Abstract
We developed an insomnia classification algorithm by interrogating an electronic medical records (EMR) database of 314,292 patients. The patients received care at Massachusetts General Hospital (MGH), Brigham and Women’s Hospital (BWH), or both, between 1992 and 2010. Our algorithm combined structured variables (such as International Classification of Diseases 9th Revision [ICD-9] codes, prescriptions, laboratory observations) and unstructured variables (such as text mentions of sleep and psychiatric disorders in clinical narrative notes). The highest classification performance of our algorithm was achieved when it included a combination of structured variables (billing codes for insomnia, common psychiatric conditions, and joint disorders) and unstructured variables (sleep disorders and psychiatric disorders). Our algorithm had superior performance in identifying insomnia patients compared to billing codes alone (area under the receiver operating characteristic curve [AUROC] = 0.83 vs. 0.55 with 95% confidence intervals [CI] of 0.76–0.90 and 0.51–0.58, respectively). When applied to the 314,292-patient population, our algorithm classified 36,810 of the patients with insomnia, of which less than 17% had a billing code for insomnia. In conclusion, an insomnia classification algorithm that incorporates clinical notes is superior to one based solely on billing codes. Compared to traditional methods, our study demonstrates that a classification algorithm that incorporates physician notes can more accurately, comprehensively, and quickly identify large cohorts of insomnia patients.
Similar content being viewed by others
Introduction
Sleep-related complaints are second only to complaints of pain as a reason to seek medical attention1. Characterized by difficulty falling asleep, staying asleep, or waking unrefreshed, insomnia has a strong impact on the daily lives of affected individuals. In the United States, insomnia is associated with 252.7 million days of lost work per year and an annual cost of $63.2 billion2.
Insomnia has been described as an underdiagnosed and undertreated disease in multiple studies3,4,5 and its prevalence has been estimated between 10% and 40%, depending on the definition of insomnia used6. These prevalence estimates suggest a potentially high medical burden of insomnia and associated conditions, but existing data are typically from smaller studies in selected populations7,8,9. Furthermore, insomnia studies are insufficient in number, and limited evidence exists for managing more complex insomnia patients and for studying the long-term effects of insomnia treatments10. Robust studies of insomnia require long durations and considerable population sizes to detect sufficient outcomes for analysis, and the low frequency of insomnia diagnosis codes in longitudinal databases likely underestimates insomnia’s actual prevalence11,12,13. Therefore, there is a pressing need to develop large cohorts of patients with insomnia that are more thoroughly inclusive and that can be assessed over extended durations.
The capabilities of advances in data analytics with the large volume of data available in electronic medical records (EMRs) allow the use of highly accurate mechanisms to process large collections of clinical notes and enable researchers to conduct a variety of analyses that can add to insights gained from traditional cohorts14,15,16,17,18,19,20. Specifically, the development of classification algorithms that combine coded data, such as prescriptions or diagnosis codes (structured data), with narrative, textual data, such as physician narrative notes (unstructured data), has been shown to increase the accuracy of identifying patient cohorts with specific diseases21.
Our analysis of EMR data showed that documentation of insomnia symptoms in the EMR is rarely detailed enough to use formal case definitions of insomnia such as those from the Diagnostic and Statistical Manual of Mental Disorders (DSM-V)22. Therefore, we used a more empirical approach to identify insomnia patients. This definition includes criteria such as a physician-documented diagnosis of insomnia (whether via a billing code or a text note), electronic prescriptions for insomnia medications (those indicated only for insomnia), or physician documentation of sleep issues consistent with insomnia (for brevity, we used the term “physician-documented insomnia” to describe this empirical definition). Using a physician-documented insomnia definition allows for the identification of insomnia patients in the EMR who had sleep features not documented in a formal DSM-V diagnostic manner.
The objective of our study was to develop a classification algorithm to identify patients with physician-documented insomnia, even when no indication was found in their traditional structured records (such as coded insomnia). We evaluated whether a classification methodology that incorporates clinical narrative notes could be superior to one that solely relies on billing codes. We hypothesized that an EMR text-based algorithm approach allows for more accurate identification of a large number of patients with physician-documented insomnia, especially in comparison to traditional billing-code-based classification schemes. Our study emphasizes the critical need to develop more efficient methods to classify patients with insomnia.
Results
Identifying a Physician-Documented Insomnia Cohort using EMR Data
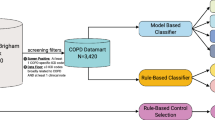
Applying penalized logistic regression to a training set of patients manually annotated for their physician-documented insomnia status led to the selection of seven variables in the final algorithm (Fig. 1). Structured variables in the algorithm, for instance, included the number of ICD-9 codes for insomnia and the number of prescriptions for sleep-related medications. Unstructured variables included the number of narrative note mentions of sleep difficulties as well as the number of narrative note mentions of psychiatric disorders. An additional structured covariate called “# of EMR facts” included the total number of data entries associated with the patient, including, for example, medications, laboratory measurements, notes, comorbidities, and office and emergency room visits. This covariate was able to estimate the patient’s degree of utilization of the care system. The rationale was that patients with a larger number of data entries tend to be sicker than others and thus associated with a higher level of utilization. The algorithm had an area under the receiver operating characteristic curve (AUROC) = 0.83 [95% CI, 0.76–0.90] based on a manual chart review (Fig. 2). Applying this algorithm to the entire insomnia candidate datamart led to a cohort of 36,810 physician-documented insomnia patients (associated with our chosen specificity threshold of 97%).
The classification used a logistic regression algorithm that included both structured and unstructured variables. Of note, only 6,159 of 36,810 patients with physician-documented insomnia had an insomnia-related billing code in their EMR history (17%). We constructed analogous logic regression algorithms with different combinations of data subtypes to explore the effect of different data types on algorithm performance. In contrast to the AUROC of 0.83 [95% CI, 0.76–0.90] for our algorithm using both unstructured and structured variables, the AUROC was 0.78 [95% CI, 0.70–0.85] for an algorithm using structured data only, 0.73 [95% CI, 0.67–0.79] using unstructured data only, and only 0.55 [95% CI, 0.51–0.58] using ICD-9 codes only (Fig. 2). Based on a manual chart review, the positive predictive value (PPV) was 0.81 (see Supplementary Table 4). The similar values of AUROCs in the derivation and validation sets in the combined structured and unstructured scenario support the assumption that no overfitting occurred (0.85 [Standard Error: 0.0036] vs. 0.83).
Physician-Documented Insomnia Cohort Characteristics
Patient characteristics for our cohort are presented in Table 1. In particular, the mean age in our cohort was 62.0 years with 59.4% females. Our cohort was multiethnic, with Caucasian (72.9%), African American (10.4%), Hispanic (10.8%), and Asian (2.0%) patients. The average duration of follow up (time between the first encounter and either the last encounter or death) was 14.1 years; 76.6% of the patients had 10 or more years of follow up.
Joint disorders, hypertension, and disorders of lipid metabolism and diabetes (type 1 or type 2) were the highest prevalent comorbidities, at 79.3%, 75.4%, 66.5%, and 55.9%, respectively. A high proportion of patients suffered from anxiety/depression (46.9%) or a psychiatric disorder (38.4%), and 1.9% of patients suffered from Alzheimer’s disease (including the broader definition for dementia). Several highly prevalent chronic conditions were also highly represented in this cohort, including obesity (34.5%), congestive heart failure (32.7%), coronary artery disease (27.9%), asthma (23.9%), and chronic obstructive pulmonary disease (23.5%).
Discussion
Sleep disorders are commonly assessed using standardized survey instruments, including the Insomnia Severity Index23,24,25 and the Pittsburgh Sleep Quality Index26,27,28. However, these questionnaires are laborious to administer, rely on patient recall, and are often administered to narrowly define patient populations (e.g.2,29,30). In contrast, the development of classification algorithms that rely on EMR data may serve as efficient mechanisms to accurately assess sleep disorders at both the individual and population levels.
Our study describes the development of an algorithm that was used to define a large, longitudinal EMR cohort of patients with a high likelihood for physician-documented insomnia. These patients had an average follow up of 14.1 years, and 76.6% of them had greater than or equal to 10 years of follow-up data, highlighting the breadth of data made available by this algorithm-based cohort identification approach. To our knowledge, our cohort is the largest cohort of insomnia patients (n = 36,810).
As a reassuring validation of our approach, our cohort’s female:male predominance of 1.5 among physician-documented insomnia patients was very similar to the female:male ratio of 1.4 reported in an insomnia meta-analysis31. Additionally, the identified insomnia patients had a high prevalence of insomnia-associated diseases, such as psychiatric diseases32,33,34,35,36,37,38,39,40, joint disorders41,42,43,44,45, diabetes46,47, and stroke/cerebrovascular diseases48,49,50.
Our algorithm demonstrated superiority in identifying insomnia patients over using billing codes alone, which is in line with previous text-inclusive algorithm results for other diseases21. Our algorithm performed best when it combined classic structured elements, like billing codes and medication prescriptions, with unstructured data from narrative notes (AUROC = 0.83 [95% CI, 0.76–0.90]). Among our physician-documented insomnia patients, only 17% had one or more billing codes for insomnia, and the AUROC for identification using billing data alone was 0.55 [95% CI, 0.51–0.58]. The markedly lower AUROC with a billing-code-only identification methodology demonstrates the inferiority of this traditional classification scheme and highlights the benefits that a note-incorporating algorithm-based identification can provide. Furthermore, because only 17% of the physician-documented insomnia patients had billing codes for insomnia, this suggests that studies based on billing codes alone may fail to identify a large proportion of insomnia patients. Relying on inaccurate identification of insomnia patients may result in biased experiments that are not representative of the general insomnia population.
Interestingly, even an algorithm incorporating only unstructured data had an AUROC higher than that of a solely billing-code-based identification, validating the effectiveness of features indicating a sleep disorder found in clinical notes. Our algorithm demonstrates the need to increase the use of the large amount of data on insomnia documented in EMR text. This also points to the potential of analyzing narrative text as a powerful new data source to understand insomnia. Extracting disease concepts from clinical narrative notes may more accurately characterize an individual’s health status, especially for patient symptoms or conditions that are not the principal reason for the physician visit (and thus may be less likely to be coded for billing).
Notably, a simplified version of our algorithm, one that considers only the unstructured sleep disorder variable (and ignores the other six variables) can quickly identify patients with insomnia. For instance, per Equations 1 and 2, when a patient has one note indicating a sleep disorder, his or her insomnia probability equals 0.60. When the patient has two notes indicating a sleep disorder, his or her insomnia probability equals 0.94, exceeding the 0.828 threshold and classifying the patient as having insomnia. In another scenario, even when no notes are available, it is possible to use our algorithm based on insomnia billing codes alone. To exceed the insomnia threshold in such a scenario, a patient needs 5 or more insomnia billing codes, having an insomnia probability of 0.89. On the one hand, using simplified versions of our algorithm may not be as accurate as using it with all seven variables. On the other hand, this will allow a rapid initial assessment to estimate the number of patients with insomnia in a given EMR repository, even if it does not contain notes.
EMR documentation did not allow for thorough identification of patients based on traditional DSM-V criteria. We believe this enhances our study’s relevance to clinical practice, because our empiric definition (which includes the ability to mine the content of physician notes) reflects the reality of how insomnia is discussed in patient–doctor interactions. It also suggests that traditional studies based on DSM-V criteria may be significantly underusing a large volume of the patient health information available in EMR notes and that new methods for classifying insomnia for research purposes are needed.
Although our study describes analyses of retrospective medical databases, our proposed algorithm can be used to identify patients who are at a high-risk of insomnia in real time and thus may inform therapeutic decision-making before the patient has actually developed the condition. In a desirable scenario, our algorithm can calculate the risk of insomnia automatically as an integrated component of an EMR system; the clinician would see a risk score (probability) or a risk rank (low, medium, high) associated with the patient, and this can be used to guide inpatient and outpatient monitoring strategies.
Although extended time frames of EMR data were available for patients in our identified cohort, it is likely that this EMR data was incomplete because various health records may have been stored in other databases not available to us. This is an important limitation of our study. Further, despite the large number of patients in our cohort, our population was derived from urban tertiary-referral hospitals and thus may not be representative of patients seen in other health care contexts. Also, given the integration of prescription and comorbidity data into our algorithm, further studies using this cohort should apply matching methodologies to account for the level of utilization of the health system for insomnia cases vs. controls.
Another limitation of our study is algorithmic. On the one hand, the adaptive least absolute shrinkage and selection operator (LASSO) method identified seven variables and left out potential variables that may also be correlated with insomnia vs. lack of insomnia. Feature selection algorithms are known to be blind to the clinical importance of variables, and when highly correlated variables are identified, the algorithm randomly selects one. Adaptive LASSO has been highly effective in variable selection for the prediction of outcomes related to liver disease51, and we thus chose it for our study as well. Additional feature selection algorithms are available, including those based on supervised and unsupervised approaches. Each such algorithm has advantages and disadvantages regarding its ability to identify the most efficient covariates associated with a certain outcome52,53.
We do not propose our algorithm as a complete replacement for gold-standard methods for identifying patients with insomnia. However, we do demonstrate a method capable of quickly identifying a large number of patients with insomnia that can be used to find associations traditional studies of smaller scale and shorter duration cannot. We also demonstrate the inferiority of traditional billing code-based classification schemes. Because our algorithm allows for a large-scale and quick approach to insomnia classification, findings resulting from the identified patients can be used to guide hypothesis generation in insomnia research.
In conclusion, EMR approaches may prove to be powerful complementary methods to study the impact of insomnia in large populations, especially if the breadth and depth of knowledge available in clinical notes is used to its fullest potential. We demonstrated a physician-documented insomnia classification algorithm that outperforms billing codes in identifying of physician-documented insomnia and highlighted the importance of finding new approaches for classifying insomnia. Using our clinical note-based approach, we identified the largest insomnia cohort to date, which can be used to conduct large-scale data analyses of this highly prevalent and important medical condition. As a subsequent step, we plan to apply our algorithm on additional populations to further explore the characteristics of patients suffering from insomnia compared to the general population as a whole.
Methods
Study Population
We analyzed a previously defined dataset of 314,292 patients from Massachusetts General Hospital and Brigham and Women’s Hospital who received care between 1992 and 201054,55. Patients in this cohort were at increased risk for metabolic syndrome—they had at least one type 2 diabetes mellitus (T2DM) diagnosis code, a T2DM medication, an Hemoglobin A1c (HbA1c) level ≥6.5 percent, or plasma glucose ≥200 mg/dl. From the 314,292 patients, we first created an intermediate dataset of patients (our insomnia candidate mart), based on insomnia-related billing codes, insomnia-related medication prescriptions, and narrative note mentions of sleep-related keywords. Criteria for inclusion in this intermediate cohort included presence of an insomnia-related billing code (307.41, 307.42, 327.00, 327.01, 327.02, 327.09, 780.52) or a sleep-related medication. Additionally, our initial inclusion criteria included patients who had at least one note with a mention of either “sleep” or “insomnia.” The definition identified a broad set of patients who truly had documentation of sleep disorder as well as patients who did not suffer from any sleep difficulties (e.g., “patient sleeps well.”) Only patients with at least two notes of 500 characters or more were included. A total of 164,349 patients made up the insomnia candidate mart (Fig. 3).
Insomnia algorithm and cohort development. A total of 600 patients were manually labeled: 230 patients had insomnia, 270 patients did not have insomnia, and 100 patients did not have a clear insomnia status. The 500 patients with known insomnia status were used to develop the algorithm; 7 of these 500 patients were excluded because their age was below 18 (date of death or date of the end of the study). Additionally, two-thirds of these (328) served as a training set, while the rest (165) served as a validation set.
A medical student performed a physician-supervised manual chart review to assess the feasibility of using a DSM-V-based manual classification scheme. Based on a manual chart review of approximately 50 random insomnia patients, details such as the frequency of symptoms per week or the duration of symptoms, which would be necessary for a DSM-V diagnosis, were not documented for the vast majority of patients. These observations from our data are consistent with a previous report56. As a result, criteria such as DSM-V would be impractical to classify the insomnia status of patients in the EMR. Therefore, we supplemented DSM-V with more empirical criteria, considering the criteria presented in Supplementary Table 1. For brevity, we used the term “physician-documented insomnia” to describe this empirical definition. All manual chart reviews were performed by RA under the guidance of SYS.
A total of 600 randomly selected patients from the insomnia candidate datamart were subjected to manual EMR chart review. This chart review was physician supervised and done by a medical student to ensure consistency of labeling. Patients were classified as “physician-documented insomnia” (230), “no evidence of physician-documented insomnia” (270), or “undetermined” (100). Among the 500 patients with known insomnia status (positive or negative), seven were excluded because they were younger than 18 either at their date of death or at the date of the end of the study. Two-thirds of the patients (328) served as a training set and one-third (165) were designated as a validation set for algorithm development (Fig. 3).
Because EMR documentation did not support use of formal case definitions for insomnia (see Results), our insomnia case definition supplemented formal insomnia diagnostic criteria (DSM-V) with additional empirical criteria. These empirical criteria included a physician-documented diagnosis of insomnia, or a physician documenting sleep-related difficulty consistent with insomnia on different occasions throughout the patient’s life-time. Additionally, the criteria included a physician prescribing a medication that was indicated only for insomnia (Ambien/Zolpidem, Ambien CR/Zolpidem CR, Lunesta/Eszopiclone, Restoril/Temazepam, Sonata/Zaleplon, Dalmane/Flurazepam, and ProSom/Eurodin/Estazolam). We selected these medications because they are known primarily for treatment of troubled sleeping (as opposed to medications primarily used to treat other conditions, such as depression or anxiety, whose second use can also help with insomnia, such as Desyrel/trazodone). Furthermore, we considered only medications that were available during our study period (between 1992 and 2010) and not newer ones irrelevant to our study (e.g., Belsomra/Suvorexant). The presence of any one of the classification criteria was sufficient for an empiric classification of a patient as physician-documented insomnia (Supplementary Table 1).
Covariate Definition
Structured variables were defined using ICD9 codes and current procedural terminology codes and included a broad range of comorbidities (Supplementary Table 2). To extract unstructured variables, we used text nailing (TN), a text-processing method that members of our group developed. TN is based on using an interactive human-in-the-loop mechanism to identify nonnegated clinical and behavioral descriptors, and it was proved to be highly accurate compared to traditional machine learning algorithms57,58. One advantage of TN is that it is not sensitive to negations; thus, it can identify expressions that truly indicate the existence of a certain condition or behavior. As in our recent study published in Scientific Reports59, we used the top 10 expressions with the highest prevalence to define a sleep disorder incorporated by our algorithm. The expressions were: “poor sleep,” “has trouble sleep,” “reduced sleep,” “increased sleep,” “decreased sleep,” “excessive sleep,” “fragmented sleep,” “sleeplessness,” “sleep disruption,” and “sleeps poorly.” All the definitions for our unstructured variables can be seen in Supplementary Table 3.
Classification Modeling
We applied logistic regression with the adaptive LASSO to develop a classification algorithm for physician-documented insomnia. We evaluated different combinations of variable subtypes, including (1) ICD-9 codes related to insomnia, (2) structured variables (e.g., ICD-9 codes of variety of comorbidities, prescriptions, demographics), (3) only narrative (unstructured) variables, and (4) a combination of structured and unstructured variables. We used these four combinations to assess the optimal algorithm for identifying patients with physician-documented insomnia. We chose the adaptive LASSO over other feature selection methods because it is considered an efficient algorithm for parsimoniously ranking variables in clinical predictive modeling60,61.
We used a specificity threshold of 97% for classifying physician-documented insomnia patients, which corresponded to a probability threshold (for physician-documented insomnia) of 0.828. To calculate 95% confidence intervals, we applied the bootstrap procedure with 1,000 replicates. We calculated the AUROCs to measure the model’s accuracy in the validation set. Additionally, we evaluated for overfitting by comparing the AUROC in the validation set to an average AUROC value for 100 permutations of randomly selected sub-derivation and sub-validation sets in the training set. We then applied our algorithm to the 164,349 patients in the insomnia candidate datamart using Equations 1 and 2 to calculate the probability of having physician-documented insomnia for each patient.
Validation
To validate our algorithm, we randomly selected a distinct set of 300 patients from the intermediate dataset of 164,349 individuals. Manual review identified 200 patients who could be labeled as having insomnia or not; of these, 88 patients had insomnia, and 112 patients did not have insomnia. We excluded two of the patients because they were younger than 18 either at their date of death or at the date of the end of the study. A performance summary to assess the algorithm performance in the remaining 198 patients is presented in Supplementary Table 4. Based on this, the final PPV of our algorithm was calculated.
The institutional review board of Partners HealthCare approved this study and all its methods, including the EMR cohort assembly, data extraction, and analyses. The present project was reviewed and approved with a waiver of informed consent from the institutional review board at Partners HealthCare. Furthermore, we confirm that all methods were performed in accordance with the relevant guidelines and regulations of Scientific Reports. Data contain potentially identifying information and may not be shared publicly. Deidentified data may be requested from The Partners Human Research Committee, the Institutional Review Board of Partners HealthCare (Address: 399 Revolution Drive, Suite # 710, Somerville MA, 02145, USA, Telephone: 857-282-1900).
References
Mahowald, M. W. & Schenck, C. H. Insights from studying human sleep disorders. Nature. 437(7063), 1279–1285 (2005).
Kessler, R. C. et al. Insomnia and the performance of US workers: results from the America Insomnia survey. Sleep. 34(9), 1161–1171 (2011).
Park, S. C. et al. Prevalence and clinical correlates of insomnia in depressive disorders: The CRESCEND Study. Psychiatry Investig. 10(4), 373–381 (2013).
Sunderajan, P. et al. Insomnia in patients with depression: a STAR*D report. CNS Spectr. 15(6), 394–404 (2010).
Wong, S. H. & Ng, B. Y. Review of sleep studies of patients with chronic insomnia at a sleep disorder unit. Singapore Med J. 56(6), 317–323 (2015).
Mai, E. & Buysse, D. J. Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med Clin. 3, 167–174 (2008).
Manber, R. et al. Cognitive behavioral therapy for Insomnia enhances depression outcome in patients with comorbid major depressive disorder and Insomnia. Sleep. 31(4), 489–495 (2008).
Dikeos, D. & Georgantopoulos, G. Medical comorbidity of sleep disorders. Curr Opin Psychiatry. 24(4), 346–354 (2011).
Mysliwiec, V. et al. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 36(2), 167–174 (2013).
Benca, R. M. Diagnosis and treatment of chronic insomnia: a review. Psychiatr Serv. 56(3), 332–343 (2005).
Wallander, M. A., Johansson, S., Ruigómez, A., García Rodríguez, L. A. & Jones, R. Morbidity associated with sleep disorders in primary care: a longitudinal cohort study. Prim Care Companion J Clin Psychiatry. 9(5), 338–345 (2007).
Meltzer, L. J., Johnson, C., Crosette, J., Ramos, M. & Mindell, J. A. Prevalence of diagnosed sleep disorders in pediatric primary care practices. Pediatrics. 125(6), e1410–e8 (2010).
Brass, S. D., Li, C. S. & Auerbach, S. The underdiagnosis of sleep disorders in patients with multiple sclerosis. J Clin Sleep Med. 10(9), 1025–1031 (2014).
Wilke, R. A. et al. The emerging role of electronic medical records in pharmacogenomics. Clin Pharmacol Ther. 89(3), 379–386 (2011).
Kohane, I. S., Drazen, J. M. & Campion, E. W. A glimpse of the next 100 years in medicine. N Engl J Med. 367, 2538–2539 (2012).
Denny, J. C. et al. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat Biotechnol. 31(12), 1102–1110 (2013).
Liao, K. P. et al. Associations of autoantibodies, autoimmune risk alleles, and clinical diagnoses from the electronic medical records in rheumatoid arthritis cases and non-rheumatoid arthritis controls. Arthritis Rheumatol. 65(3), 571–581 (2013).
Bowton, E. et al. Biobanks and electronic medical records: enabling cost-effective research. Sci Transl Med. 6(234), 234 cm3 (2014).
Doshi-Velez, F., Ge, Y. & Kohane, I. Comorbidity clusters in autism spectrum disorders: an electronic health record time-series analysis. Pediatrics. 133(1), e54–e63 (2014).
Corey, K. E., Kartoun, U., Zheng, H., Chung, R. T. & Shaw, S. Y. Using an electronic medical records database to identify non-traditional cardiovascular risk factors in nonalcoholic fatty liver disease. Am J Gastroenterol. 111(5), 671–676 (2016).
Liao, K. P. et al. Development of phenotype algorithms using electronic medical records and incorporating natural language processing. The BMJ. 350, h1885 (2015).
Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Association. Arlington, VA: American Psychiatric Publishing (2013).
Morin, C. M., Belleville, G., Bélanger, L. & Ivers, H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 34(5), 601–608 (2011).
Cho, Y. W., Song, M. L., Morin & C.M. Validation of a Korean version of the insomnia severity index. Journal of Clinical Neurology. 10(3), 210–215 (2014).
Taylor, S. S. et al. Prevalence of and characteristics associated with insomnia and obstructive sleep apnea among veterans with knee and hip osteoarthritis. BMC Musculoskelet Disord. 19(1), 79 (2018).
Buysse, D. J., Reynolds, C. F. 3rd, Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 28(2), 193–213 (1989).
Black, D. S., O’Reilly, G. A., Olmstead, R., Breen, E. C. & Irwin, M. R. Mindfulness meditation and improvement in sleep quality and daytime impairment among older adults with sleep disturbances: a randomized clinical trial. JAMA Internal Medicine. 175(4), 494–501 (2015).
Mollayeva, T. et al. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev. 25, 52–73 (2016).
Calem, M. et al. Increased prevalence of insomnia and changes in hypnotics use in England over 15 years: analysis of the 1993, 2000, and 2007 National Psychiatric Morbidity Surveys. Sleep. 35(3), 377–384 (2012).
Calhoun, S. L., Fernandez-Mendoza, J., Vgontzas, A. N., Liao, D. & Bixler, E. O. Prevalence of insomnia symptoms in a general population sample of young children and preadolescents: gender effects. Sleep Medicine. 15(1), 91–95 (2014).
Zhang, B. & Wing, Y. K. Sex differences in insomnia: a meta-analysis. Sleep. 29(1), 85–93 (2006).
Thase, M.E. Antidepressant treatment of the depressed patient with insomnia. Journal of Clinical Psychiatry. 60 (Suppl 17: 28-31; discussion 46-4) (1999).
Taylor, D. J., Lichstein, K. L., Durrence, H. H., Reidel, B. W. & Bush, A. J. Epidemiology of insomnia, depression, and anxiety. Sleep. 28(11), 1457–1464 (2005).
Stewart, R. et al. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 29(11), 1391–1397 (2006).
Buysse, D. J. et al. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 31(4), 473–480 (2008).
Staner, L. Comorbidity of insomnia and depression. Sleep Medicine Reviews. 14(1), 35–46 (2010).
Lai, L. L., Tan, M. H. & Lai, Y. C. Prevalence and factors associated with off-label antidepressant prescriptions for insomnia. Journal of Drug, Healthcare and Patient Safety. 3, 27–36 (2011).
Soehner, A. M. & Harvey, A. G. Prevalence and functional consequences of severe insomnia symptoms in mood and anxiety disorders: results from a nationally representative sample. Sleep. 35(10), 1367–1375 (2012).
Buysse, D. J. Insomnia. The Journal of the American Medical Association. 309(7), 706–716 (2013).
Finan, P. H. & Smith, M. T. The comorbidity of insomnia, chronic pain, and depression: Dopamine as a putative mechanism. Sleep Medicine Reviews. 17(3), 173–183 (2013).
Luyster, F. S., Chasens, E. R., Wasko, M. C. & Dunbar-Jacob, J. Sleep quality and functional disability in patients with rheumatoid arthritis. J Clin Sleep Med. 7(1), 49–55 (2011).
Louie, G. H., Tektonidou, M. G., Caban-Martinez, A. J. & Ward, M. M. Sleep disturbances in adults with arthritis: prevalence, mediators, and subgroups at greatest risk. Data from the 2007 National Health Interview Survey. Arthritis Care Res (Hoboken). 63(2), 247–260 (2011).
Irwin, M. R. et al. Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. Sleep. 35(4), 537–543 (2012).
Parmelee, P. A., Tighe, C. A. & Dautovich, N. D. Sleep disturbance in osteoarthritis: linkages with pain, disability, and depressive symptoms. Arthritis Care Res (Hoboken). 67(3), 358–365 (2015).
Pickering, M. E., Chapurlat, R., Kocher, L. & Peter-Derex, L. Sleep disturbances and osteoarthritis. Pain Pract. 16(2), 237–244 (2016).
Gottlieb, D. J. et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 165(8), 863–867 (2005).
Knowler, W. C. et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 346(6), 393–403 (2002).
Wallace, D. M., Ramos, A. R. & Rundek, T. Sleep disorders and stroke. Int J Stroke. 7(3), 231–242 (2012).
Wu, M. P. et al. Insomnia subtypes and the subsequent risks of stroke: report from a nationally representative cohort. Stroke. 45(5), 1349–1354 (2014).
Ferro, J. M., Caeiro, L. & Figueira, M. L. Neuropsychiatric sequelae of stroke. Nat Rev Neurol. 12(5), 269–280 (2016).
Kartoun, U. et al. The MELD-Plus: A generalizable prediction risk score in cirrhosis. PLOS ONE. 12(10), e0186301 (2017).
Lu, F. & Petkova, E. A comparative study of variable selection methods in the context of developing psychiatric screening instruments. Stat Med. 33(3), 401–421 (2014).
Dy, J. G. & Brodley, C. E. Feature selection for unsupervised learning. Journal of Machine Learning Research. 5, 845–889 (2004).
Kumar, V. et al. Natural language processing improves phenotypic accuracy in an electronic medical record cohort of type 2 diabetes and cardiovascular disease. Journal of the American College of Cardiology. 63(12), A1359 (2014).
Kartoun, U. et al. Demonstrating the advantages of applying data mining techniques on time-dependent electronic medical records. American Medical Informatics Association 2015 Annual Symposium, Nov 2015, San Francisco, CA (2015).
Mann-Jiles, V., Thompson, K. & Lester, J. Sleep impairment and insomnia in sickle cell disease: a retrospective chart review of clinical and psychological indicators. J Am Assoc Nurse Pract. 27(8), 441–449 (2015).
Kartoun, U. Text nailing: an efficient human-in-the-loop text-processing method. ACM Interactions. 24(6), 44–49 (2017).
Kartoun, U. Beyond brute force. Communications of the ACM. 60(10), 8–9 (2017).
Beam, A. L. et al. Predictive modeling of physician-patient dynamics that influence sleep medication prescriptions and clinical decision-making. Sci Rep. 7, 42282 (2017).
Liao, K. P. et al. Electronic medical records for discovery research in rheumatoid arthritis. Arthritis Care Res (Hoboken). 62(8), 1120–1127 (2010).
Ananthakrishnan, A. N. et al. Improving case definition of Crohn’s disease and ulcerative colitis in electronic medical records using natural language processing: a novel informatics approach. Inflamm Bowel Dis. 19(7), 1411–1420 (2013).
Acknowledgements
This work was supported by a research grant from Merck & Co., Inc. I.S.K. was supported in part by NIH grant U54HG007963. IBM neither provided author U.K. salaries related to the study nor played any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. U.K. received honoraria and travel funding from The American Association for the Study of Liver Diseases (2017), and received travel funding from Merck & Co., Inc. (2017).
Author information
Authors and Affiliations
Contributions
U.K., R.A., A.L.B.: conception and design of the study; interpreting data; writing the manuscript. J.K.P., A.K.C., T.P.F.: conception and design of the study; interpreting data; writing the manuscript. I.S.K., S.Y.S.: conception and design of the study; interpreting data; writing the manuscript; study supervision.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kartoun, U., Aggarwal, R., Beam, A.L. et al. Development of an Algorithm to Identify Patients with Physician-Documented Insomnia. Sci Rep 8, 7862 (2018). https://doi.org/10.1038/s41598-018-25312-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-25312-z
This article is cited by
-
Enhancing Clinical Prediction Performance by Incorporating Intuition
Journal of Medical Systems (2021)
-
Application of data mining for predicting hemodynamics instability during pheochromocytoma surgery
BMC Medical Informatics and Decision Making (2020)
-
Toward an accelerated adoption of data-driven findings in medicine
Medicine, Health Care and Philosophy (2019)
-
Validation of the Use of Electronic Medical Records for Identification of Post-gastric Bypass Hypoglycemia Cases
Obesity Surgery (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.