Abstract
Cisplatin is a commonly used chemotherapeutic agent in the treatment of different types of malignant tumors, but nephrotoxicity limits its usage. Therefore, in this study, we aimed to determine the possible protective effect of Huaiqihuang (HQH) extractum, a kind of Chinese herbal complex that consists of Trametes robiniophila Murr., Lycium barbarum and Polygonatum sibiricum, against nephrotoxicity induced by cisplatin in mice. We found that pretreatment with HQH significantly attenuated the cisplatin-induced increase in blood urea nitrogen (BUN), interstitial congestion, acute renal tubular injury and tubular cell apoptosis and necroptosis. It was further shown that HQH administration reduced cisplatin-induced release and nuclear-cytoplasmic translocation of HMGB1 and inactivated its downstream signaling molecules, TLR4 and NFκB, in renal tubular cells; as a result, HQH repressed cisplatin-induced TNF-α production. As dexamethasone (Dex) exerts renoprotective effects in severe Acute kidney injury (AKI), we compared it with HQH and found that HQH showed similar renoprotective effects to dexamethasone via similar mechanisms. Considering the potential side effects of corticosteroids, reducing the effectiveness of treatment and shortening survival in solid tumor patients, we suggest administration of HQH as a potential adjuvant for cisplatin therapy in solid tumor patients to preserve renal function.
Similar content being viewed by others
Introduction
Cisplatin is a widely used anti-cancer agent that has been used in non-small cell lung, esophagus, bladder, endometrium, ovary and testicular cancers, as well as many other types of cancers1,2. However, its side effects, such as ototoxicity, renal toxicity and neurotoxicity, limit its clinical use3,4,5; among these effects, nephrotoxicity is the most concerning. Nephrotoxicity induced by cisplatin mainly occurs in renal tubular proximal tubular epithelial cells and is characterized by tubular cell necrosis, oxidative damage, inflammatory injury and acute renal failure6,7,8, which is related to high morbidity and mortality9. As the mechanisms implicated in cisplatin-induced AKI are still unclear, an effective clinical agent for preventing cisplatin-induced AKI is needed. Recently, dexamethasone (Dex) has been used as a potent therapeutic agent for acute kidney injury induced by cardiac surgery10,11, multiple myeloma12,13 and drug-induced nephrotoxicity14,15. However, the shortened survival of solid tumor patients due to corticosteroids restricts its application16.
Huaiqihuang (HQH) extractum is a Chinese herbal complex that consists of Trametes robiniophila Murr., Lycium barbarum and Polygonatum sibiricum, all of which have been used intensively in China for more than 1, 000 years. Among them, Trametes robiniophila Murr. is one of the important anti-tumor herbs and is widely used in the adjuvant therapy of primary liver cancer, breast cancer, stomach cancer, colon cancer and other cancers17,18,19,20. HQH extractum has been used in the treatment of nephrotic syndrome, bronchial asthma and recurrent upper respiratory tract infection in children. An in vitro study showed that HQH extractum protects against podocyte injuries by regulating the p-ERK/CHOP signaling pathway to promote podocyte proliferation and suppress podocyte apoptosis21. Liu H et al. reported that HQH extractum could increase podocyte nephrin expression, inhibit the NF-κB signaling pathway and suppress glomerular and tubular apoptosis in rats with adriamycin-induced nephropathy22. All these studies suggest that HQH extractum is a potent nephroprotective agent; however, little is known about the protective effect of HQH in AKI.
In this study, the renoprotective effects of HQH against cisplatin-induced AKI was surveyed in mice and we found that pretreatment with HQH demonstrated renoprotective effects similar to those of Dex via similar mechanisms to relieve cisplatin-induced renal injury. Furthermore, it was shown that HQH did not impair cisplatin-mediated killing of HeLa tumor cells in vitro. Taken together, these results suggest that HQH is a potent renoprotective agent for preventing the renal toxicity caused by cisplatin without affecting the anti-tumor effects of cisplatin.
Results
Effects of Huaiqihuang (HQH) Extractum on Renal Damage in Cisplatin-Treated Mice
The experimental design of the renoprotective effect of HQH on cisplatin-induced renal injury in C57BL 6 male mice is summarized in Fig. 1A. Urea levels were significantly increased in the cisplatin-treated group, but this effect was significantly reversed by HQH and Dex pretreatment (Fig. 1B). An obvious medullary congestion zone was seen in cisplatin-treated kidneys when the mice were sacrificed on day 3, which was significantly attenuated after HQH pretreatment (Fig. 1C).
HuaiQihuang extractum (HQH) or Dexamethasone (Dex) pretreatment attenuated acute renal injury induced by cisplatin (Cis). (A) Scheme: The group of HQH + Cis received 6 g/kg HQH for two consecutive days before intraperitoneal injection with 20 mg/kg cisplatin and the group of Dex + Cis intraperitoneally injected with 4 mg/kg Dex 30 minutes before intraperitoneal injection with cisplatin. (B) Serum urea was measured at day 3 after cisplatin injection (*P < 0.05, **P < 0.01, n = 5/group). (C) Representative medullary congestion after Cisplatin injury with or without HQH and Dex pretreatment. Graphs indicate congestion area. (D) Representative photomicrographs and graphs of Cisplatin induced kidney injury, with or without HQH and Dex pretreatment. Injured tubules was showed by PAS staining, Bar = 50 μm. LTL (lotus tetragonolobus lectin) identifying proximal tubules (green), Bar = 100 μm. Kim-1 expression (red) identifying tubular injury, Bar = 50 μm. Statistics analyzed using the Mann-Whitney U test. **P < 0.01, ****P < 0.0001, n = 5/group. Values are means ± SEM.
The morphological changes in the kidneys are shown in Fig. 1D. Cisplatin-induced cast formation, tubular dilation and brush border loss were significantly alleviated by HQH pretreatment (Fig. 1D). The HQH group showed less tubular cast than the Dex group.
To assess the injury of proximal renal tubule cells (TECs), we detected the brush border with immunofluorescence staining of lotus tetragonolobus lectin (LTL) and kidney injury molecule 1 (Kim-1). As shown in Fig. 1D, the loss of LTL-positive tubules in the cisplatin-treated kidneys was restored by HQH pretreatment. Compared to the Dex group, the HQH group had more LTL-positive tubules. Compared to its expression in cisplatin-treated control mice, Kim-1 was less expressed in the HQH group, similar to the Dex group, (Fig. 1D). These data suggested that HQH pretreatment alleviated cisplatin-induced AKI better than the dexamethasone.
Effects of Huaiqihuang (HQH) Extractum on Proliferation, Apoptosis and Necroptosis of TECs in Cisplatin-Treated Mice
Previous studies have shown that cisplatin-induced AKI is accompanied by proliferation of renal TECs23,24,25. We found that cisplatin induced a significant increase in Ki67-positive nuclei in TECs, which was not further increased in the HQH- and Dex-pretreated group (Fig. 2A), suggesting that HQH pretreatment-induced renoprotection does not occur through the increased renal regeneration capacity related to TEC proliferation. Apoptosis in TECs was assessed using the terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) assay (Fig. 2B). TUNEL-positive cells were almost undetectable in the control kidneys, while the number of the TUNEL-positive cells was significantly increased after cisplatin administration. Pretreatment of both HQH and Dex reduced the cisplatin-induced apoptosis; however, there was no difference between the HQH-pretreated group and the Dex-pretreated group. We further examined the expression of the pro-apoptotic protein Bax and the anti-apoptotic protein Bcl-2, which showed that HQH pretreatment attenuated cisplatin-induced Bax expression (Fig. 2C) and enhanced the expression of anti-apoptotic Bcl-2. This result suggests that HQH pretreatment protected the kidneys against cisplatin-induced apoptosis. Tubular necroptosis is another characteristic of kidney tissues in cisplatin-treated mice and is characterized by high levels of receptor interacting-protein 3(RIP3) and mixed-lineage kinase domain-like protein (MLKL) in TECs26. To assess this necroptosis, we observed the expression of RIP3 and MLKL by western blotting (Fig. 2C). We found that both HQH and Dex pretreatment attenuated kidney necroptosis induced by cisplatin, suggesting that HQH treatment protected the kidneys against cisplatin-induced necroptosis.
HQH pretreatment protected mice from cisplatin induced tubular apoptosis and necroptosis, but not improved renal tubular epithelial cell proliferation. (A) Representative photomicrographs and graphs of immunofluorescence staining for Ki-67. Bar = 50 μm. (**P < 0.01 versus control, n = 8/group). Ki-67 was stained in red and nucleus was counterstained in blue by DAPI. (B) Representative photomicrographs and graphs of TUNEL (green) assay for analysis of tubular apoptosis (**P < 0.01, n = 5/group). Bar = 50 μm. (C) Representative Western blot and graphs for pro-apoptotic proteins Bax (C and D, *P < 0.05, n = 3/group), anti-apoptotic protein Bcl-2 (E and F, *P < 0.05, n = 3/group) and the key element of necroptosis RIP3 (*P < 0.05, n = 3/group) and MLKL (*P < 0.05, n = 3/group). Each group of blots is the same exposure of a gel, each group of results repeated more than three times.
Effects of Huaiqihuang (HQH) Extractum on inflammation of kidneys in Cisplatin-Treated Mice
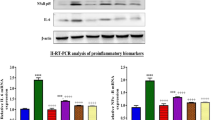
Previous study confirmed that cisplatin-induced acute renal injury is related to the release of pro-inflammatory cytokines, including high mobility group box-1 protein (HMGB1) and tumor necrosis factor-α (TNF-α)27. As shown in Fig. 3A, cisplatin injection resulted in obvious release of HMGB1 into the cytoplasm in TECs, which was restored by HQH and Dex pretreatment (Fig. 3A). We further used RT-PCR to detect the expression of TNF-h (Fig. 3B). We found that both HQH and Dex pretreatment reduced the expression of TNF-N induced by cisplatin. These results were further confirmed by Western blotting, as shown in Fig. 3C. NFκB and TNF-N are the downstream signaling pathways of HMGB1 and TLR428,29,30. We found that HMGB1 in the cytoplasm, NF-κB P56 in the nucleus and TLR4 in total protein, were highly induced after cisplatin administration and these effects were reversed by HQH and Dex pretreatment (Fig. 3C). We also assessed the MAPK signaling pathway; however, the expression of p-Erk/Erk was increased in all three groups and there was no significant difference between them (Fig. 3C). This result suggests that HQH pretreatment protected the kidneys against cisplatin-induced inflammation through the HMGB1/TLR4/NFκB pathway.
HQH protected the kidney through inhibition of HMGB1/TLR4/NF-κB/TNF-α pathway. (A) Representative photomicrographs and graphs for the expression of HMGB1 by immunohistochemical staining (**P < 0.01, n = 5/group). Bar = 50 μm. (B) Real-time PCR analyses of TNF-α (**P < 0.01, n = 3 ~ 7/group). (C) Representative Western blot and graphs for HMGB-1 in cytoplasmprotein (**P < 0.01, *P < 0.05, n = 3 ~ 4/group) and p65 in nucleoprotein (*P < 0.05, n = 3 ~ 4/group), and TLR4 (*P < 0.05, n = 4/group), p-Er and Erk (*P < 0.05, n = 3/group) in total protein. Each group of blots is the same exposure of a gel, each group of results repeated more than three times.
HQH Extractum does not Bind to Cisplatin
To observe the change in HQH after the addition of cisplatin, we applied nuclear magnetic resonance to detect the change in the one-dimensional proton spectrum. If cisplatin binds to HQH, it will cause chemical shifts, which will cause a change in the proton spectrum. However, we found that these two lines substantially overlapped (the red line indicates the hydrogen spectra of HQH and the blue line indicates the hydrogen spectra of Cis added to HQH) (Fig. 4), suggesting that HQH and cisplatin do not undergo chemical or physical reactions and that neither agent produces chelates or degradation. In other words, HQH does not react with cisplatin to prevent cisplatin from entering the cell.
HQH does not bind with cisplatin. (A) The nuclear magnetic resonance showed that HQH and Cis does not interact with each other. The red line indicates the hydrogen spectra of HQH. The blue line indicates the hydrogen spectra of which Cis into HQH. These two lines substantially overlapped, suggesting that HQH does not interact with Cis. (nuclear magnetic resonance spectrometer, agilent 600 DD2).
HQH Extractum does not Impair Cisplatin-Mediated Killing of HeLa Tumor Cells In Vitro
To verify the protective effect of HQH on the kidney at the cellular level, we performed a CCK 8 experiment in cisplatin-treated renal tubular epithelial cells after HQH pretreatment, which showed that HQH pretreatment increased the viability of TECs in a dose-dependent manner from 0 mg/mL to 12 mg/mL (Fig. 5a). Therefore, we pretreated TECs with HQH at a concentration of 12 mg/mL and showed that, at such a dose, HQH pretreatment significantly inhibited cisplatin-induced necroptosis in vitro (Fig. 5b). Thus, we used 12 mg/mL HQH for the following in vitro experiments in tumor cells (HeLa cells). We found that HQH does not inhibit the anti-tumor activity of cisplatin in HeLa cells (Fig. 5c), which suggests that HQH pretreatment protects against cisplatin-induced renal toxicity without affecting the anti-tumor effect of cisplatin.
HQH reduced the damage of renal tubular epithelial cells induced by cisplatin, but did not affect the antitumor activity of cisplatin in Hela cells. (a) CCK 8 experiment in renal proximal tubules after HQH (0/6/12/18 mg/ml) pretreatment 12 h before cisplatin (75 μM) treatment 12 h (*P < 0.05, n = 5/group). (b) RIP3 expression are decreased in renal proximal tubules after HQH (12 mg/ml) pretreatment 12 h before cisplatin (75 μM) treatment 12 h (*P < 0.05, n = 5/group). (c) CCK8 cell proliferation assay for Hela cell. Con: Without stimulus; Cis: 30 mM; HQH: 10 mg/ml; Dex: 50 μM; (**P < 0.01, n = 5/group).
HQH Extractum Pretreatment Attenuates AKI In Two Other Models
To verify that Huaiqihuang also provides protection in other nephrotoxic models, we established a folic acid (FA)-induced AKI model and an ischemia-reperfusion (IRI)-induced AKI model. The experimental design of the renoprotective effect of HQH on FA- or IRI-induced renal injury is summarized in Fig. 6A and C, respectively. FA-induced (Fig. 6C) and IRI-induced (Fig. 6D) cast formation, tubular dilation and brush border loss were significantly alleviated with HQH and Dex pretreatment. Urea levels were significantly increased by FA and IRI treatment, which were restored by HQH and Dex pretreatment (Fig. 6C and D). There was no difference between the HQH- and Dex-pretreated groups, suggesting that HQH pretreatment attenuated acute renal tubular injury and improved kidney function in various models of AKI in mice.
HQH and Dex pretreatment attenuate renal injuries induced by folic acid (FA) or unilateral kidney ischemia reperfusion (IRI). (A) Scheme: Male C57BL/6 mice with body weight about 25 g underwent intraperitoneal injection with NaHCO3(150 mM) or 150 mg/kg folic acid (n = 4/group). Mice received gavage of 6 g/kg HQH extractum every day or one dose of Dex with 4 mg/kg before intraperitoneal injection with 150 mg/kg folic acid. (B) Representative photomicrographs and graphs of kidney injury outcomes induced by folic acid (*P < 0.05, n = 4/group). Bar = 100 μm. Serum urea was measured at day 3 (*P < 0.05, n = 4/group). (C) Scheme: Male C57BL/6 mice underwent left kidney Ischemia Reperfusion Injury (IRI) (ischemia 20 min, n = 5/group). The group of HQH + IRI mice received 10 g/kg HQH extractum gavage two consecutive days before IRI surgery on left kidney and sacrificed three days later (ischemia 20 min, n = 5/group). The group of Dex + IRI mice intraperitoneal injected with 4 mg/kg Dex one hour before left kidney IRI surgery (ischemia 20 min, n = 5/group). (D) Representative photomicrographs and graphs of the kidney outcomes of the IRI models (*P < 0.05,**P < 0.01, n = 4 ~ 5/group). Bar = 50 μm. Serum urea was measured at day 3 after IRI (*P < 0.05, n = 4 ~ 5/group).
Discussion
This study identified the renoprotective properties of HQH extractum on cisplatin-induced acute kidney injury. HQH does not bind to cisplatin directly in vivo. It ameliorated cisplatin-induced TECs apoptosis and necrosis and preserved renal function by inhibition of the HMGB1/TLR4/NFκB/TNF-α pathway. We found that HQH pretreatment did not inhibit the anti-tumor activity of cisplatin and it demonstrated similar renoprotective effects in various models of AKI, similar to Dex. In considering the reports that corticosteroids retard the treatment effectiveness of solid tumors and shorten survival to a certain extent16,31,32, the present findings opened a new market for the prevention of kidney injury in patients with solid tumors.
Cisplatin-induced nephrotoxicity involves a complex multifactorial process. First, cisplatin has a direct toxic effect on the renal tubular cells and renal blood vessels, resulting in a decline in renal blood flow and glomerular filtration rate. Second, cisplatin induces renal interstitial inflammation and chronic interstitial fibrosis, leading to irreversible renal damage. Additionally, cisplatin activates a variety of cellular pathways, cytokines and signaling molecules in TECs, which causes renal inflammation, apoptosis and necrosis9,33,34,35.
Huaiqihuang (HQH) extractum is a Chinese herbal complex composed of Trametes robiniophila Murr. (Huaier), Lycium barbarum (Gouqi) and Polygonatum sibiricum (Huangjing). It has been identified that Huaiqihuang may protect against proteinuria by preventing MPC5 podocyte damage via targeting the p-ERK/CHOP pathway21 and enhancing Nephrin expression and the necrosis factor κg signaling pathway22. HQH not only activates macrophages, neutrophils, natural killer cells but also promotes T lymphocyte division, proliferation, maturation and differentiation36. Based on the immunoregulatory function of HQH extractum and the possible direct protective effect on injured kidneys, mice were pretreated with HQH before the injection of cisplatin. As a result, the number of renal tubular epithelial cell injuries, swelling, apoptosis and necrosis was decreased and after pretreatment with HQH, the renal tubular lesion was alleviated, which was significantly lower than that of the cisplatin-treated control group (P < 0.05). Such protective effects were further identified in FA- and IRI-induced AKI models (Fig. 6), suggesting that HQH is a broad-spectrum nephroprotective agent for AKI.
Glucocorticoids have been largely used in the clinical treatment of acute renal injury with a significant protective effect37,38,39, but the side effects of glucocorticoids for cancer patients, such as hair loss, endocrine disorders, weight gain and osteoporosis, make them unsuitable for long-term use. The more serious concern is that corticosteroids even inhibit the effectiveness of cancer treatment and shorten survival of solid tumor patients16,31,32. However, the key component of HQH, Trametes robiniophila Murr. (Huaier), has been identified as an effective chemotherapeutic agent for certain tumors, ‘with safe and broad anti-cancer spectrum’40,41,42,43,44. In the present study, we found that pretreatment with HQH and dexamethasone showed a similar renoprotective effect for cisplatin-induced acute kidney injury treatment. Thus, HQH can eventually be used in clinical prevention or treatment of cisplatin chemotherapy-induced kidney injury.
In summary, the current study indicated that HQH extractum protects the kidneys from cisplatin-induced injuries by inhibiting the HMGB1/TLR4/NFκB/TNF-α pathway in renal tubular cells. It shows potent therapeutic activity superior to that of dexamethasone for AKI in cancer patients. HQH extractum might be a potential adjuvant for clinical cisplatin therapy.
Materials and Methods
Cisplatin-Induced Acute Kidney Injury Model
Male C57BL/6 mice (7 weeks old, 20–23 g) were purchased from the Hubei Experimental Animal Research Center. All mice were housed in the experimental animal center at the Tongji Medical College, Huazhong University of Science and Technology with a 12/12-h light/dark cycle. After a minimum of 1 week of acclimatization, the mice (n = 5/group) were randomly divided into four groups. Two groups of mice were gavage pretreated daily with normal saline (NS) for 3 days. The third group was gavage pretreated daily with HQH (6 g/kg; Qidong GaiTianLi Pharmaceutical Co.; dissolved by NS, 0.4 g/ml) for 3 days. A single nephrotoxic dose of cisplatin (20 mg/kg; Nanjing Pharmaceutical Co.) was administered via intraperitoneal injection (ip) to the HQH-pretreated group and one NS-pretreated group on the third day 30 minutes before the pretreatment. A single dose of NS (0.015 ml/g) was administered ip to one NS-pretreated group as a control. The last group was administered ip a single dose of dexamethasone (Dex) (4 mg/kg; MaAnShan Fengyuan Pharmaceutical Co.) 30 minutes before cisplatin (20 mg/kg) ip. The mice were sacrificed on Day 3. All studies were approved by the Animal Care and Use Committee (ACUC) in Tongji Hospital and conducted in accordance with NIH guidelines.
FA-Induced Acute Kidney Injury Model
Male C57BL/6 mice (7 weeks old, 20–23 g) were purchased from the Hubei Experimental Animal Research Center. All mice were housed in the experimental animal center at the Tongji Medical College, Huazhong University of Science and Technology with a 12/12-h light/dark cycle. After a minimum of 1 week of acclimatization, the mice (n = 4/group) were randomly divided into four groups. Two groups of mice were gavage pretreated daily with normal saline (NS) for 3 days. The third group was gavage pretreated with HQH (6 g/kg; Qidong GaiTianLi Pharmaceutical Co.; dissolved by NS, 0.4 g/ml) daily for 3 days. A single nephrotoxic dose of FA (150 mg/kg; SIGMA; dissolved by 150 mM NaHCO3) was administered ip to the HQH-pretreated group and one NS-pretreated group per day 30 minutes after the gavage. A single dose of NaHCO3 (150 mM, 0.2 ml) was administered ip to one NS-pretreated group as a control. The last group was administered ip a single dose of Dex (4 mg/kg; MaAnShan Fengyuan Pharmaceutical Co.) 30 minutes before FA (150 mg/kg) ip. The mice were sacrificed on Day 3. All studies were approved by the Animal Care and Use Committee (ACUC) in Tongji Hospital and conducted in accordance with NIH guidelines.
IRI-Induced Acute Kidney Injury Model
Male C57BL/6 mice (7 weeks old, 20–23 g) were purchased from the Hubei Experimental Animal Research Center. All mice were housed in the experimental animal center at the Tongji Medical College, Huazhong University of Science and Technology with a 12/12-h light/dark cycle. After a minimum of 1 week of acclimatization, the mice (n = 5/group) were randomly divided into four groups. Two groups of mice were gavage treated daily with normal saline (NS) for 5 days. The third group was gavage treated with HQH (10 g/kg; Qidong GaiTianLi Pharmaceutical Co.; dissolved by NS, 0.4 g/ml) daily for 5 days. Left renal ischemia-reperfusion surgery was performed on mice in the HQH-pretreated group and one NS-pretreated group one hour after the gavage on Day 0. No surgery was performed on the mice in one NS-treated group, used as controls. The last group was administered ip of a single dose of Dex (4 mg/kg; MaAnShan Fengyuan Pharmaceutical Co.) one hour before the left renal ischemia-reperfusion surgery. The mice were sacrificed on Day 3. All studies were approved by the Animal Care and Use Committee (ACUC) in Tongji Hospital and conducted in accordance with NIH guidelines.
Serum Metabolites
Commercial kits were used to measure urea (Bioassay Systems, Hayward, CA).
Histologic, immunohistochemical and immunofluorescence staining
Paraffin-embedded renal sections (3 μm) were subjected to periodic acid-Schiff (PAS) staining to evaluate tubular injury. The tubule injuries include cast formation, tubular dilation and brush border loss, which was analyzed by the quantities of injury tubules in >10 random high-power fields (HPF). LTL (Lotus Tetragonolobus Lectins) carrying green fluorescence could be seen on the tubular brush border with specific coloring at the proximal end. However, when external factors or internal immune factors lead to renal tubular injury the brush edge is removed and there is no fluorescence expression. Therefore, LTL low coloring can be used as an indicator of renal tubular injury. For immunohistochemical (IHC) analysis, renal sections (3 μm) were incubated with 3% H2O2 for 20 min and nonspecific proteins were blocked with 10% goat serum for 30 min at room temperature. The slides were incubated with primary antibodies against either Kim-1 (1:1000, R & D), Ki-57 (1:200, Abcam), or HMGB-1 (1:200, Abcam) at 4 °C overnight. The slides were then incubated with the appropriate secondary antibody (HRP-conjugated secondary antibody for IHC and fluorescent-labeled secondary antibody for IF).
Western blot analysis
Renal tissues were homogenized in RIPA lysis buffer containing two kinds of protease inhibitors: cocktail (50×) and phenylmethanesulfonyl fluoride (PMSF, 100×). The extraction of nucleoprotein and cytoplasm protein was performed according to the manufacturer’s instructions (KeyGEN BioTECH). Total protein concentrations were determined using a BCA assay kit according to the manufacturer’s instructions. The kidney tissue total protein was separated by SDS-PAGE and the separated proteins were transferred to PVDF membranes. The membranes were blocked with 5% nonfat milk in TBS with 0.1% Tween-20 (TBST) for 1 h at room temperature and then probed with antibodies against either Bax (1:1000, Abclonal), Bcl-2 (1:1000, Abclonal), RIP3 (1:200, BioVision), MLKL (1:1000, Abgent), TLR-4 (1:500, Abgent), β-actin (1:2000, Google Bio.), p-Er/Erk or GAPDH (1:2000, Google Bio.) at 4 °C overnight and with p65 (1:1000, Rui Ying Bio.) for nucleoprotein and HMGB-1 (1:1000, Abcam) for cytoplasmic proteins. After being washed with TBST, the blots were incubated with an HRP-conjugated anti-IgG and the target bands were visualized with ECL plus reagents according to the manufacturer’s instructions. The intensities of the target bands were analyzed by densitometry and were normalized to GAPDH or β-actin using Quantity One software (BioRad, CA, USA); prior to analysis, the intensities of the target bands were normalized to the corresponding values of the control group.
Real-time quantitative RT-PCR
Total RNA extraction and reverse transcription were conducted using a GoScript reverse transcription system (Promega, USA). PCR enzymes and master mixes (Thermo Scientific, USA) were used for real-time PCR with primers specific to mouse GAPDH and TNF-α. The sequences for all primers are shown in Table 1. Relative expression levels were normalized to GAPDH and calculated using the 2−ΔΔCt method.
Nuclear Magnetic Resonance (NMR)
First, 450 μl cisplatin (1 mg/ml) was taken and then 50 μl D2O was added; the samples were thoroughly mixed and placed in NMR tubes. One-dimensional 1H NMR spectra were obtained. Then, 500 μl H2O (containing 10% D2O) was added to 152.9 mg HQH and thoroughly mixed and after centrifugation, the supernatant was placed in NMR tubes. One-dimensional 1H NMR spectra were obtained. Next, 450 μl cisplatin with 50 μl D2O was added to 156.5 mg HQH and thoroughly mixed and after centrifugation, the supernatant was placed in NMR tubes. One-dimensional 1H NMR spectra were obtained.
In vitro cell culture in TECs
Male C57BL/6 mice (4 weeks old, 12–15 g) were sacrificed and kidneys were removed; then, tubular epithelial cells were isolated and cultured in DMEM/F12 (Gibco) with 10% FBS medium at 37 °C in 5% CO2. Tubular epithelial cells were split into 96-well plates with HQH (0/6/12/18 mg/ml) when the cells were confluent. Then, a CCK8 experiment in renal proximal tubules was performed after HQH (0/6/12/18 mg/ml) pretreatment 12 h before cisplatin (75 μM) treatment for 12 h. The CCK8 experiment was performed according to the manufacturer’s instructions.
In vitro cell culture in HeLa cells
HeLa cells were cultured in DMEM-H (Gibco) with 10% FBS medium at 37 °C in 5% CO2. The HeLa cells were split into 96-well plates with DMEM-H medium (with 1% FBS), 30 mM cisplatin, 10 mg/ml HQH + 30 mM cisplatin, or 50 μM dexamethasone + 30 mM cisplatin when the cells were confluent; then, a CCK8 cell proliferation assay for HeLa cells was performed. The CCK8 experiment was performed according to the manufacturer’s instructions.
Statistical analysis
All statistics were analyzed using the Mann-Whitney U test; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Values are the means ± SEM. *P < 0.05 was considered to indicate a significant difference.
References
Istvan, A. & Robert, L. Safirstein, Cisplatin Nephrotoxicity. Seminars in Nephrology. 23, 460–64 (2003).
Wang, D. & Lippard, S. J. Cellular Processing of Platinum Anticancer Drugs. Nat Rev Drug Discov. 4, 307–20 (2005).
Milner, R., Barabas, K., Lurie, D. & Adin, C. Cisplatin: A Review of Toxicities and Therapeutic Applications. Vet Comp Oncol. 6, 1–18 (2008).
Sastry, J. & Kellie, S. J. Severe Neurotoxicity, Ototoxicity and Nephrotoxicity Following High-Dose Cisplatin and Amifostine. Pediatr Hematol Oncol. 22, 441–5 (2005).
Al-Fayea, T., Tsang, R. Y. & Au, H. J. Cisplatin Overdose: Toxicities and Management. Drug Saf. 32, 1109–22 (2009).
Ljubanovic, D. et al. Caspase-1-Deficient Mice Are Protected against Cisplatin-Induced Apoptosis and Acute Tubular Necrosis. Kidney Int. 66, 2202–13 (2004).
Okada, K. et al. Amelioration of Cisplatin-Induced Nephrotoxicity in Peroxiredoxin I-Deficient Mice. Cancer Chemotherapy and Pharmacology. 71, 503–09 (2012).
Costa, M. J., Werner, M., Mitchell, L. G. & Nayar, R. Nephrotoxicity of Xenobiotics. Clin Chim Acta. 237, 107–54 (1995).
Pabla, N. & Dong, Z. Cisplatin Nephrotoxicity: Mechanisms and Renoprotective Strategies. Kidney Int. 73, 994–1007 (2008).
Jacob, K. A. et al. Dexamethasone for Cardiac Surgery Study, Intraoperative High-Dose Dexamethasone and Severe Aki after Cardiac Surgery. J Am Soc Nephrol. 26, 2947–51 (2015).
Schaub, J. A. & Parikh, C. R. Acute Kidney Injury: Steroids for Prevention of Aki after Cardiopulmonary Bypass. Nat Rev Nephrol. 11(9), 509–10 (2015).
Shi, H., Chen, Z., Xie, J. & Chen, N. The Prevalence and Management of Multiple Myeloma-Induced Kidney Disease in China. Kidney Diseases. 1, 235–40 (2016).
Yadav, P., Cook, M. & Cockwell, P. Current Trends of Renal Impairment in Multiple Myeloma. Kidney Dis (Basel). 1, 241–57 (2016).
Thompson, D. M., Shord, S. S., Krempl, G. A. & Hanigan, M. H. Effect of Concurrent Medications on Cisplatin-Induced Nephrotoxicity in Patients with Head and Neck Cancer. Anticancer Drugs. 17(2), 207–15 (2006).
Tessoulin, B. et al. Carboplatin Instead of Cisplatin in Combination with Dexamethasone, High-Dose Cytarabine with or without Rituximab (Dhac +/−R) Is an Effective Treatment with Low Toxicity in Hodgkin’s and Non-Hodgkin’s Lymphomas. Ann Hemato. 96, 943–50 (2017).
Pitter, K. L. et al. Corticosteroids Compromise Survival in Glioblastoma. Brain. 139, 1458–71 (2016).
Lei, J. Y., Yan, L. N., Zhu, J. Q. & Wang, W. T. Hepatocellular Carcinoma Patients May Benefit from Postoperative Huaier Aqueous Extract after Liver Transplantation. Transplant Proc. 47, 2920–4 (2015).
Ren, J. et al. Inhibitory Effect of Extract of Fungi of Huaier on Hepatocellular Carcinoma Cells. Journal of Huazhong University of Science and Technology [Medical Sciences]. 29, 198–201 (2009).
Wei, Q. et al. Anticancer Effects Of huaier are Associated with Down-Regulation of P53. Asian Pac J Cancer Prev. 12, 2251–4 (2011).
Zhang, N., Kong, X., Yan, S., Yuan, C. & Yang, Q. Huaier Aqueous Extract Inhibits Proliferation of Breast Cancer Cells by Inducing Apoptosis. Cancer Sci. 101, 2375–83 (2010).
Li, T. et al. Huaiqihuang May Protect from Proteinuria by Resisting Mpc5 Podocyte Damage Via Targeting P-Erk/Chop Pathway. Bosn J Basic Med Sci. 16, 193–200 (2016).
Liu, H. et al. Huaiqihuang Granules () Reduce Proteinuria by Enhancing Nephrin Expression and Regulating Necrosis Factor Kappab Signaling Pathway in Adriamycin-Induced Nephropathy. Chin J Integr Med. 23, 279–87 (2017).
Price, P. M., Safirstein, R. L. & Megyesi, J. The Cell Cycle and Acute Kidney Injury. Kidney Int. 76, 604–13 (2009).
Qi, S. & Wu, D. Bone Marrow-Derived Mesenchymal Stem Cells Protect against Cisplatin-Induced Acute Kidney Injury in Rats by Inhibiting Cell Apoptosis. Int J Mol Med. 32, 1262–72 (2013).
Miyaji, A. K. T., Yasuda, H., Fujigaki, Y. & Hishida, A. Role of the Increase in P21 in Cisplatin-Induced Acute Renal Failure in Rats. J Am Soc Nephrol. 12, 900–08 (2001).
Xu, Y. et al. A Role for Tubular Necroptosis in Cisplatin-Induced Aki. J Am Soc Nephrol. 26, 2647–58 (2015).
Benedetti, G. et al. Tnf-Alpha-Mediated Nf-Kappab Survival Signaling Impairment by Cisplatin Enhances Jnk Activation Allowing Synergistic Apoptosis of Renal Proximal Tubular Cells. Biochem Pharmacol. 85, 274–86 (2013).
Weng, Y. D. H., Xie, Y., Liu, H. & Gong, F. Expression and Significance of Hmgb1, Tlr4 and Nf-Κb P65 in Human Epidermal Tumors. BMC Cancer. 13, 311 (2013).
Tadie, J. M. et al. Toll-Like Receptor 4 Engagement Inhibits Adenosine 5′-Monophosphate-Activated Protein Kinase Activation through a High Mobility Group Box 1 Protein-Dependent Mechanism. Mol Med. 18, 659–68 (2012).
Lotze, M. T. & Tracey, K. J. High-Mobility Group Box 1 Protein (Hmgb1):Nuclear Weapon in the Immune Arsenal. Nat Rev Immunol. 5(4), 331–42 (2005).
Shields, L. B. et al. Dexamethasone Administration During Definitive Radiation and Temozolomide Renders a Poor Prognosis in a Retrospective Analysis of Newly Diagnosed Glioblastoma Patients. Radiat Oncol. 10, 222 (2015).
Yecies, D. et al. Near-Fatal Gastrointestinal Hemorrhage in a Child with Medulloblastoma on High Dose Dexamethasone. Cureus. 9, e1442 (2017).
dos Santos, N. A., Carvalho Rodrigues, M. A., Martins, N. M. & dos Santos, A. C. Cisplatin-Induced Nephrotoxicity and Targets of Nephroprotection: An Update. Arch Toxicol. 86, 1233–50 (2012).
Faubel, S. et al. Cisplatin-Induced Acute Renal Failure Is Associated with an Increase in the Cytokines Interleukin (Il)-1beta, Il-18, Il-6 and Neutrophil Infiltration in the Kidney. J Pharmacol Exp Ther. 322, 8–15 (2007).
Yang, Y., Liu, H., Liu, F. & Dong, Z. Mitochondrial Dysregulation and Protection in Cisplatin Nephrotoxicity. Arch Toxicol. 88, 1249–56 (2014).
Ruichuan, W. J. C. Huaier Development and Clinical Application. Journal of Kunming Medical College. 24(1), 101–03 (2003).
Choi, H. M. et al. Glucocorticoids Attenuate Septic Acute Kidney Injury. Biochem Biophys Res Commun. 435, 678–84 (2013).
Rusai, K. et al. Dexamethasone Protects from Renal Ischemia/Reperfusion Injury: A Possible Association with Sgk-1. Acta Physiol Hung. 100, 173–85 (2013).
Yang, J. et al. The Protective Effect of Different Doses of Dexamethasone on Acute Kidney Injury Induced by Sepsis in Mice. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 25, 424–8 (2013).
Bin, C. M. H. & Yanbo, W. Clinical Study on the Effect of Huaier Granule on Quality of Life and Immune Function of Chemotherapy Patients. Liaoning Journal of Traditional Chinese Medicine. 34(7), 943–44 (2007).
Ji Delin, M. D. Effect of Huaier Granule on Immunity and Quality of Life in Patients with Gastric Cancer Undergoing Postoperative Concurrent Radiochemotherapy. Chinese Journal of Cancer. 19(1) (2010).
Yi, Z. Study on Anti - Cancer New Medicine Huaier Granules. Chinese Journal of Pharmaceutical Sciences. 33(5) (1988).
Ming, H. G. Y., Zhigang, L. & Wei, D. Effect of Huaier Granule Combined an Cdhemotherapy on Immunity in Patients with Colorectal Carcinoma Postoperation. Chinese Journal of Cancer. 14(7) (2005).
G-qiang, Z. & Yu-bao, Z. et al. Function and Clinical Application of Huaier Plaster in Comprehensive Therapy of Breast Cancer. Chinese Journal of Clinical Oncology and Rehabilitation. 11(6) (2004).
Acknowledgements
This work was supported by Qidong Gaitianli Pharmaceutical Co., Ltd. Thanks to Prof. Gang Chen, Professor of Gynecologic Oncology Laboratory, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, who donated HELA cells.
Author information
Authors and Affiliations
Contributions
The experiments were designed by Ying Yao, Rui Zeng, Gang Xu and Yujiao Guo and performed by Yujiao Guo, Meng Wang, Jingyi Mou and Zhi Zhao. Data analysis was carried out by Yujiao Guo, Guangchang Pei, Juan Yang, Fengming Zhu, Han Zhu, Meng Wang, Jingyi Mou and Zhi Zhao. Reagents were contributed by Guangchang Pei, Juan Zhan, Meng Wang, Zhi Zhao, Jingyi Mou, Yuxi Wang and Qian Yang. The manuscript was written by Yujiao Guo and Rui Zeng. The manuscript was read and approved by all of the authors.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, Y., Wang, M., Mou, J. et al. Pretreatment of Huaiqihuang extractum protects against cisplatin-induced nephrotoxicity. Sci Rep 8, 7333 (2018). https://doi.org/10.1038/s41598-018-25610-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-25610-6
This article is cited by
-
NLRP3 inflammasome in rosmarinic acid-afforded attenuation of acute kidney injury in mice
Scientific Reports (2022)
-
Natural products: potential treatments for cisplatin-induced nephrotoxicity
Acta Pharmacologica Sinica (2021)
-
Efficacy and safety of Huaiqihuang granule as adjuvant treatment for primary nephrotic syndrome in children: a meta-analysis and systematic review
World Journal of Pediatrics (2021)
-
Risk factors and clinical characteristics of tacrolimus-induced acute nephrotoxicity in children with nephrotic syndrome: a retrospective case-control study
European Journal of Clinical Pharmacology (2020)
-
Disulfiram and Its Copper Chelate Attenuate Cisplatin-Induced Acute Nephrotoxicity in Rats Via Reduction of Oxidative Stress and Inflammation
Biological Trace Element Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.