Abstract
Growing evidence suggests that the cerebellum is not only involved in motor functions, but it significantly contributes to sensory and cognitive processing as well. In particular, it has been hypothesized that the cerebellum identifies recurrent serial events and recognizes their violations. Here we used transcranial magnetic stimulation (TMS) to shed light on the role of the cerebellum in short-term memory of visual sequences. In two experiments, we found that TMS over the right cerebellar hemisphere impaired participants’ ability to recognize the correct order of appearance of geometrical stimuli varying in shape and/or size. In turn, cerebellar TMS did not affect recognition of highly familiar short sequences of letters or numbers. Overall, our data suggest that the cerebellum is involved in memorizing the order in which (concatenated) stimuli appear, this process being important for sequence learning.
Similar content being viewed by others
Introduction
Recent literature indicates that the cerebellum plays a significant role in the perceptual and cognitive domain, in addition to its established role in motor planning and behavior1,2,3,4. For instance, converging findings suggest that the cerebellum contributes to the recognition of (recurrent) temporal and spatial relations among stimuli. Indeed, cerebellar lesions impair the ability to recognize serial events as sequences and to identify correct vs. violated sequences of different kind (e.g., verbal, spatial and action sequences)5,6,7,8,9,10,11,12,13. Schubotz and von Cramon’s study14 indicates that the cerebellum is also a critical structure in identifying serial events and their violations. Participants in this study attended to the size-based sequential order of a series of circles and had to detect possible sequence violations, whereas in the control task they had just to indicate whether the same circles had same or different color. Results indicate that the cerebellum responded preferentially when the order of the stimuli was attended (sequence task) rather than when the color was. Accordingly, Bubic and colleagues15 observed preferential activation of the cerebellum while processing serial stimuli, with the posterior cerebellum being especially activated during detection of sequence violations.
The hypothesized role of the cerebellum in perceptual sequential patterns’ processing originates from the motor domain where the cerebellum has been repeatedly proven to underpin memory of movements’ sequences1. Indeed, cerebellar patients did not show implicit (motor) learning for repeated patterns while performing tasks requiring to repeat on a keyboard the sequences of visual stimuli appearing on the screen16,17,18. Accordingly, neuroimaging evidence shows consistent activation in the cerebellum during several tasks involving sequences: visual-motor learning19, learning of sequences of hand keypresses20 and learning alternating wrist flexion and extension movements21. Crucially, the cerebellum seems to be differentially engaged in the successive phases of motor sequence learning, with predominant cerebellar contribution during sequence acquisition rather than sequence execution1. Indeed, once a motor sequence is learned (hence it is automatically performed), the cerebellum shows lower activity in comparison to when the sequence has not been learnt as yet1,21. In this regard, it has been suggested that the cerebellum sustains gradual learning of motor sequences by implementing and testing internal feedforward models22. Accordingly, the effects of cerebellar lesions upon motor skills may also depend on suboptimal implementation of these models rather than exclusively on failures of motor control per se4.
Brain stimulation evidence also supports a role of the cerebellum in the acquisition of motor sequences18. However, no brain stimulation studies have directly investigated whether cerebellar structures are also involved in processing perceptual sequences. Here we addressed this issue, by specifically investigating using transcranial magnetic stimulation (TMS) whether the cerebellum is involved in maintaining in short-term memory the order in which stimuli appear. Indeed, sequence learning relies on the ability to maintain in memory the temporal characteristics of events23,24. In two experiments, we asked participants to memorize new sequences of geometrical shapes (Experiment 1 and 2) or to detect violations in highly familiar alphabetic or numerical strings (Experiment 1) while TMS was delivered over the cerebellum, the visual cortex and the vertex (control condition). The familiarity of the sequences was manipulated in order to directly test whether the cerebellum plays a different role in relation to the learning phase of the to-be-processed input21. Indeed, there is evidence that the cerebellum is more activated when the structure of the motor pattern has not been completely understood16,17,19,25 compared to when the motor patterns are consolidated in memory. Therefore, we expected cerebellar TMS to affect memory of unfamiliar sequences (where acquisition of the sequence is still occurring), and to have no effect on the monitoring of familiar (well known) sequences. TMS was delivered over the right cerebellar hemisphere (rather than medial portions, i.e., vermis) in light of consistent evidence suggesting that perceptual and cognitive processing occurs more in lateral vs. medial cerebellar structures4,26.
Experiment 1
Method
Participants
Eighteen Italian students (3 males, mean age = 21.7 years, SD = 1.5) participated in the experiment. Each participant filled in a questionnaire27 to evaluate their compatibility with TMS before undergoing the experiment. None of the participants reported neurological problems or history of seizures. None was taking medications that could interfere with neuronal excitability. Written informed consent was obtained from all participants before the experiment. The protocol was approved by the psychological ethical committee of the University of Pavia and participants were treated in accordance with the Declaration of Helsinki.
Stimuli and Procedure
Participants were seated comfortably at a distance of 57 cm from a 17″ (1024 × 768 pixels resolution) TFT-LCD computer monitor and wore earplugs to minimize TMS click sound interference. They were required to detect possible irregularities of unfamiliar visual sequences composed by geometrical shapes (new sequence condition) and alphabetic or numerical visual sequences (familiar sequence condition).
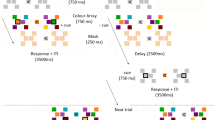
In the new sequence condition, stimuli consisted of 3 visual shapes (circles, triangles, squares), each appearing in three different sizes throughout the experiment: small (approx. 2*2 deg of visual angle), medium (approx. 4*4 deg) and large (approx. 8*8 deg). The timeline of an experiment trial is presented in Fig. 1. Each trial consisted of a sample sequence and of a test sequence. Circles were only used in sample sequences, triangles and squares were only used in test sequences (we used different shapes in the test compared to the sample sequences to avoid any possible visual adaptation effect). The sample sequence consisted of the consecutive presentation of three circles varying in size (e.g., small-large-medium; small-small-large). Each sample sequence started with a central fixation cross (presented for 1000 ms), followed by the first item of the series (500 ms duration), a blank screen (200 ms), the second item (500 ms), a blank screen (200 ms), and the third item (500 ms). The sample sequence was immediately followed by the test sequence. In the test sequence either 3 triangles or 3 squares were presented. Stimuli duration was the same as in the sample sequence except for the latest stimulus of the sequence that was visible until response. The first two shapes of the test sequence were always identical in size to those of the sample sequence, whereas the size of the last shape could be the same as that used in the sample sequence (i.e., sample and test were identical in terms of size-sequence) or different (violation of the sample sequence). Three shapes of the same size were never presented in the same sequence; furthermore, sequences organized in entirely ascending or descending order (i.e., small-medium-large; large-medium-small) were not used. Participants were instructed to indicate as quickly as possible whether the test size-sequence was identical to the sample one by left/right key pressing using their right hand (with response keys counterbalanced among participants). This condition consisted of 72 trials (half in which the test size-sequence was identical to the sample one, and half in which it was different).
The timeline of an experimental trial in new sequence condition of Experiment 1. Participants were required to indicate whether stimuli of the test and sample sequences followed the same size-order. In the example shown here this was not the case, with the test sequence violating the predictable order. TMS was delivered 150 ms before the onset of the last item of the test sequence.
Familiar sequences consisted of either 4 letters or 4 digits (in the 1–9 range), presented in black ink, 16-point Calibri font and measured approx. 4*4 deg of visual angle. The first three items of the sequence were always presented in correct consecutive order, that is ascending for digits (e.g., 3-4-5) and alphabetic for letters (e.g., L-M-N). The fourth item could be either the correct consecutive item of the sequence (e.g., 3 4 5 6) or the incorrect (e.g., 3 4 5 1): when incorrect, half of them represented the item preceding in the series (as in the example above) and the other half represented the item following in the series. In the familiar sequence condition, only test sequences were presented (with time parameters identical to those of the new sequence condition) and there were no sample sequences (since sequences were already known). This condition consisted of 40 trials presented in random order, half in which the correct sequence order was respected, and half in which it was violated. A fixation cross (1000 ms) was presented during the intertrial interval.
For both types of sequence condition, TMS was delivered before the onset of the last stimulus (the last stimulus of the test sequence of the new sequences where possible violations occurred and the last stimulus of the familiar sequences). The experiment consisted of six experimental blocks, one for each TMS site (TMS over the right cerebellum, TMS over the early visual cortex and TMS over the vertex, see below) and sequence condition (new and familiar sequence condition). A short practice session was presented at the beginning of the experiment to familiarize participants with the stimuli used. Order of TMS site and sequence condition was counterbalanced across participants.
Transcranial Magnetic Stimulation (TMS)
Online neuronavigated TMS was performed with a Magstim Rapid2 stimulator (Magstim Co., Ltd, Whitland, UK) connected to a 70-mm butterfly coil. At the beginning of each session single pulse TMS was applied at increasing intensities to determine individual motor threshold (MT). MT was defined as the lowest TMS intensity capable of evoking a muscle twitch in the controlateral hand in 5/10 consecutive trials28,29. During the experiment, participants were stimulated at 100% of their MT (mean TMS intensity delivered: 56.1% of the maximum stimulator output, SD = 2.9%). No participants reported phosphene perception.
Triple-pulse 20 Hz TMS was delivered 150 ms before the presentation of the last stimulus of the test sequence (so that the last TMS pulse was given 50 ms before the onset of the stimulus), with similar parameters of stimulation used in previous TMS studies targeting the cerebellum28,30,31,32. TMS was delivered over the right cerebellum, the early visual cortex and the vertex (control condition). Early visual cortex was chosen as additional control area since prior evidence suggests that cerebellar stimulation may spread to primary visual cortex33. The cerebellum and the early visual cortex were localized by means of stereotaxic navigation on individual estimated magnetic resonance images (MRI) obtained through a 3D warping procedure fitting a high-resolution MRI template with the participant’s scalp model and craniometric points (Softaxic, EMS, Bologna, Italy). This procedure has been proven to ensure a global localization accuracy of about 5 mm, a level of precision closer to that obtained using individual MRI scans34, and has been successfully used in many prior studies35,36,37. Anatomical Talairach coordinates38 used for neuronavigation were x = 22, y = −75, z = −21 for the right cerebellum (corresponding to cerebellar loci of activation reported in a previous neuroimaging study investigating processing of visual sequences violation15, see Fig. 2) and x = −2, y = −81.4, z = 1.4 for early visual cortex39. The region effectively affected by cerebellar TMS corresponded to the superficial layers of the cerebellar cortex40,41: in fact, deeper sites were unlikely to be directly reached by the stimulation as the intensity of the electrical field induced by TMS drops dramatically as a function of the distance from the coil42. The vertex was localized as the point falling half the distance between the nasion and the inion on the same43,44 midline. The coil was placed tangentially to the scalp and held parallel to the midsagittal line, with the handle pointing backward in the vertex and with the handle pointing superiorly in the cerebellum and early visual cortex TMS stimulation.
Data Availability
All data collected in this experiment are available upon request.
Results
Mean accuracy rates and mean reaction times (RT) for correct responses were computed for each participant in each TMS condition, and are shown in Fig. 3. Data were submitted to repeated-measures ANOVAs with task condition (new vs. familiar sequences) and TMS (cerebellum, early visual cortex and vertex) as within-subjects variables. Mauchly’s test indicated that the assumption of sphericity has not been violated (ps > 0.23 for accuracy and ps > 0.31 for RT).
(A) Mean percentage accuracy scores and (B) mean correct RT as a function of TMS site (right cerebellum, early visual cortex and vertex) and task condition (new vs. familiar sequences) in Experiment 1. TMS over the cerebellum selectively impaired participants’ accuracy compared to early visual cortex and vertex stimulation in memory for new but not familiar sequences. RT were not affected by TMS. Error bars represent ±SEM. Asterisks indicate significant differences between conditions (*p < 0.05).
The ANOVA on mean accuracy scores revealed a significant main effect of task condition, F(1, 17) = 19.06, p < 0.001, ηp2 = 0.53, but not of TMS, F(2, 34) < 1, p = 0.42. Critically, the interaction task condition by TMS was significant, F(2, 34) = 7.53, p = 0.002, ηp2 = 0.31. The significant two-way interaction was investigated by looking at the simple main effect of TMS within each task condition. TMS significantly affected detection of irregularities of new sequences, F(2, 34) = 4.69, p = 0.016, ηp2 = 0.22. In particular, post-hoc comparisons showed that cerebellar TMS impaired participants’ accuracy compared to both early visual cortex TMS, t(17) = 2.63, p = 0.018 (with Bonferroni-Holm correction, p = 0.054) and vertex TMS, t(17) = 2.34, p = 0.032 (with Bonferroni-Holm correction, p = 0.064). The effect of TMS over the early visual cortex and vertex was comparable (p = 0.70). In turn, TMS did not affect detection of irregularities of familiar sequences, F(2, 34) = 1.38, p = 0.26.
The ANOVA on mean correct RT revealed a significant main effect of task condition, F(1, 17) = 22.95, p < 0.001, ηp2 = 0.57, indicating that participants were faster (mean correct RT = 445 ms, SD = 47) in familiar compared to new sequences (497 ms, SD = 56). Neither the main effect of TMS, F(2, 34) = 1.87, p = 0.17, nor the interaction task condition by TMS, F(2, 34) < 1, p = 0.67, reached significance.
Experiment 2
In Experiment 1, TMS selectively affected processing of new/unfamiliar sequences without affecting familiar sequences processing. The lack of cerebellar TMS effects on processing of familiar sequences supports the view that the cerebellum is differently involved in serial pattern detection depending on the familiarity of the sequence. Moreover, Experiment 1 ruled out the possibility that the detrimental effect of TMS depended on indirect stimulation of the visual cortex.
An important aspect to consider in interpreting results of Experiment 1 is that only the last item of the sequence could be violated. Hence, one may object that no effective serial processing was required, since in order to correctly perform the task one could just focus on the last item of the sequence. To investigate this possibility, we conducted Experiment 2 in which we presented a new group of participants with sequences composed by three geometrical shapes appearing consecutively as in the new sequences condition of Experiment 1. Hence, one of the three shapes composing the sequence was presented and participants had to indicate its order of appearance in the sequence (first, second or third). Importantly, to successfully perform the task, participants had to pay attention to all the elements of the sequence since they could all be potential targets. If the cerebellum plays a role in coding and maintain in memory the sequential order of the serial elements we should replicate the detrimental effect of cerebellar TMS over participants’ performance found in Experiment 1. In turn, if the cerebellum is involved in short-term memory of the single elements composing the sequence but not in their temporal relationship, cerebellar TMS should not effectively modulate participants’ performance.
Methods
Participants
Eighteen Italian students (6 males, mean age = 23.1 years, SD = 2.3) participated in the experiment. None of them had participated in Experiment 1. Each participant filled in a questionnaire27 to evaluate their compatibility with TMS before undergoing the experiment. None of the participants reported neurological problems or history of seizures. None was taking medications that could interfere with neuronal excitability. Written informed consent was obtained from all participants before the experiment. The protocol was approved by the psychological ethical committee of the University of Pavia and participants were treated in accordance with the Declaration of Helsinki.
Material and Procedure
The experimental setting was the same as in Experiment 1. Stimuli consisted of the same visual shapes used in Experiment 1 but only the medium size (4*4 deg) was used. Each trial started with a central fixation cross (2500 ms) followed by the presentation of a sequence of three shapes. Each shape lasted for 500 ms and was separated by the consecutive one by a blank screen (200 ms). The last shape was followed by a blank screen (200 ms) and then by the presentation of the target shape (i.e., one of the elements of the sequence). Below each target shape an ordinal number (1^, 2^ or 3^) appeared. The number indicated in which order position the target element displayed above was presented in the just presented triplet (e.g., 2^ to indicate the second element of the sequence). In half of the trials, the number indicated the correct order position of the target element, whereas in the other half the number indicated an incorrect position. Importantly, the target element depicted with equal frequency all the order positions (first, second and last) of the sequence. In each trial, participants had to indicate by left/right key pressing with their right hand whether the number corresponded to the position in which the target element was presented in that sequence. Response keys assignment was counterbalanced among participants. The target shape remained on the screen until participants responded. Participants were instructed to respond as quickly as possible. Each block consisted of 108 trials and each participant performed the same block three times (once for each TMS site, see below). A practice session consisting of 16 trials was presented before the experiment.
TMS
TMS parameters and TMS sites were identical to Experiment 1. Triple-pulse 20 Hz TMS was delivered 150 ms before the presentation of the target element (so that the last TMS pulse was given 50 ms before the onset of the stimulus). Mean TMS intensity used was: 53.7% (SD = 4.0%). No participants reported phosphene perception during the experiment.
Data Availability
All data collected in this experiment are available upon request.
Results
Mean accuracy rates and mean RT for correct responses were computed for each participant in each TMS condition (Fig. 4). Accuracy and RT were analyzed via repeated-measures ANOVAs with TMS (cerebellum, early visual cortex and vertex) as within-subjects variable. Mauchly’s test indicated that the assumption of sphericity has not been violated (ps > 0.41 for accuracy and ps > 0.43 for RT).
(A) Mean percentage accuracy scores and (B) mean correct RT as a function of TMS site (right cerebellum, early visual cortex and vertex) in Experiment 2. TMS over the cerebellum decreased participants’ accuracy rates compared to early visual cortex and vertex stimulation. TMS did not affect RT. Error bars represent ±SEM. Asterisks indicate significant differences between conditions (*p < 0.05).
The ANOVA on mean accuracy scores revealed a significant main effect of TMS, F(2, 34) = 4.12, p = 0.025, ηp2 = 0.20, indicating that TMS impaired participants’ performance (see Fig. 4A). Post-hoc comparisons showed that accuracy rates were lower in the cerebellar TMS condition compared to both TMS over the early visual cortex, t(17) = 2.64, p = 0.017 (with Bonferroni-Holm correction, p = 0.051) and over the vertex, t(17) = 2.08, p = 0.053 (with Bonferroni-Holm correction, p = 0.11). The effect of TMS over early visual cortex and vertex was comparable, t(17) < 1, p = 0.72.
The ANOVA on mean correct RT revealed a non-significant main effect of TMS, F(2, 34) < 1, p = 0.18.
Discussion
We found that TMS applied over the cerebellar hemisphere interfered with participants’ ability to memorize the order of appearance of a series of geometrical shapes (Experiment 1 and 2), without affecting processing of familiar alphanumeric sequences (Experiment 1). Importantly, this effect did not depend on indirect stimulation of early visual cortex.
Although our effects were overall of small size, they are in line with prior neuroimaging and neuropsychological evidence suggesting that the cerebellum is involved in processing perceptual sequences10,14,15,17,45,46,47,48,49. It is important to note that our experiments did not directly test sequence learning or violation that are typically assessed with deviant detection tasks (e.g., oddball paradigms, see Kotz et al., 201450). Nonetheless, the storage in memory of the temporal association between incoming stimuli is critical to perceive events as linked in a sequence23,50. Moreover, predictive processes – in which the cerebellum seems to be involved - cooperate and actively build on mnemonic ones, helping to generate goal-directed and adapted behavior23.
In Experiment 1 only the last element of the sequence could vary between the sample and test sequences, and subjects could thus selectively focus on that to successfully perform the task (ignoring the preceding items). To control for this possibility, Experiment 2 measured participants’ ability to retain in memory the order of appearance of all the items of the series, forcing to process the whole sequence. Results of Experiment 2 suggest that the cerebellum is relevant in specifically coding the way stimuli are concatenated, in line with prior evidence28,50,51,52. In turn, cerebellar TMS did not affect recognition of familiar alphabetic or numerical progressive sequences. This supports prior neuroimaging findings showing preferential cerebellar response when participants had to decide whether the order of two probe stimuli (e.g., F, C) was consistent with that of a previous (just presented) sequence (e.g, D, C, I, F, J, A), but not when they had to decide whether the probes appeared in alphabetical or numerical progressive order52. Accordingly, in the motor domain, Doyon et al.19 found that the neural representation of a new sequence of movements becomes gradually less dependent on the cerebellum with learning and taps more onto striatal–cortical circuits53. In line with this, cerebellar patients are more impaired in procedural motor learning than in implementing sequences acquired via explicit instructions17. This is also paralleled by animal evidence showing that hemicerebellectomy in rats impairs the acquisition and execution of new spatial sequences but not the execution of sequences learnt before the lesion54.
In our experiments, we targeted cerebellar hemispheres and not medial structures in light of prior evidence indicating that perceptual and cognitive processing mainly occur in lateral vs. medial cerebellar regions4,26. Moreover, it is important to note that although TMS directly affects neurons in the targeted area, stimulation may also indirectly modulate neural responses in other proximal or distal regions (e.g., motor cortex or the prefrontal cortex55,56). Specifically, changes in the excitability of the cerebellar cortex may affect excitability of the Purkinje cells, hence modulation by deep cerebellar nuclei over cortical regions41,57. Although the lack of neuroimaging data in our study does not allow to disentangle whether our findings are due to direct effects of TMS on the cerebellar hemisphere or to more indirect effects, our data show that neurons located in the posterior lateral cerebellum contribute to the extended network underpinning visual sequence processing. Furthermore, our participants were overall faster and more accurate with familiar sequences, accuracy being overall higher than 95%. Ceiling performances are less likely to be modulated by TMS58 and cerebellar involvement in cognitive tasks has been found to depend on the complexity of the patterns to be processed14,59,60, response uncertainty25, and cognitive load61. In line with this, it remains to be investigated whether the cerebellum is involved also in retrieving familiar material when the task is sufficiently demanding.
In sum, by showing that the cerebellum plays a role in short-term memory for the order of incoming visual stimuli, our data contribute to a broader investigation on cerebellar involvement in extracting deterministic and probabilistic regularities in incoming sensory information (e.g., sequence detection) and in using this information to generate predictions about future events15,62.
References
Manto, M. et al. Consensus Paper: Roles of the Cerebellum in Motor Control — The Diversity of Ideas on Cerebellar Involvement in Movement. Cerebellum 11, 457–487 (2012).
Koziol, L. F. et al. Consensus Paper: The Cerebellum’s Role in Movement and Cognition. Cerebellum 13, 151–177 (2014).
Mariën, P. et al. Consensus Paper: Language and the Cerebellum: an Ongoing Enigma. The Cerebellum 386–410, https://doi.org/10.1007/s12311-013-0540-5 (2014).
Baumann, O. et al. Consensus Paper: The Role of the Cerebellum in Perceptual Processes. Cerebellum 14, 197–220 (2015).
Tedesco, A. M. et al. The cerebellar cognitive profile. Brain 134, 3669–3683 (2011).
Petrosini, L., Leggio, M. G. & Molinari, M. The cerebellum in the spatial problem solving: A co-star or a guest star? Prog. Neurobiol. 56, 191–210 (1998).
Restuccia, D., Della Marca, G., Valeriani, M., Leggio, M. G. & Molinari, M. Cerebellar damage impairs detection of somatosensory input changes. A somatosensory mismatch-negativity study. Brain 130, 276–87 (2007).
Molinari, M. et al. Cerebellum and detection of sequences, from perception to cognition. Cerebellum 7, 611–5 (2008).
Leggio, M. G., Chiricozzi, F. R., Clausi, S., Tedesco, A. M. & Molinari, M. The neuropsychological profile of cerebellar damage: The sequencing hypothesis. Cortex 47, 137–144 (2011).
Shin, J. C. & Ivry, R. B. Spatial and Temporal Sequence Learning in Patients with Parkinson’ s Disease or Cerebellar Lesions. J. Cogn. Neurosci. 15, 1232–1243 (2002).
Tedesco, A. M. et al. Does the Cerebellum Contribute to Human Navigation by Processing Sequential Information? Neuropsychology 31, 564–574 (2017).
Leggio, M. G. et al. Cognitive sequencing impairment in patients with focal or atrophic cerebellar damage. Brain 131, 1332–43 (2008).
Leggio, M. G. et al. Cerebellar contribution to spatial event processing: Characterization of procedural learning. Exp. Brain Res. 127, 1–11 (1999).
Schubotz, R. & von Cramon, D. Y. A Blueprint for Target Motion: fMRI Reveals Perceived Sequential Complexity to Modulate Premotor Cortex. Neuroimage 16, 920–935 (2002).
Bubic, A., von Cramon, D. Y., Jacobsen, T., Schröger, E. & Schubotz, R. I. Violation of expectation: neural correlates reflect bases of prediction. J. Cogn. Neurosci. 21, 155–168 (2009).
Pascual-Leone, A. et al. Procedural Learning in Parhnson’s Disease and Cerebellar Degeneration. Ann Neurol 34, 594–602 (1993).
Molinari, M. et al. Cerebellum and procedural learning: Evidence from focal cerebellar lesions. Brain 120, 1753–1762 (1997).
Torriero, S., Oliveri, M., Koch, G., Caltagirone, C. & Petrosini, L. Interference of Left and Right Cerebellar rTMS with Procedural Learning. J. Cogn. Neurosci. 16, 1605–1611 (2004).
Doyon, J. et al. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc. Natl. Acad. Sci. USA 99, 1017–1022 (2002).
Jenkins, I. H., Brooks, D. J., Frackowiak, R. S. J. & Passingham, F. E. Motor Sequence Tomography Learning: A Study with Positron. J. Neurosci. 14, 3775–3790 (1994).
Ellerman, J. M. et al. Spatial patterns of functional activation of the cerebellum investigated using high field (4 T) MRI. NMR Biomed 7, 63–68 (1994).
D’Angelo, E. & Casali, S. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front. Neural Circuits 6, 116 (2012).
Bubic, A., von Cramon, D. Y. & Schubotz, R. I. Prediction, cognition and the brain. Front. Hum. Neurosci. 4, 1–15 (2010).
LaBerge, D. Attentional Processing: The Brain’s Art of Mindfulness. (Press, Harvard University, 1995).
Volz, K. G., Schubotz, R. I. & von Cramon, D. Y. Predicting events of varying probability: uncertainty investigated by fMRI. Neuroimage 19, 271–280 (2003).
Keren-Happuch, E., Chen, S., Ho, M. & Desmond, J. A meta‐analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum. Brain Mapp. 35, 593–615 (2014).
Rossi, S., Hallett, M., Rossini, P. M. & Pascual-Leone, A. Screening questionnaire before TMS: an update. Clin. Neurophysiol. 122, 1686 (2011).
Koch, G. et al. Repetitive TMS of cerebellum interferes with millisecond time processing. Exp. Brain Res. 179, 291–299 (2007).
Hanajima, R. et al. Comparison of different methods for estimating motor threshold with transcranial magnetic stimulation. Clin Neurophysiol 118, 2120–2122 (2007).
Cattaneo, Z. et al. Cerebellar vermis plays a causal role in visual motion discrimination. Cortex. 58, 272–80 (2014).
Gamond, L., Ferrari, C., La Rocca, S. & Cattaneo, Z. Dorsomedial prefrontal cortex and cerebellar contribution to in-group attitudes: a transcranial magnetic stimulation study. Eur. J. Neurosci. 45, 932–939 (2017).
Ferrari, C., Oldrati, V., Gallucci, M., Vecchi, T. & Cattaneo, Z. The role of the cerebellum in explicit and incidental processing of facial emotional expressions: A study with transcranial magnetic stimulation. Neuroimage 169, (2018).
Renzi, C., Vecchi, T., D’ Angelo, E., Silvanto, J. & Cattaneo, Z. Phosphene induction by cerebellar transcranial magnetic stimulation. Clin. Neurophysiol. 125, 2132–2133 (2014).
Carducci, F. & Brusco, R. Accuracy of an individualized MR-based head model for navigated brain stimulation. Psychiatry Res. 203, 105–8 (2012).
Cattaneo, Z., Devlin, J. T., Salvini, F., Vecchi, T. & Silvanto, J. The causal role of category-specific neuronal representations in the left ventral premotor cortex (PMv) in semantic processing. Neuroimage 49, 2728–34 (2010).
Cattaneo, Z., Mattavelli, G., Platania, E. & Papagno, C. The role of the prefrontal cortex in controlling gender-stereotypical associations: a TMS investigation. Neuroimage 56, 1839–46 (2011).
Mattavelli, G., Cattaneo, Z. & Papagno, C. Transcranial magnetic stimulation of medial prefrontal cortex modulates face expressions processing in a priming task. Neuropsychologia 49, 992–8 (2011).
Talairach, J. & Tournoux, P. Co-planar Stereotaxic Atlas of the Human Brain. (Thieme Medical, 1988).
Alink, A., Schwiedrzik, C. M., Kohler, A., Singer, W. & Muckli, L. Stimulus predictability reduces responses in primary visual cortex. J. Neurosci. 30, 2960–6 (2010).
Del Olmo, M. F., Cheeran, B., Koch, G. & Rothwell, J. C. Role of the cerebellum in externally paced rhythmic finger movements. J. Neurophysiol. 98, 145–52 (2007).
Koch, G. Repetitive transcranial magnetic stimulation: a tool for human cerebellar plasticity. Funct. Neurol. 25, 159–163 (2010).
Zangen, A., Roth, Y., Voller, B. & Hallett, M. Transcranial magnetic stimulation of deep brain regions: Evidence for efficacy of the H-Coil. Clin. Neurophysiol. 116, 775–779 (2005).
Bona, S., Cattaneo, Z. & Silvanto, J. The Causal Role of the Occipital Face Area (OFA) and Lateral Occipital (LO) Cortex in Symmetry Perception. J. Neurosci. 35, 731–738 (2015).
Ferrari, C. et al. The Dorsomedial Prefrontal Cortex Plays a Causal Role in Integrating Social Impressions from Faces and Verbal Descriptions. Cereb. Cortex 26, 156–165 (2016).
Schubotz, R. I. & von Cramon, D. Y. Interval and ordinal properties of sequences are associated with distinct premotor areas. Cereb. Cortex 11, 210–222 (2001).
Schubotz, R. I. & von Cramon, D. Y. Sequences of Abstract Nonbiological Stimuli Share Ventral Premotor Cortex with Action Observation and Imagery. J. Neurosci. 24, 5467–5474 (2004).
Timmann, D. et al. Use of sequence information in associative learning in control subjects and cerebellar patients. Cerebellum 3, 75–82 (2004).
Hoen, M., Pachot-Clouard, M., Segebarth, C. & Dominey, P. F. When Broca experiences the janus syndrome: An ER-fMRI study comparing sentence comprehension and cognitive sequence processing. Cortex 42, 605–623 (2006).
Schubotz, R. I. Prediction of external events with our motor system: towards a new framework. Trends Cogn. Sci. 11, 211–218 (2007).
Kotz, S. A., Stockert, A. & Schwartze, M. Cerebellum, temporal predictability and the updating of a mental model. Philos. Trans. R. Soc. B Biol. Sci. 369, 20130403–20130403 (2014).
Ivry, R. Exploring the role of the cerebellum in sensory anticipation and timing: Commentary on Tesche and Karhu. Hum. Brain Mapp. 9, 115–118 (2000).
Attout, L., Fias, W., Salmon, E. & Majerus, S. Common neural substrates for ordinal representation in short-term memory, numerical and alphabetical cognition. PLoS One 9, e92049 (2014).
Houk, J. C. & Wise, S. P. Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning and controlling action. Cereb. cortex 5, 95–110 (1995).
Leggio, M. G. et al. Representation of actions in rats: the role of cerebellum in learning spatial performances by observation. Proc. Natl. Acad. Sci. USA 97, 2320–2325 (2000).
Farzan, F., Pascual-Leone, A., Schmahmann, J. D. & Halko, M. Enhancing the Temporal Complexity of Distributed Brain Networks with Patterned Cerebellar Stimulation. Sci. Rep. 6, 23599 (2016).
Esterman, M. et al. Network-targeted cerebellar transcranial magnetic stimulation improves attentional control. Neuroimage 156, 190–198 (2017).
Pinto, A. D. & Chen, R. Suppression of the motor cortex by magnetic stimulation of the cerebellum. Exp. Brain Res. 140, 505–510 (2001).
Robertson, E. M., Théoret, H. & Pascual-Leone, A. Studies in Cognition: The Problems Solved and Created by Transcranial Magnetic Stimulation. J. Cogn. Neurosci. 15, 948–960 (2003).
Boecker, H. et al. A H215O Positron Emission Tomography Study on Mental Imagery of Movement Sequences—The Effect of Modulating Sequence Length and Direction. Neuroimage 17, 999–1009 (2002).
D’Agata, F. et al. The recognition of facial emotions in spinocerebellar ataxia patients. Cerebellum 10, 600–610 (2011).
Kirschen, M. P., Chen, S. H., Schraedley-Desmond, P. & Desmond, J. E. Load- and practice-dependent increases in cerebro-cerebellar activation in verbal working memory: an fMRI study. Neuroimage 24, 462–472 (2005).
Leggio, M. & Molinari, M. Cerebellar Sequencing: a Trick for Predicting the Future. The Cerebellum 14, 35–38 (2015).
Acknowledgements
This work was supported by Ministry of Education – Italy, University and Research (FIRB FR12F0BD to Z.C, PRIN 2015WXAXJF to Z.C., and PRIN 2015AR52F9 to T.V.). This work was supported by Italian Ministry of Health (Ricerca Corrente 2015–2017) to ED and LC. LC was supported by Progetto HBP (Grant agreement 720270)-Regione Lombardia.
Author information
Authors and Affiliations
Contributions
C.F., Z.C., E.D. and T.V. designed the study. C.F., V.O., and L.C. collected the data. C.F. and Z.C. analyzed the data. C.F., Z.C. and F.C. wrote the manuscript. C.F., Z.C., V.O. and F.C. contributed to the revision process. Scientific discussion and paper validation: all.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ferrari, C., Cattaneo, Z., Oldrati, V. et al. TMS Over the Cerebellum Interferes with Short-term Memory of Visual Sequences. Sci Rep 8, 6722 (2018). https://doi.org/10.1038/s41598-018-25151-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-25151-y
This article is cited by
-
The Possibility of Increasing the Effectiveness of Correcting Motor Skills and Cognitive Functions Using Noninvasive Brain Stimulation in Humans
Neuroscience and Behavioral Physiology (2023)
-
Probing cerebellar involvement in cognition through a meta-analysis of TMS evidence
Scientific Reports (2021)
-
New Horizons on Non-invasive Brain Stimulation of the Social and Affective Cerebellum
The Cerebellum (2021)
-
Cerebellar disruption impairs working memory during evidence accumulation
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.