beckenbestrahlungen. Strahlentherapie Und Onkologie 191, 726–733 (2015).
Giuliano, A. E. et al. Breast Cancer—Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA: A Cancer Journal for Clinicians 67, 290–303, https://doi.org/10.3322/caac.21393 (2017).
Van, H. M. Errors and margins in radiotherapy. Seminars in Radiation Oncology 14, 52–64 (2004).
Acknowledgements
This work has been supported by the Fund: Science and technology projects of clinical medicine for Jiangsu province (BL2014040).
Author information
Authors and Affiliations
Contributions
W.-D.G., X.-B.W. and M.-J.L. conceived and designed the study. M.-J.L. performed the analysis, prepared the figures and tables and wrote the main manuscript. All of the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wei, X., Liu, M., Ding, Y. et al. Setup errors and effectiveness of Optical Laser 3D Surface imaging system (Sentinel) in postoperative radiotherapy of breast cancer. Sci Rep 8, 7270 (2018). https://doi.org/10.1038/s41598-018-25644-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-25644-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.