Abstract
Wheat yield components vary between different ecological regions and yield levels. Grain number responses to pre-anthesis dry matter (DM) and nitrogen (N) in increasing yield were always investigated in spike organs, neglecting the effect of non-spike organ nutrition or overall distribution. This paper determined the relationships between grain number and pre-anthesis DM and N in spike and non-spike organs under different yield levels, with using two sorts of field experiments (different water-nitrogen modes and cultivation management patterns) from 2012–2015 in Huang-Huai plain. The results indicated that improving yield under yield of <7500 kg ha−1 depends on increasing grain number per spike (GNs) or spike number (SN) or both, increased yield under higher yield of >7500 kg ha−1 mainly depends on GNs. GNs showed significant positive relationships with above-ground DM accumulation from jointing to anthesis under high or low yield levels. Rapid DM growth in spring achieves higher GNs. Spike and non-spike DM and N contents both demonstrated strong positive relationships with GNs, spike DM distribution also shows a positive correlation, but spike N distribution ratio show negatively correlation with GNs. Improved N distribution in non-spike organs and DM partition in spike organs conduce to increasing GNs.
Similar content being viewed by others
Introduction
In recent years, the burgeoning world population means that a substantial increase in grain yield is needed1,2. In wheat (Triticum aestivum L.) production, yield is an extremely complex trait, determined by agricultural manipulation and breeding attributes1,3. Research efforts focusing on physiological characteristics of wheat and improved production technologies aimed at breeding of new high-yielding wheat varieties are therefore necessary4,5. Wheat yield is determined by its components such as spike number per unit area (SN), grain number per spike (GNs) and thousand grain weight (TGW)4,6,7. Increased coordination between yield components is required to improve yield potential. Some studies in America and Europe have shown that both SN and GNs determine grain number per unit area (GNa)8,9,10, but the above three indicators were analyzed respectively in China. GNa and TGW are determined at different periods of growth. While GNa is largely dependent on floret set at pre-anthesis, TGW relies on post-anthesis grain-filling11. Yield potential can be improved by increasing GNa or TGW2,8,9,12, however, during the past century, increased yield have been largely attributed to increases in GNa13,14, frequently relating sink limitations in wheat15,16,17,18. With improvements in breeding technology, large spike varieties have been widely bred, and therefore, such the characters of sink may have some variation in increased grain yield. In addition, improvements in planting technology and increased sowing quantity have caused wheat population spikes to reach saturation, suggesting that improved yield is perhaps dependent on increased GNs. Nevertheless, the optimal approach to increase yield in modern cultivars remains unclear, as does the current role of GNs in increasing wheat yield.
The month before flowering is a critical period of floret differentiation in wheat, determining the GNs19. The process of floret primordia differentiation into fertile florets is dependent on nutrient supplements, with a strong positive relationship between floret survival and aboveground DM (especially spike DM) at anthesis20,21. Grain formation is therefore dependent on the pre-anthesis growth status14,22. Previous studies also revealed a closely relationship between spike DM at anthesis and grains number in wheat23,24. This suggested that adequate nutrition is the foundation for achieving higher grain number.
Nitrogen (N) is an important constituent of chlorophyll and metabolic enzyme activities, and one of the main limiting factors of crop production25,26. Previous studies have revealed a reduction in GNa with N deficiency before anthesis27, and a strong correlation between GNa and spike N content at anthesis20. Furthermore, Abbate et al.28 revealed that GNa is more strongly related to spike N than spike DM accumulation at anthesis. However, others confirmed the strongly relationship between GNa and spike DM, and further considered that spike DM dominated the determination of spike N to GNa20,29. To sum up, above-mentioned studies showed the importance of N to GNa. However, the above results mainly focus on the relationship between spike nutrient matter and grain formation, with few studies documenting the partition of nutrients in different organs. For example, Demotes-Mainard et al.30 studied changes in the proportion of DM and N in the spikes, but not the relationship between GNa and nutrient matter partitioning during spike growth. In addition, although previous research suggests that GNa is related to both spike N and DM, few studies have examined the role of non-spike organ N or DM during the process of floret differentiation and grain formation.
Huang-Huai Plain is the main producing area of winter wheat in China. With improvements in wheat varieties and soil fertility, effective spikes become saturated (over 6, 750, 000 spike ha−1), and the TGW reaches levels of 40~52 g. In the current experimental region, the long young ear differentiation period (160~170d) and more differentiated florets (about 180 spikes−1) suggest that GNs has great potential for improvement. In order to illuminate the contribution of GNs to yield and the effect of dry matter and nitrogen on GNs, we carried out a series of experiments in the Huang-Huai plain, China. The aims were to: (i) identify the main factors of yield components in increasing grain yield under different production levels (>7500 and <7500 kg ha−1); (ii) describe the relationship between aboveground DM and GNs during pre-anthesis at each production level; (iii) clarify the characteristics of spike and non-spike organ DM in relation to GNs during spike growth; and (IV) characterize the relationship between spike and non-spike organ N in terms of GNs during spike growth before anthesis.
Results
Changes in yield components and the impact on grain yield
Yield components included SN, GNs, and TGW. These components were not independent, but rather, correlated in a complex manner, combining to generate yield. Figure 1(A–D) showed varying relationships between yield components and yield. Above or below 7500 kg ha−1, relationship between yield components and yield showed larger differences, spike number showed significant negative correlation with grain yield when yield was above 7500 kg ha−1, but significant positive correlation under yield levels below 7500 kg ha−1; thousand grain weight showed poor correlation with grain yield under yield levels above 7500 kg ha−1, but significant negative correlation under yield below 7500 kg ha−1. And 7500 kg ha−1 could be as the boundary of analyzing relationships between each yield components and yield. Here we define yield of <7500 kg ha−1 as low yield levels and yield of >7500 kg ha−1 as higher yield levels. Both the two groups yield showed significantly positive relationship between GNa and yield (R2 = 0.240, >7500 kg ha−1; R2 = 0.711, <7500 kg ha−1), while the R2 of higher yield levels was lower than that of low yield levels. GNa could be further divided into two sub-components, i.e., SN and GNs. Low yield levels generated a significantly positive linear relationship between yield and SN (R2 = 0.629), while the higher yield level was poor negative correlation. Both the two yield levels resulted in significantly relationship between yield and GNs (R2 = 0.615, P < 0.01 for >7500 kg ha−1; R2 = 0.512, P < 0.01 for <7500 kg ha−1). The above higher R2 of SN than GNs indicated that SN was primary in increasing yield under low yield levels compared to GNs. Under higher yield levels, coefficient of determination between GNs and yield was higher than that between GNa and yield (Fig. 1A–C). So we mainly studied the effect of GNs on increasing yield. The correlation between TGW and yield was poor at higher yield levels, and significantly negative at low yield levels (R2 = 0.180, p < 0.01). These results suggest that improvements in yield of <7500 kg ha−1 are attributable to increases in both GNs and SN, the latter being the primary factor. However, in contrast, increases in yield of >7500 kg ha−1 are mainly dependent on a marked increase in GNs.
Relationships between GNs and pre-anthesis DM and DM growth rate
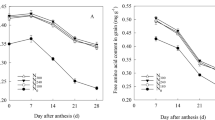
The difference between the whole data set and subsets representing different yield levels was noticeable in terms of the relationship between GNs and pre-anthesis aboveground DM at different stages. As shown in Table 1, GNs was significantly positively related to aboveground DM at different growth stages (r = 0.529~0.654, P < 0.01) when the experimental data was pooled. However, the relationships between DM and GNs differed when subsets of the two yield rates were applied. Coefficients of determination were significant except at wintering and turning green at higher yield levels, and at all stages at low yield levels. In addition, higher coefficients of determination (R2 = 0.248~0.508) were observed at higher yield levels than the low (R2 = 0.122~0.329) from jointing to anthesis (Table 2 and Fig. 1E and F). Coefficients of determination increased with growth from jointing to anthesis regardless of whether the data set was pooled or divided into subsets, reaching a maximum at anthesis (R2 = 0.508 at higher yield levels and R2 = 0.329 at the low, Fig. 1F). From the above, higher aboveground DM before anthesis was important in increased GNs, especially at higher yield levels.
Growth rates at different developmental stages affected aboveground DM accumulation. GNs was significantly positively related to DM growth rates at all stages expect wintering to turning green, regardless of whether the data was pooled or divided into subsets (Table 2). Coefficients of determination were higher (r = 0.316~0.650) at higher yield levels than the low (r = 0.259~505) from turning green to anthesis, reaching a maximum (r = 0.50~0.65) from jointing to anthesis (i.e. stem elongation stage), especially at higher yield levels. As shown in Fig. 1G, increasing DM accumulation from jointing to anthesis was important in increasing GNs.
Relationships between GNs and the dry weights of spike and non-spike organs before anthesis
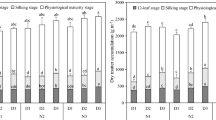
Comparisons of the relationships between GNs and organ nutrients at each yield level revealed a similar tendency at both higher yield levels and low yield levels. The respective datasets were therefore pooled. In this study, the aboveground organs were divided into two, spike and non-spike organs. The DM of both spike and non-spike organs showed a significantly positive relationship with GNs from booting to anthesis (R2 = 0.325~0.428). Furthermore, coefficients of determination for spike DM (R2 = 0.373~0.428) were higher than those for non-spike organs (R2 = 0.325~0.358) (Fig. 2A1–B3), suggesting that DM is really important factor affecting young ear development, especially spike DM. Moreover, the distribution ratio of spike DM showed a significantly positive correlation with GNs (R2 = 0.166~0.229; Fig. 2C1–C3). With growth, the demand for nutrients increases, with prior distribution to spike organs meeting the requirements of floret differentiation and grain development, and achieving higher GNs.
Relationships between GNs and N contents of spike and non-spike organs before anthesis
The partitioning of aboveground N to spike and non-spike organs was also investigated from booting to anthesis. GNs was significantly positively associated with N content and accumulation in both spike and non-spike organs at all growth phases up to anthesis (R2 = 0.360~0.528) (Figs 3A1–B3 and 4A1–B3). Considering organ N accumulation as a product of N concentration and DM, stronger relationships were observed between GNs and N accumulation in both spike and non-spike organs (R2 = 0.399~0.528, Fig. 3A1–B3) compared with N concentration (R2 = 0.360~0.466, Fig. 4A1–B3). Compared with spike organs (R2 = 0.360~0.472, Figs 3A1–A3 and 4A1–A3), non-spike organs showed stronger association (R2 = 0.394~0.528, Figs 3B1–B3 and 4B1–B3) with GNs in terms of N content and accumulation from booting to anthesis. Moreover, the distribution ratio of spike N showed a significantly negative relationship with GNs (R2 = 0.274~0.344, Fig. 4C1–C3), suggesting that preferential N distribution to non-spike organs and suitable reduction in nitrogen accumulation in spike organs are conducive to increases in GNs.
There was only a negligible difference between N concentrations in terms of the correlation between GNs and spike organ nutrients (R2 = 0.360~0.437, Fig. 3A1–A3) and DM (R2 = 0.373~0.428, Fig. 2A1–A3). However, in non-spike organs, N concentration had a higher coefficients of determination (R2 = 0.394~0.466, Fig. 3B1–B3) with GNs compared to DM (R2 = 0.325~0.358, Fig. 2B1–B3). Organ N accumulation is determined by both N concentration and DM, with coefficients of determination between GNs and N accumulation (R2 = 0.399~0.528, Fig. 4A1–B3) higher than those with DM (R2 = 0.325~0.428, Fig. 2A1–B3) in both spike and non-spike organs. Moreover, a higher R2 seemed to be gained from non-spike than spike organs.
Discussion
In general, increasing one or more main yield-components traits can improve yield for wheat, and the main factor usually varied in different eco-climatic regions2,4,8,9,12. Previous studies suggest that wheat yield improvements are primarily attributable to increases in grains number in the UK, Argentina, Mexico and Australia31,32,33, and grain weight concomitantly decreases with increasing GNa3. While in northern China, Zhou et al.34 found that from 1960 to 2000 improvements were primarily attributable to increased grain weight per spike. In this study, both low and higher yield levels showed significantly positive relationship between GNa and yield. Wheat yield was significantly positively related to GNa and its sub-components: GNs (R2 = 0.512) and SN (R2 = 0.629), and weakly with grain weight (R2 = 0.180) at low yield level. This suggests that increased yield is attributable to increases in GNa or GNs or SN, the latter being the primary factor at low yield levels. However, at higher yield levels, yield was significantly positively correlated with GNa (R2 = 0.240) and its sub-components GNs (R2 = 0.615), and poor correlation with SN (R2 = 0.040) and TGW (R2 = 0.007). These findings suggested that the function of GNs to improve yield is stronger than GNa and yield improvements are mainly attributable to marked increases in GNs at high yield levels.
Previous research suggests that yield components vary depending on plant growth conditions, with GNa being more plastic and responsive to resource availability24,35,36,37. DM production and spike weight during period of spike growth are really important components in enhancing GNa24,35,38,39. Arduini et al.40 reported that higher aboveground DM at heading resulted in higher remobilization of resources to the developing grains, promoting floret development and grain set. In our study, GNs showed a significant relationship with aboveground DM from jointing to anthesis. Related research confirms that crop growth rate (aboveground DM per unit area per day) during the critical period before anthesis is positively associated with spikelet and grain number41. In our study, the rate of aboveground DM production was strongly related to GNs during stem elongation stage from jointing to anthesis. During this period of young ear differentiation, higher DM accumulation rate led to more DM accumulation at anthesis, which was beneficial to increasied GNs. In the study region, wheat growth starts early, reaching an exuberant rate in spring (March to April), resulting in high vegetative mass and a superior canopy structure, thereby increasing radiation interception and radiation-use efficiency (RUE). It has been suggested that increased RUE before anthesis is directly related to increases in GNa31,41,42. Moreover, research suggests that improved total GNa is largely associated with more grains per spikelet in both wheat and rice43,44, and strongly positively related to spike dry weight at flowering20,45,46. In the present study, strong relationships between GNs and spike DM were observed from booting to anthesis. Furthermore, a significant relationship between GNs and non-spike DM was observed at three stages consistent with previous findings19,35. Overall, the coefficients of determination between spike DM and GNs were greater than that with non-spike DM. Our results also suggest that distribution ratio of spike DM is significantly correlated with GNs at different growth stages, with increased partitioning of assimilates to spike organs increasing the GNs. Compared to non-spike organs, spike DM (both the accumulative amount and distribution ratio) is therefore more beneficial to GNs.
N is an essential element of plant growth and one of the main limiting factors of crop production25. Rational application of N fertilizer can promote the synthesis of chlorophyll, increase leaf area and improve photosynthesis during crop growth47. When deficient, aerial DM and / or aerial N content decreases, thereby decreasing DM partitioning to the spikes and subsequent GNa in both wheat and barley27,48. N nutrition strongly affects N absorption and distribution in both spike and non-spike organs. Pre-anthesis spike N can be considered the result of partitioning of aboveground N accumulation between spike and non-spike organs. It was previously revealed that spike N content at anthesis is strongly related to grain number27,28,49. In our study, GNs was strongly related to spike N concentration and accumulation during spike growth (R2 = 0.360~0.472). Moreover, compared to spike organs, N concentration and accumulation in the non-spike organs was more strongly correlated with GNs before anthesis (R2 = 0.394~0.528). Increased partitioning of N to non-spike organs such as the leaf and stem is therefore conducive to increased green leaf area and physiological activities that improve radiation interception, RUE and DM production31,42,50, which would help to provide adequate nutrition for floret development and grain formation. Analysis of N distribution between spike and non-spike organs further revealed that GNs was significantly negatively related to the N distribution ratio of the spikes. This phenomenon was most likely caused by the balance between plant carbon(C) and nitrogen (N) metabolism, with a decreased proportion of carbon - nitrogen in non-spike organs contributing to the transportation of photosynthetic products to the spikes51. Qiu et al.52 observed that C/N value increased from booting to grain filling stage in spike organ, and the C/N value in spike organ was higher than that in non-spike organs in wheat. This indicated that more transported photosynthate to spike organ and much allocated N in non-spike organs would be conducive to promoting floret development to grain during spike growth stages. Furthermore, more N distribution in non-spike organs such as stem and leaves was beneficial to improve photosynthetic enzyme activities and photosynthesis, and subsequently produce more photosynthate53. Previous studies found that higher photosynthate transported to spike organ from non-spike organs was conducive to spike development, and the number of surviving florets and final grains were highly related to the proportion of photosynthate assimilates partitioned to the spikes54,55. These suggest that although spike N concentration and accumulation are beneficial to grain formation, an increased N distribution ratio in non-spike organs, is more beneficial to increased GNs.
GNs is a sub-component of GNa and certainly associated with GNa. Conxita et al.56 found that GNs could explain the improvement of GNa well. In our experiments, the correlation coefficient (r) between GNs and GNa reached 0.84 (P < 0.01). Previous studies have examined the role of plant N and DM in determining GNa or GNs. For GNa, Abbate et al.28 revealed that GNa was more closely related to spike N than spike DM accumulation. In contrast, Prystupa et al.23 revealed that GNa was more closely related to spike DM accumulation than spike N content. For GNs, Demotes-Mainard et al.27 found that GNs was related to both spike N and DM. Qiu et al.52 reported that GNs was more closely related to spike DM. In our study, we mainly focused on GNs. The relationship between GNs and spike DM (R2 = 0.373~0.428) was only slightly different compared with that between spike N concentration (R2 = 0.360~0.437). However, the N concentration and N accumulation in non-spike organs were both more strongly correlated with GNs compared to non-spike DM. The above differences may be due to differences in the wheat cultivar or organs examined, and thus, further research is needed to confirm the most important component in terms of GNs. This study provides a good theoretical basis for wheat production practice, further studies are now required to determine how to better promote the development of florets and grain setting.
Conclusions
The major factors affecting yield improvements showed differences under different yield levels in the Huang-Huai Plain. Improvements in yield at low yield of <7500 kg ha−1 were attributed to increases in GNs and SN, the latter being the primary factor. Under a higher yield of >7500 kg ha−1, increased yield was mainly dependent on marked increases in GNs. GNs was significantly related to above-ground DM and DM accumulation from jointing to anthesis (stem rapidly elongate), higher DM growth rate results in an increase in DM accumulation at anthesis, which is highly beneficial to increased GNs and a subsequent increase in yield. For pre-anthesis nutrient (DM and N) accumulation and distribution to different organs, GNs demonstrated strong relationships with both spike and non-spike DM and N. Spike DM (both accumulative amount and distribution ratio) was more beneficial to improvements in GNs than non-spike organs. While spike N distribution ratio shows a negative correlation with GNs, and increased N accumulation and distribution ratio to non-spike organs were more conducive to improved GNs. Thus, nutrient production and distribution differ between non-spike and spike organs in terms of improvements in GNs, with increased N allocation in non-spike organs contributing to the transportation of photosynthetic products to the spikes. Generally recognizing that improved spike DM production is therefore more beneficial in promoting floret development into kernels. So, accelerated DM accumulation in the spring and improved partitioning of N to non-spike organs and DM to spike organs via cultivation management during jointing to anthesis would benefit GNs, thereby increasing grain yield.
Materials and Methods
Experimental site and production situation
Two field experiments were carried out with two cultivars Yumai 49–198 and Aikang58 at three sites from October 2012 to June 2015 in the Huang-huai plain, China: Shangshui (114°29′E, 33°32′N), Kaifeng (114°37′E, 34°44′N) and Wenxian (112°99′E, 34°92′N), located in southern, central and northern Henan Province, respectively. The cultivar Yumai 49–198 and Aikang 58 both are multi spike cultivars and widely planted in this experimental area. Both the two cultivars have characters of strong tillering ability (6–8 tillers per plant at jointing stage; 3–5 spikes per plant at maturity), high lodging resistance, cold resistance and high yield potentiality in field production. The yield levels of this region increased from 3000 kg ha−1 (1950s) to 5500 kg ha−1 (1980s) and now above 7500 kg ha−1 (after 2010 year)57,58. In addition, for analyzing the 161 wheat cultivars passed the examination and approval in Henan Province from 2001 to 2015, the yields of 58.3% cultivars exceed 7500 kg ha−1. 7500 kg ha−1 is classified as a higher yield standard to analyze the approaches to higher yield under different yield levels in this region58. The experimental sites have a warm temperate semi-humid continental monsoon climate, which result in a long tillering stage (110~120 d) and young ear differentiation period (160~170 d). Specific properties of plough layer soil and meteorological conditions during wheat growing period are shown in Table 3. The preceding crop grown in all three experimental sites was maize, with the straw returned to the field by machine after harvest.
Experimental Design
Cultivation patterns experiment
Four cultivation patterns were tested using the wheat cultivar Yumai 49–198 as follows: local traditional cultivation pattern (TCP); optimized cultivation pattern in comparison with TCP (OCP); super high yielding cultivation pattern (SHP); and high yielding high efficiency pattern (HEP). In all cases, the date of sowing was Oct. 12~17. Under TCP, rotary tillage (about 15 cm) was carried out before sowing and no rolling was carried out after seeding. Irrigation was applied after sowing and during returning green stage. Equal row spacing of 20 cm and a large sowing quantity (225 kg ha−1) of seeds were applied. Under OCP, SHP and HEP, mechanical deep ploughing (over 25 cm deep) was adopted, with multiple rotary harrowing and rolling after seeding. Irrigation was applied after sowing and during jointing stage. Equal row spacing of 20 cm was adopted in OCP, and of 17 cm in SHP and HEP. A sowing quantity of 150 kg ha−1 was applied in OCP, and 120 kg ha−1 in both SHP and HEP. Irrigation was carried out, and organic, microelement and phosphate and potassium fertilizers applied before sowing (Table 4). Under TCP, all of N, phosphate (P) and potassium (K) fertilizers were applied before sowing as a base fertilizer. Under OCP and SHP, 50% of N fertilizer and all of P and K fertilizers were applied before sowing, with the remaining 50% N fertilizer applied at jointing stage. Under HEP, 40% of N fertilizer and 70% of P and K fertilizers were applied before sowing, with the remaining 60% N fertilizer and 30% P and K fertilizers applied at jointing stage. In addition, all of the organic and microelement fertilizers were applied before sowing. All four treatments followed a randomized block design with three replications, each plot 165 m2 (15 m in length and 11 m wide).
Water-nitrogen mode experiments
Water-nitrogen mode experiments were carried out using the wheat cultivar Aikang 58 (sowing quantity was 150 kg ha−1), sown on Oct 12~17 at Shangshui and Wenxian. The experimental design in Shangshui consisted of a combination of two irrigation regimes (no irrigation and single irrigation at jointing stage (750 m3 ha−1), respectively) and four N rates (0,180, 240 and 300 N kg ha−1, respectively). In Wenxian, the design consisted of three irrigation regimes (no irrigation; single irrigation at the jointing stage (750 m3 ha−1), and irrigation at jointing plus anthesis stages (750 m3 ha−1 each), respectively) and four N rates (as above). As base fertilizers, 50% N fertilizer (Wenxian) or 70% N fertilizer (Shangshui) was applied with, all of P (100 kg P ha−1) and K (150 kg K ha−1) fertilizers before sowing. The remaining N fertilizer was applied at jointing stage. The experimental plots were arranged in a split-plot design with three replicates, each plot 40.6 m2 (7 m in length and 5.8 m in width). The main plot was assigned to the irrigation regime and subplots to the N rate.
Sampling and Measurements
Dry matter
Aboveground DM was measured at different growth stages (wintering, turning green, jointing, booting, heading and anthesis). Twenty plants were randomly sub-sampled and divided into stems and leaves during wintering to jointing stages, and into stems, leaves (non-spike organs: stems + leaves) and spike organs during booting to anthesis stages. All samples were oven dried at 65 °C and DM weighed. The distribution ratio of spike DM was calculated as the ratio of spike DM accumulation to total above-ground DM.
Nitrogen content
N content was determined using the Kjeldahl method for each of the organs for which DM was determined during spike growth period (booting, heading and anthesis). Spike N accumulation was calculated as the product of spike N content and spike DM. Non-spike N accumulation was calculated as the product of non-spike N content and non-spike DM. The distribution ratio of spike N was calculated as the ratio of spike N accumulation to total N in the above-ground DM.
Grain yield and yield components
Prior to harvest, the number of spikes per unit area was determined using fixed sampling points for 1 m’s double row for each treatment. At maturity, 80 wheat plants were sampled for determination of GNs, two samples of 500 grains were counted and weighed to calculate TGW. An area of 5 m2 was harvested, and the harvested samples threshed and dried for calculation of grain yield (kg ha−1).
Based on the above data, yield component data were obtained from experiments of different cultivation patterns and water-nitrogen modes from 2012–2015. DM and growth rate data were obtained from the experiments of cultivation patterns from 2012–2015 and water-nitrogen mode experiments from 2013–2015. Spike and non-spike data were obtained came from experiments of cultivation patterns and water-nitrogen modes from 2013–2015.
Statistical analysis
Experimental data were analyzed and processed using Microsoft Office 2013. Correlations among physiological factors were determined using Pearson correlation analysis with SPSS 19.0, with significance at P < 0.05 and P < 0.01.
References
Sehgal, D. et al. Identification of genomic regions for grain yield and yield stability and their epistatic interactions. Sci Rep 7, 41578 (2017).
Wu, W. et al. Genetic progress in wheat yield and associated traits in China since 1945 and future prospects. Euphytica 196, 155–168 (2014).
Acreche, M. M. & Slafer, G. A. Grain weight response to increases in number of grains in wheat in a Mediterranean area. Field Crops Res. 98, 52–59 (2006).
Qin, X. et al. Wheat yield improvements in China: Past trends and future directions. Field Crops Res. 177, 117–124 (2015).
Foley, J. A. et al. Solutions for a cultivated planet. Nature 478, 337 (2011).
Slafer, G. Genetic basis of yield as viewed from a crop physiologist’s perspective. Ann. Appl. Biol. 142, 117–128 (2003).
Sadras, V. O. & Slafer, G. A. Environmental modulation of yield components in cereals: heritabilities reveal a hierarchy of phenotypic plasticities. Field Crops Res. 127, 215–224 (2012).
Brancourthulmel, M. et al. Genetic Improvement of Agronomic Traits of Winter Wheat Cultivars Released in France from 1946 to 1992. Crop Sci. 43, 37–45 (2003).
Pedró, A., Savin, R. & Slafer, G. A. Crop productivity as related to single-plant traits at key phenological stages in durum wheat. Field Crops Res. 138, 42–51 (2012).
Bustos, D. V., Hasan, A. K., Reynolds, M. P. & Calderini, D. F. Combining high grain number and weight through a DH-population to improve grain yield potential of wheat in high-yielding environments. Field Crops Res. 145, 106–115 (2013).
Peltonen-Sainio, P., Kangas, A., Salo, Y. & Jauhiainen, L. Grain number dominates grain weight in temperate cereal yield determination: evidence based on 30 years of multi-location trials. Field Crops Res. 100, 179–188 (2007).
Sun, Y. et al. Changes in the yield and associated photosynthetic traits of dry-land winter wheat (Triticum aestivum L.) from the 1940s to the 2010s in Shaanxi Province of China. Field Crops Res. 167, 1–10 (2014).
Fischer, R. A. Number of kernels in wheat crops and the influence of solar radiation and temperature. J. Agr. Sci. 105, 447–461 (1985).
Sadras, V. O. & Lawson, C. Genetic gain in yield and associated changes in phenotype, trait plasticity and competitive ability of South Australian wheat varieties released between 1958 and 2007. Crop Pasture Sci. 62, 533–549 (2011).
Slafer, G. A. & Savin, R. Source—sink relationships and grain mass at different positions within the spike in wheat. Field Crops Res. 37, 39–49 (1994).
Miralles, D. J. & Slafer, G. A. Individual grain weight responses to genetic reduction in culm length in wheat as affected by source-sink manipulations. Field Crops Res. 43, 55–66 (1995).
Sinclair, T. R. & Jamieson, P. D. Grain number, wheat yield, and bottling beer: an analysis. Field Crops Res. 98, 60–67 (2006).
Fischer, R. A. The importance of grain or kernel number in wheat: A reply to Sinclair and Jamieson. Field Crops Res. 105, 15–21 (2008).
Bancal, P. Positive contribution of stem growth to grain number per spike in wheat. Field Crops Res. 105, 27–39 (2008).
Fischer, R. A. Wheat physiology: a review of recent developments. Crop Pasture Sci. 62, 95–114 (2011).
González, F. G., Miralles, D. J. & Slafer, G. A. Wheat floret survival as related to pre-anthesis spike growth. J. Exp. Bot. 62, 4889–4901 (2011).
Sadras, V. O. & Denison, R. F. Do plant parts compete for resources? An evolutionary viewpoint. New Phytol. 183, 565 (2009).
Prystupa, P., Savin, R. & Slafer, G. A. Grain number and its relationship with dry matter, N and P in the spikes at heading in response to N × P fertilization in barley. Field Crops Res. 90, 245–254 (2004).
Xie, Q., Mayes, S. & Sparkes, D. L. Preanthesis biomass accumulation of plant and plant organs defines yield components in wheat. Eur. J. Agron. 81, 15–26 (2016).
Hirel, B., Le, G. J., Ney, B. & Gallais, A. The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Exp. Bot. 58, 2369–2387 (2007).
Kong, L. et al. Excessive nitrogen application dampens antioxidant capacity and grain filling in wheat as revealed by metabolic and physiological analyses. Scientific Reports 7 (2017).
Demotesmainard, S., Jeuffroy, M. H. & Robin, S. Spike dry matter and nitrogen accumulation before anthesis in wheat as affected by nitrogen fertilizer: relationship to kernels per spike. Field Crops Res. 64, 249–259 (1999).
Abbate, P. E., Andrade, F. H. & Culot, J. P. The effects of radiation and nitrogen on number of grains in wheat. J. Agr. Sci. 124, 351–360 (1995).
Demotes-Mainard, S. & Jeuffroy, M.-H. Effects of nitrogen and radiation on dry matter and nitrogen accumulation in the spike of winter wheat. Field Crops Res. 87, 221–233 (2004).
Demotesmainard, S. & Jeuffroy, M. H. Partitioning of dry matter and nitrogen to the spike throughout the spike growth period in wheat crops subjected to nitrogen deficiency. Field Crops Res. 70, 153–165 (2001).
Foulkes, M. J., Reynolds, M. P. & Sylvester-Bradley, R. Genetic Improvement of Grain Crops: yield potential. Crop Physiology: Applications for Genetic Improvement and Agronomy, 355–385 (2009).
Zhang, H., Turner, N. C. & Poole, M. L. Increasing the harvest index of wheat in the high rainfall zones of southern Australia. Field Crops Res. 129, 111–123 (2012).
Gaju, O. et al. Relationships between physiological traits, grain number and yield potential in a wheat DH population of large spike phenotype. Field Crops Res. 164, 126–135 (2014).
Zhou, Y. et al. Genetic improvement of grain yield and associated traits in the southern China winter wheat region: 1949 to 2000. Euphytica 157, 465–473 (2007).
Bindraban, P. S., Sayre, K. D. & Solismoya, E. Identifying factors that determine kernel number in wheat. Field Crops Res. 58, 223–234 (1998).
Sadras, V. O. Evolutionary aspects of the trade-off between seed size and number in crops. Field Crops Res. 100, 125–138 (2007).
Foulkes, M. J. et al. Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. J. Exp. Bot. 62, 469–486 (2011).
Miralles, D. J. & Slafer, G. A. Radiation interception and radiation use efficiency of near-isogenic wheat lines with different height. Euphytica 97, 201–208 (1997).
Pedro, A., Savin, R., Habash, D. Z. & Slafer, G. A. Physiological attributes associated with yield and stability in selected lines of a durum wheat population. Euphytica 180, 195–208 (2011).
Arduini, I., Masoni, A., Ercoli, L. & Mariotti, M. Grain yield, and dry matter and nitrogen accumulation and remobilization in durum wheat as affected by variety and seeding rate. Eur. J. Agron. 25, 309–318 (2006).
Horie, T. et al. Physiological traits associated with high yield potential in rice. Rice Science: Innovations and Impact for Livelihood. IRRI, Manila, 117–146 (2003).
Shearman, V. J., Sylvesterbradley, R., Scott, R. K. & Foulkes, M. J. Physiological processes associated with Wheat Yield Progress in the UK. Crop Sci. 45, 175–185 (2005).
Fischer, R. A. & Stockman, Y. M. Increased Kernel Number in Norin 10-Derived Dwarf Wheat: Evaluation of the Cause. Functional Plant Biology 13, 767–784 (1986).
Peng, S. et al. Grain yield of rice cultivars and lines developed in the Philippines since 1966. Crop Sci. 40, 307–314 (2000).
Fischer, R. A. Understanding the physiological basis of yield potential in wheat. J. Agr. Sci. (Cambridge) 145, 99–114 (2007).
Miralles, D. & Slafer, G. A. Sink limitations to yield in wheat: how could it be reduced? J. Agr. Sci. 145, 139–149 (2007).
Lauerer, M. et al. Decreased ribulose-1,5-bisphosphate carboxylase-oxygenase in transgenic tobacco transformed with “antisense” rbcS. Planta 190, 332–345 (1993).
Fischer, R. A. Irrigated spring wheat and timing and amount of nitrogen fertilizer. II. Physiology of grain yield response. Field Crops Res. 33, 57–80 (1993).
Prystupa, P., Slafer, G. A. & Savin, R. Leaf appearance, tillering and their coordination in response to NxP fertilization in barley. Plant Soil 255, 587–594 (2003).
Bingham, I. J., Blake, J., Foulkes, M. J. & Spink, J. Is barley yield in the UK sink limited?: I. Post-anthesis radiation interception, radiation-use efficiency and source–sink balance. Field Crops Res. 101, 198–211 (2007).
Lyu, X. et al. Effect of exogenous polyamines on mechanism of floret degeneration in wheat. Acta Agron. Sin. 42, 1391–1401 (2016).
Qiu, Z. et al. The relation between floret development and carbon-nitrogen metabolism in winter wheat. Acta Agron. Sin. 6, 139–146 (1980).
Dong, C. et al. Influence of nitrogen source and concentrations on wheat growth and production inside “Lunar Palace-1”. Acta Astronaut 144, 371–379 (2018).
Slafer, G. A. & Andrade, F. H. Physiological attributes related to the generation of grain yield in bread wheat cultivars released at different eras. Field Crops Res. 31, 351–367 (1993).
Miralles, D. J., Katz, S. D., Colloca, A. & Slafer, G. A. Floret development in near isogenic wheat lines differing in plant height. Field Crops Res. 59, 21–30 (1998).
Conxita, R. et al. Genetic changes in durum wheat yield components and associated traits in Italian and Spanish varieties during the 20th century. Euphytica 155, 259–270 (2007).
Ru, Z. et al. High-yield potential and effective ways of wheat in Yellow &Huai River Valley facultative winter wheat region. Sci. Agric. Sin. 48, 3388–3393 (2015).
Wei, Y. et al. Advances in study of quality property improvement of winter wheat in China. Sci. Agric. Sin. 46, 4189–4196 (2013).
Acknowledgements
This work was supported by the Special Fund for Agro-scientific Research in the Public Interest [grant number 201203096], the National Natural Science Foundation of China [grant numbers 31671624, 31571607], the Program of Science & Technology Innovation Talents in Universities of Henan Province, China [grant number 17HASTIT036], the Thirteenth Five-year Plan of National Key Research Project of China [grant number 2016YFD0300105], and the National Agriculture Technology Research System of China [grant number CARS-03-01-22].
Author information
Authors and Affiliations
Contributions
W.F., Y.J.Z. and T.G. designed experiments; J.D., Y.W., Y.Z. and X.R. carried out experiments; J.D. and W.F. analyzed experimental results and wrote the manuscript; Y.S. and Y.H.W. assisted manuscript writing and editing.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Duan, J., Wu, Y., Zhou, Y. et al. Grain number responses to pre-anthesis dry matter and nitrogen in improving wheat yield in the Huang-Huai Plain. Sci Rep 8, 7126 (2018). https://doi.org/10.1038/s41598-018-25608-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-25608-0
This article is cited by
-
Chromosomal mapping of a major genetic locus from Agropyron cristatum chromosome 6P that influences grain number and spikelet number in wheat
Theoretical and Applied Genetics (2024)
-
Exploring the Association Between Inherent Soil Nutrient Availability and Crop Responses Following Long-term Cessation of Fertilizer Inputs in a Wheat–Maize Cropping System
Journal of Soil Science and Plant Nutrition (2022)
-
Nitrogen Fertilization and Precipitation Affected Wheat Nitrogen Use Efficiency and Yield in the Semiarid Region of the Loess Plateau in China
Journal of Soil Science and Plant Nutrition (2022)
-
Synchronizing nitrogen supply and uptake by rainfed maize using mixed urea and slow-release nitrogen fertilizer
Nutrient Cycling in Agroecosystems (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.