Abstract
This study investigated the characteristics of patients with different chronic kidney disease (CKD) stages according to various body mass index (BMI) categories and determined the influence of BMI in renal function deterioration. We conducted a multicenter, longitudinal cohort study based on the Epidemiology and Risk Factors Surveillance of CKD project (2008–2013) and National Health Insurance Research Database (2001–2013). A total of 7357 patients with CKD aged 20–85 years from 14 hospitals were included in the study. A higher male sex, diabetes mellitus (DM) and hypertension were noted among overweight and obese CKD patients, while more cancer prevalence was noted among underweight CKD patients. Charlson comorbidity index was significantly higher and correlated with BMI among late CKD patients. Patients with BMI < 18.5 kg/m2 exhibited non-significantly higher events of eGFR decline events in both early and late CKD stages than other BMI groups. BMI alone is not a determinant of CKD progression among our Taiwanese CKD patients. Obesity should be re-defined and body weight manipulation should be individualized in CKD patients.
Similar content being viewed by others
Introduction
Obesity, a global pandemic problem, is associated with various metabolic disorders and results in a shortened life span related to adverse health consequences. In Taiwan, the prevalence of overweight and obesity among adults was reported to be 44.1%, of whom 50.8% were men and 36.9% were women, according to 2005–2008 data1. Moreover, in a survey performed in 2002 and 2007 (Taiwanese Survey on Hypertension, Hyperglycemia, and Hyperlipidemia), the prevalence of obesity increased from 19.2% to 23.3% among men and from 13.4% to 19.0% among women2. The overweight and obesity rates in Taiwan are substantially higher than those in Japan, Singapore, and other neighboring Asian countries. On the other hand, the prevalence of chronic kidney disease (CKD) in Taiwan is also increasing, with nearly 6 million people undergoing dialysis and approximately 2,000 people newly diagnosed as having end-stage renal disease (ESRD) annually (http://www.tsn.org.tw/UI/K/K008.aspx). Since the numbers of obese patients with CKD and those undergoing dialysis are also increasing in parallel, the diagnosis and precise management of obesity have become critical among these patients.
Many studies have demonstrated that obesity is an important risk factor for incident CKD3,4,5,6,7 and increased risk of ESRD8,9,10,11,12. Paradoxically, obesity itself in CKD and ESRD has been found to be associated with more favorable outcomes13,14. A reverse obesity–mortality association has been consistently observed in patients with ESRD15,16,17; however, conflicting results have been observed among patients with CKD13,18,19,20. Body mass index (BMI) is a globally accepted anthropometric measure for obesity classification. Recently, many studies have questioned the accuracy of BMI in obesity and excess body fat assessment21,22,23. Whether BMI can influence the CKD progression among all stages of CKD in the Taiwanese population remains unclear.
We conducted a multicenter, longitudinal cohort study to investigate the characteristics of patients at all CKD stages (CKD stages 1–5 nondialysis [ND]) according to various BMI categories and to determine the influence of BMI in renal function deterioration by using the data from the Epidemiology and Risk Factors Surveillance of CKD project (2008–2013) and National Health Insurance Research Database (NHIRD) (2001–2013).
Results
Demographic characteristics of the patients
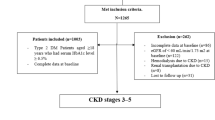
A total of 7357 patients with CKD aged 20–85 years from 14 hospitals were included in the study. Patients with ESRD, defined as either receiving maintenance dialysis during this period or having a kidney transplant, were excluded. After the exclusion of patients with less than 1 year of follow-up (n = 2789) and those with missing or incomplete data (n = 999), 4022 patients with CKD were finally enrolled in this study (Fig. 1). Among these patients, 2008 had early-stage CKD (CKD stages 1, 2, and 3a) and 2014 had late-stage CKD (CKD stages 3b, 4, and 5ND). The patients were continually traced from the baseline date to the end of the study period (June 18, 2015).
The mean age of the cohort was 62.86 ± 14.93 years, and 56.9% of the patients were men. The mean baseline eGFR was 51.5 ± 36.15 mL/min per 1.73 m2. The baseline characteristics of patients according to baseline BMI are presented in Table 1. For further analysis, we also present the baseline characteristics of patients with early-stage CKD (CKD stages 1–3a) in Table 2 and late-stage CKD (CKD stages 3b–5) in Table 3. The highest prevalence rates of overweight (24 ≤ BMI <27 kg/m2) and obesity (BMI ≥ 27 kg/m2) were observed among patients aged 45–64 years, with the values being 45% and 39.38% respectively. We also noted a male predominance in BMI (62.7% for overweight and 56.43% for obesity). Patients with higher BMI exhibited significantly higher baseline DM and hypertension than those with lower BMI (16.15% in the underweight group, compared with 36.05%, 47.29%, and 52.23% in the normal, overweight, and obesity groups, respectively). Furthermore, patients with BMI < 18.5 kg/m2 exhibited a characteristically higher cancer prevalence rate (18.46%) than the other groups; the prevalence rates were 9.75%, 9.16%, and 8.22% in the normal, overweight, and obesity groups, respectively. Baseline coronary artery disease (CAD) and stroke did not differ between the groups. Similarly, the baseline Charlson comorbidity index was significantly lower in the low and normal BMI groups (3.38 ± 2.50 and 3.53 ± 2.39, respectively), compared with the overweight and obesity groups (3.81 ± 2.46 and 3.73 ± 2.41, respectively). Furthermore, the higher BMI groups had higher medication use and more smoking, alcohol consumption, and betel nut chewing habits. The biochemical analysis revealed significantly higher hemoglobin and hematocrit levels, AC sugar, triglyceride, and uric acid levels in the higher BMI groups. No significant difference was observed in baseline cholesterol, electrolytes, albumin, UPCR, and eGFR between the groups (Table 1). We subgrouped our cohort into early- and late-stage CKD (CKD stages 1–3a and 3b–5) and studied their characteristics according to different BMI categories (Tables 2 and 3). Similar age and sex prevalence rates were observed after subgrouping. Among patients with early-stage CKD, those in the lower BMI group exhibited significantly higher cancer prevalence (16.67% in with the underweight group) than those in the higher BMI groups (8.64% in the normal, 8.95% in the overweight, and 6.76% in the obesity groups; p = 0.032). However, among patients with late-stage CKD, those in the lower BMI group had nonsignificantly higher cancer prevalence than those in the higher BMI groups (20% in the underweight group vs. 10.8% in the normal group, 11.39% in the overweight group, and 9.8% in the obesity group; p = 0.08). Higher DM and hypertension rates were observed in overweight and obese patients with early- and late-stage CKD. The Charlson comorbidity index did not differ significantly with BMI among patients with early-stage CKD (2.57 ± 2.31 in the underweight group vs. 2.69 ± 2.12 in the normal group, 2.92 ± 2.19 in the overweight group, and 2.86 ± 2.12 in the obesity group; p = 0.1773). However, a significantly higher Charlson comorbidity index was observed among overweight and obese patients with late-stage CKD (4.07 ± 2.47 in the underweight group vs. 4.32 ± 2.37 in the normal group, 4.71 ± 2.39 in the overweight group, and 4.67 ± 2.35 in the obesity group; p = 0.003). The initial stages of CKD did not differ significantly among patients with early-stage CKD; by contrast, they differed significantly among patients with late-stage CKD according to BMI (p = 0.0004). More prevalent CKD stage 3b (18.57% in the underweight group vs. 26.51% in the normal group, 33.3% in the overweight group, and 31.58% in the obesity group) and stage 4 (28.5% in the underweight group vs. 36.43% in the normal group, 36.35% in the overweight group, and 38.11% in the obesity group) were observed among patients in the higher BMI group; by contrast, 52.8% of patients in the underweight group had CKD stage 5 compared with 37.06%, 30.32%, 30.31% in the normal, overweight, and obesity groups, respectively.
We analyzed the proportion of eGFR progression events during the follow-up period among patients with CKD stages 1–5 in different BMI groups. We also executed further subgroup analysis of the proportion of eGFR progression among patients with early-stage CKD (stages 1–3a) and late-stage CKD (stages 3b–5) in different BMI groups.
Correlation between BMI and CKD progression among patients with CKD stages 1–5
Table 4 presents the proportion of eGFR progression events in patients with CKD stages 1–5. The study outcomes are presented as ORs, and the normal group was used as the reference group to calculate the OR for each group. The underweight group exhibited the highest proportion of events (25%) compared with the normal (19%), overweight (19%), and obesity (18%) groups. The ORs of eGFR progression events were 1.44 (0.95, 2.18), 0.99 (0.81, 1.2), and 0.95 (0.78, 1.16) in the underweight, overweight, and obesity groups, respectively. After adjusting for age, sex, previous diabetes, CAD, stroke, cancer, high blood pressure, Charlson score, TB, COPD, ACEI, ARB, fibrate, smoking, alcohol consumption, betel nut chewing, baseline UPCR, and baseline eGFR, we observed that the OR was 1.35 (0.87, 2.10) in the underweight group compared with 1.02 (0.83, 1.25) and 0.95 (0.77, 1.18) in the overweight and obesity groups, respectively (Fig. 2).
Correlation between BMI and CKD progression among patients with early-stage CKD (stages 1–3a)
Table 5 presents the proportion of eGFR deterioration events in patients with CKD stages 1–3a. The study outcomes are presented as ORs, and the normal group was used as the reference group to calculate the OR for each group. The underweight group exhibited the highest proportion of eGFR deterioration events (20%) compared with the normal (13%), overweight (13%), and obesity (12%) groups. The ORs of eGFR progression events were 1.67 (0.86, 3.25), 0.98 (0.71, 1.34), and 0.91 (0.66, 1.26) in the underweight, overweight, and obesity groups, respectively. After adjusting for age, sex, previous diabetes, CAD, stroke, cancer, high blood pressure, Charlson score, TB, COPD, ACEI, ARB, fibrate, smoking, alcohol consumption, betel nut chewing, baseline UPCR, and baseline eGFR, we determined that the ORs were 1.42 (0.70, 2.88), 1.06 (0.76, 1.47), and 0.92 (0.66, 1.30) in the underweight, overweight, and obesity groups, respectively (Fig. 3).
Correlation between BMI and CKD progression among patients with late-stage CKD (stage 3b–5)
Table 6 presents the proportion of eGFR deterioration events in patients with CKD stages 3b–5. The study outcomes are presented as ORs, and the normal group was used as the reference group to calculate the OR for each group. The underweight group exhibited the highest proportion of eGFR deterioration events (30%) compared with the normal (25%), overweight (25%), and obesity (25%) groups. The ORs of the eGFR progression events were 1.29 (0.76, 2.21), 1.01 (0.79, 1.30), and 1.02 (0.79, 1.31) in the underweight, overweight, and obesity groups, respectively. After adjusting for age, sex, previous diabetes, CAD, stroke, cancer, high blood pressure, Charlson score, TB, COPD, ACEI, ARB, fibrate, smoking, alcohol consumption, betel nut chewing, baseline UPCR, and baseline eGFR, we observed that the ORs were 1.33 (0.74, 2.39), 1.04 (0.79, 1.36), and 1.04 (0.79, 1.39) in the underweight, overweight, and obesity groups, respectively (Fig. 4).
Discussion
In this prospective cohort study, we evaluated the characteristics of a CKD cohort according to various BMI categories. Subsequently, we investigated the association between BMI and the risk of eGFR decline among patients with different CKD stages. The highest prevalence of overweight and obesity was observed among men and the working age group (45–64 years old) in both early- and late-stage CKD. Previous studies conducted on the Japanese24,25 and Malay populations26 have demonstrated a male sex-specific association between BMI and kidney disease; similarly, from our baseline data, we observed a higher male prevalence among overweight and obese CKD patients. The mechanism underlying the male sex-specific association between higher BMI and CKD remains unclear; however, several studies have identified that BMI reflects visceral fat more efficiently in men than in women27,28. Generally, men exhibit a higher risk of kidney disease and develop the disease earlier in life than women because of hormonal and lifestyle influences29,30,31,32.
We observed a significantly higher prevalence of DM and hypertension among overweight and obese patients with CKD (both early- and late-stage CKD). This observational association might not represent cause and effect; since obesity itself is associated with various adverse sequelae from metabolic syndrome, as well as from comorbidities including DM and hypertension33,34, and all these conditions are associated with CKD. Patients with early-stage CKD with a lower BMI exhibited significantly higher cancer prevalence; however, non-significantly higher prevalence was observed in patients with late-stage CKD. This might also not represent a causal relation; nevertheless, many studies have revealed a bidirectional association between CKD and cancer35,36. Cancer patients with lower BMI exhibited associated nutritional disturbances and tended to have reduced renal function status from nutritional and specified therapies. A Korean study reported a significantly higher risk of CKD and proteinuria among cancer survivors37.
Furthermore, both CKD and ESRD are higher risks from a number of malignancies38,39. We calculated the Charlson scores at different CKD stages, which did not differ significantly among patients with early-stage CKD in different BMI categories. However, the median Charlson score increased significantly among overweight and obese patients with late-stage CKD (Fig. 5), which demonstrates the presence of more comorbidity among these patients.
The initial CKD stages did not differ significantly with BMI among patients with early-stage CKD (Table 2). However, in patients with late-stage CKD, the prevalence of the initial stages of CKD differed significantly according to BMI (Table 6), with a higher prevalence of CKD stages 3b and 4 observed in overweight and obese patients. We observed a significantly higher number of patients with CKD stage 5 to be underweight (BMI < 18.5). The reason for this finding is unknown, and additional studies are required to confirm whether any nutritional and concurrent comorbidity might play a role in such lower BMI prevalence among these patients. An obesity paradox was supposed for stage 5 CKD, because low BMI represents more uremia-associated inflammatory cachexia and high BMI represents fewer uremic consequences and more favorable health40,41,42. More emphasis on improved and adequate nutrition is required for these patients with advanced-stage CKD compared with control obesity in healthy patients.
A follow-up analysis revealed non-significantly increased CKD progression events among underweight patients compared with overweight and obese patients in both early and late CKD stages. This observed association persisted after adjustment for age, sex, previous diabetes, CAD, stroke, cancer, high blood pressure, Charlson score, TB, COPD, ACEI, ARB, fibrate, smoking, alcohol consumption, betel nut chewing, baseline UPCR, and baseline eGFR, and it was consistently present in the subgroup analysis among all patients with CKD. Our results are consistent with the so-called obesity paradox43 among patients with CKD; we found non-significantly higher kidney disease progression events among underweight patients compared with overweight and obese patients in all stages of CKD. Although several mechanisms have been proposed for patients with late-stage CKD42, we observed the same paradox among patients with early-stage CKD, which might be explained by the older age and higher cancer prevalence among patients with lower BMI. In patients with late-stage CKD, lower BMI was associated with poor nutritional status42 or higher prevalence of metabolically obese normal-weight individuals with a higher comorbidity burden44.
Our study results are consistent with those of other population studies. Data from a nationally representative cohort of US veterans with eGFR < 60 mL/min indicated a U-shaped association between BMI and the risk of renal progression20, with deteriorating outcomes observed in individuals with BMI < 25 kg/m2 and BMI ≥ 35 kg/m2; these data demonstrate that overweight or mild obesity (30–35 kg/m2) results in the most favorable outcomes and that in advanced CKD stages (eGFR < 30 mL/min), even morbid obesity (BMI ≥ 35 kg/m2) is not associated with adverse outcomes. A similar U-shaped association between an increased risk of progressive CKD and lowest BMI levels was noted in a large population-based cohort study in Israel45.
A retrospective study in the Taiwanese general population reported that waist-to-height ratio (WHtR), rather than BMI, increased as the prevalence of CKD increased46. Other studies have reported that WHtR and waist circumference, but not BMI, were associated with mortality in patients with CKD and ESRD47,48. Although the recent global definition uses BMI as a standard measure of obesity, obesity is affected by muscle mass, peripheral and abdominal adipose tissue mass, and bone; thus, the results should be considered with the condition49. Central obesity has been proved to be more vulnerable to metabolic syndrome and obesity-related diseases, whereas peripheral obesity and higher muscle mass appear protective50,51,52. BMI failed to represent central obesity because of the variation in individual body composition and contribution. This explains the nonsignificant association between BMI and CKD progression through all stages of CKD in our study.
Our study has several limitations. Because we studied a prevalent cohort of patients with CKD, we could not determine the effects of obesity on incident CKD. We used the study participants’ personal identities to link health care databases, and because the NHI database is based on the reporting data system and does not include the population not under medical health care, the study result may not represent the whole population; however, the missing population is negligible. We used only BMI to determine obesity, which may not be an ideal marker of obesity among our cohort; nevertheless, because BMI is generally accepted as a predominant index to establish obesity in clinical practice, our results have direct clinical relevance. Because the blood and urine samples of study participants were collected from individual hospitals and sent to the research center, the use of different equipment and personnel of individual hospitals may have resulted in measurement errors. Furthermore, we did not determine the influence of low and high BMI on mortality outcomes.
Materials and Methods
Ethics statement
The study was reviewed and approved by the institutional ethical committee of Taipei Medical University - Shuang Ho Hospital (TMU-JIRB 201204036), Tri-Service General Hospital (TSGHIRB100-05-197), Cardinal Tien Hospital (TMU-JIRB 201204035), Changhua Christian Hospital (CCHIRB 20405), Kaohsiung Medical University Chung-Ho Memorial Hospital (KMUHIRB 20120019), Kaohsiung Chang Gung Memorial Hospital (101-1096B), National Cheng Kung University Hospital (A-ER-101-117) and China Medical University Hospital (DMR101-IRB2-273(CR-1)). After a complete explanation of the study, written informed consent was obtained from all participants. All clinical and biological samples were collected after patient consent. All the study methods were in accordance with the guidelines approved by the joint institutional review board and aforementioned governmental regulations.
Study population
We conducted a multicenter, longitudinal cohort study using data from the Epidemiology and Risk Factors Surveillance of CKD database (2008–2013) managed by the Bureau of Health Promotion, Ministry of Health and Welfare, Taiwan. After excluding patients with incomplete or missing data, we linked the biochemical laboratory data to the NHIRD from 2001 to 2013. The same medical laboratory criteria and protocol have been used in our study hospitals, and the serum creatinine levels derived from different hospitals can be compared and standardized with each other. In this study, we measured CKD progression at the individual level. In addition, the patients were reexamined in the same hospital to control the individual variation. All patients provided informed consent before data collection.
Measurements and variable definitions
The patients’ demographic, clinical, and health-related behavior data were collected using a structured questionnaire. The questionnaire collected data on age, sex, cigarette smoking, alcohol consumption, betel nut chewing, personal and family comorbid conditions, and medication use. Physical examination included anthropometry, blood pressure measurement, pulse rate measurement, and systemic examination. Height was measured in centimeters by using a wall-mounted measuring tape, and weight was measured in kilograms by using a digital scale (SECA, model 782 2321009; Vogel & Halke, Germany). BMI was classified into the following groups: <18.5 kg/m2 (underweight), 18.5–23.9 kg/m2 (normal), 24–26.9 kg/m2 (overweight), and ≥27 kg/m2 (obesity). Glycemia, blood pressure, and lipid control conditions were classified as intensive and poor. Proteinuria status was determined using the urine protein-to-creatinine ratio (UPCR). CKD was defined according to the Kidney Disease Outcomes Quality Initiative guidelines53 and was evaluated using the estimated glomerular filtration rate (eGFR), which was calculated using the Chronic Kidney Disease-Epidemiology Collaboration equation: eGFR (mL/min/1.73 m2) = 141 × min (SCr/ƙ, 1)α × max (serum creatinine/ƙ, 1) − 1.209 × 0.993Age × 1.018 (if female) and × 1.159 (if black), where SCr denotes the serum creatinine level (mg/dL), ƙ = 0.7 (for women) and 0.9 (for men), α = −0.329 (for women) and −0.411(for men), min denotes the minimum of SCr/ƙ or 1, and max denotes the maximum of SCr/ƙ or 154. CKD was classified as follows: CKD stage 1, eGFR ≥ 90 mL/min/1.73 m2 and the presence of kidney damage (i.e., proteinuria dipsticks ≥1+, UPCR ≥ 150, or urine albumin-to-creatinine ratio [UACR] ≥30); CKD stage 2, eGFR = 60–89 mL/min/1.73 m2 and the presence of kidney damage (i.e., proteinuria dipsticks ≥1+, UPCR ≥ 150, or UACR ≥ 30); CKD stage 3a, eGFR = 45–59 mL/min/1.73 m2; CKD stage 3b, eGFR = 30–44 mL/min/1.73 m2; CKD stage 4, eGFR = 15–29 mL/min/1.73 m2; and CKD stage 5, eGFR < 15 mL/min/1.73 m2 55. Renal progression was defined as an average eGFR decline by more than 5 mL/min/1.73 m2 per year or into the dialysis stage56.
Statistical analysis
Consistent with the study hypothesis, all analyses were stratified according to BMI. We examined BMI as quartiles: <18.5, 18.5–23.9, 24–26.9, and ≥27 kg/m2. The characteristics of different BMI groups were compared using the chi-squared test for categorical variables and ANOVA for continuous variables. The odds ratio (OR) (95% confidence interval) of CKD was calculated for each BMI category. Next, we explored the data for confounding and effect modification in stratified analyses. After adjusting for all covariates, we used the multivariate logistic model with stepwise variable selection models to evaluate the association between BMI and eGFR decline. In our subsequent multivariate modeling, we considered covariates including age; sex; comorbid conditions such as diabetes mellitus (DM), stroke, and cancer; the Charlson comorbidity index; use of antihypertensive medications (e.g., ACEI/ARB and loop diuretics) within the previous 1 year; and baseline CKD stage. The SAS statistical package (Version 9.3, SAS Institute Inc., Cary, NC, USA) was used for all statistical tests. Results with P < 0.05 were considered statistically significant.
Conclusions
In conclusion, the definition and classification of obesity among patients with CKD should be intensively re-determined, because misdiagnosis can lead to inappropriate clinical decisions and might deteriorate patients’ prognosis. The anthropomorphic measures alternate to BMI should be established from randomized controlled clinical trials among the CKD population.
Change history
13 February 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Yeh, C. J., Chang, H. Y. & Pan, W. H. Time trend of obesity, the metabolic syndrome and related dietary pattern in Taiwan: from NAHSIT 1993–1996 to NAHSIT 2005–2008. Asia Pac J Clin Nutr 20, 292–300 (2011).
HPA. Taiwanese Survey on Hypertension, Hyperglycemia, and Hyperlipidemia. (Health Promotion Administration, Ministry of Health and Welfare, 2007).
Foster, M. C. et al. Overweight, obesity, and the development of stage 3 CKD: the Framingham Heart Study. Am J Kidney Dis 52, 39–48, https://doi.org/10.1053/j.ajkd.2008.03.003 (2008).
Ejerblad, E. et al. Obesity and risk for chronic renal failure. J Am Soc Nephrol 17, 1695–1702, https://doi.org/10.1681/ASN.2005060638 (2006).
Fox, C. S. et al. Predictors of new-onset kidney disease in a community-based population. JAMA 291, 844–850, https://doi.org/10.1001/jama.291.7.844 (2004).
Kramer, H. et al. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis 46, 587–594, https://doi.org/10.1053/j.ajkd.2005.06.007 (2005).
Pinto-Sietsma, S. J. et al. A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis 41, 733–741 (2003).
Pscheidt, C. et al. Sex- and Time-Dependent Patterns in Risk Factors of End-Stage Renal Disease: A Large Austrian Cohort with up to 20 Years of Follow-Up. PLoS One 10, e0135052, https://doi.org/10.1371/journal.pone.0135052 (2015).
Hsu, C. Y., McCulloch, C. E., Iribarren, C., Darbinian, J. & Go, A. S. Body mass index and risk for end-stage renal disease. Ann Intern Med 144, 21–28 (2006).
Franceschini, N. et al. Adiposity patterns and the risk for ESRD in postmenopausal women. Clin J Am Soc Nephrol 10, 241–250, https://doi.org/10.2215/CJN.02860314 (2015).
Panwar, B. et al. Obesity, metabolic health, and the risk of end-stage renal disease. Kidney Int 87, 1216–1222, https://doi.org/10.1038/ki.2014.384 (2015).
Kramer, H. et al. Waist Circumference, Body Mass Index, and ESRD in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am J Kidney Dis 67, 62–69, https://doi.org/10.1053/j.ajkd.2015.05.023 (2016).
Kovesdy, C. P., Anderson, J. E. & Kalantar-Zadeh, K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis 49, 581–591, https://doi.org/10.1053/j.ajkd.2007.02.277 (2007).
Kalantar-Zadeh, K., Abbott, K. C., Salahudeen, A. K., Kilpatrick, R. D. & Horwich, T. B. Survival advantages of obesity in dialysis patients. Am J Clin Nutr 81, 543–554 (2005).
Kakiya, R. et al. Body fat mass and lean mass as predictors of survival in hemodialysis patients. Kidney Int 70, 549–556, https://doi.org/10.1038/sj.ki.5000331 (2006).
Glanton, C. W. et al. Factors associated with improved short term survival in obese end stage renal disease patients. Ann Epidemiol 13, 136–143 (2003).
Kalantar-Zadeh, K. et al. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis 46, 489–500, https://doi.org/10.1053/j.ajkd.2005.05.020 (2005).
Madero, M. et al. Body mass index and mortality in CKD. Am J Kidney Dis 50, 404–411, https://doi.org/10.1053/j.ajkd.2007.06.004 (2007).
Kwan, B. C., Murtaugh, M. A. & Beddhu, S. Associations of body size with metabolic syndrome and mortality in moderate chronic kidney disease. Clin J Am Soc Nephrol 2, 992–998, https://doi.org/10.2215/CJN.04221206 (2007).
Lu, J. L., Kalantar-Zadeh, K., Ma, J. Z., Quarles, L. D. & Kovesdy, C. P. Association of body mass index with outcomes in patients with CKD. J Am Soc Nephrol 25, 2088–2096, https://doi.org/10.1681/ASN.2013070754 (2014).
Tomiyama, A. J., Hunger, J. M., Nguyen-Cuu, J. & Wells, C. Misclassification of cardiometabolic health when using body mass index categories in NHANES 2005–2012. Int J Obes (Lond) 40, 883–886, https://doi.org/10.1038/ijo.2016.17 (2016).
Peterson, M. D., Al Snih, S., Stoddard, J., Shekar, A. & Hurvitz, E. A. Obesity misclassification and the metabolic syndrome in adults with functional mobility impairments: Nutrition Examination Survey 2003-2006. Prev Med 60, 71–76, https://doi.org/10.1016/j.ypmed.2013.12.014 (2014).
Caleyachetty, R., Meunnig, P. & Kengne, A. P. Misclassification of cardiometabolic health when using body mass index categories. Int J Obes (Lond) 40, 1332, https://doi.org/10.1038/ijo.2016.65 (2016).
Iseki, K. Body mass index and the risk of chronic renal failure: the Asian experience. Contrib Nephrol 151, 42–56, https://doi.org/10.1159/000095318 (2006).
Iseki, K. et al. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int 65, 1870–1876, https://doi.org/10.1111/j.1523-1755.2004.00582.x (2004).
Shankar, A. et al. Association between body mass index and chronic kidney disease in men and women: population-based study of Malay adults in Singapore. Nephrol Dial Transplant 23, 1910–1918, https://doi.org/10.1093/ndt/gfm878 (2008).
Horber, F. F., Gruber, B., Thomi, F., Jensen, E. X. & Jaeger, P. Effect of sex and age on bone mass, body composition and fuel metabolism in humans. Nutrition 13, 524–534 (1997).
Kuk, J. L., Lee, S., Heymsfield, S. B. & Ross, R. Waist circumference and abdominal adipose tissue distribution: influence of age and sex. Am J Clin Nutr 81, 1330–1334 (2005).
Hopper, J. Jr, Trew, P. A. & Biava, C. G. Membranous nephropathy: its relative benignity in women. Nephron 29, 18–24 (1981).
Gretz, N., Zeier, M., Geberth, S., Strauch, M. & Ritz, E. Is gender a determinant for evolution of renal failure? A study in autosomal dominant polycystic kidney disease. Am J Kidney Dis 14, 178–183 (1989).
Tozawa, M. et al. Influence of smoking and obesity on the development of proteinuria. Kidney Int 62, 956–962, https://doi.org/10.1046/j.1523-1755.2002.00506.x (2002).
Neugarten, J., Acharya, A. & Silbiger, S. R. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol 11, 319–329 (2000).
Henegar, J. R., Bigler, S. A., Henegar, L. K., Tyagi, S. C. & Hall, J. E. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol 12, 1211–1217 (2001).
Haslam, D. W. & James, W. P. Obesity. Lancet 366, 1197–1209, https://doi.org/10.1016/S0140-6736(05)67483-1 (2005).
Stengel, B. Chronic kidney disease and cancer: a troubling connection. J Nephrol 23, 253–262 (2010).
Porta, C., Cosmai, L., Gallieni, M., Pedrazzoli, P. & Malberti, F. Renal effects of targeted anticancer therapies. Nat Rev Nephrol 11, 354–370, https://doi.org/10.1038/nrneph.2015.15 (2015).
Shin, H. Y., Linton, J. A., Shim, J. Y. & Kang, H. T. Cancer survivors aged 40 years or elder are associated with high risk of chronic kidney disease: the 2010–2012 Korean National Health and Nutrition Examination Survey. Asian Pac J Cancer Prev 16, 1355–1360 (2015).
Wong, G. et al. Association of CKD and cancer risk in older people. J Am Soc Nephrol 20, 1341–1350, https://doi.org/10.1681/ASN.2008090998 (2009).
Jorgensen, L., Heuch, I., Jenssen, T. & Jacobsen, B. K. Association of albuminuria and cancer incidence. J Am Soc Nephrol 19, 992–998, https://doi.org/10.1681/ASN.2007060712 (2008).
Morley, J. E., Thomas, D. R. & Wilson, M. M. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr 83, 735–743 (2006).
Lecker, S. H., Goldberg, A. L. & Mitch, W. E. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol 17, 1807–1819, https://doi.org/10.1681/ASN.2006010083 (2006).
Kalantar-Zadeh, K. et al. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care 10, 433–442, https://doi.org/10.1097/MCO.0b013e3281a30594 (2007).
Park, J. et al. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis 56, 415–425, https://doi.org/10.1016/j.pcad.2013.10.005 (2014).
Conus, F., Rabasa-Lhoret, R. & Peronnet, F. Characteristics of metabolically obese normal-weight (MONW) subjects. Appl Physiol Nutr Metab 32, 4–12, https://doi.org/10.1139/H07-926 (2007).
Vivante, A. et al. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch Intern Med 172, 1644–1650, https://doi.org/10.1001/2013.jamainternmed.85 (2012).
Li, W. C. et al. Association between waist-to-height ratio and chronic kidney disease in the Taiwanese population. Intern Med J 44, 645–652, https://doi.org/10.1111/imj.12459 (2014).
Kramer, H. et al. Association of waist circumference and body mass index with all-cause mortality in CKD: The REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am J Kidney Dis 58, 177–185, https://doi.org/10.1053/j.ajkd.2011.02.390 (2011).
Postorino, M., Marino, C., Tripepi, G., Zoccali, C. & Group, C. W. Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J Am Coll Cardiol 53, 1265–1272, https://doi.org/10.1016/j.jacc.2008.12.040 (2009).
Brown, R. N. et al. Body mass index has no effect on rate of progression of chronic kidney disease in non-diabetic subjects. Nephrol Dial Transplant 27, 2776–2780, https://doi.org/10.1093/ndt/gfr757 (2012).
Lee, M. J., Wu, Y. & Fried, S. K. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med 34, 1–11, https://doi.org/10.1016/j.mam.2012.10.001 (2013).
Mooney, S. J., Baecker, A. & Rundle, A. G. Comparison of anthropometric and body composition measures as predictors of components of the metabolic syndrome in a clinical setting. Obes Res Clin Pract 7, e55–66, https://doi.org/10.1016/j.orcp.2012.10.004 (2013).
Chang, S. H., Beason, T. S., Hunleth, J. M. & Colditz, G. A. A systematic review of body fat distribution and mortality in older people. Maturitas 72, 175–191, https://doi.org/10.1016/j.maturitas.2012.04.004 (2012).
National Kidney, F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39, S1–266 (2002).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 150, 604–612 (2009).
Levey, A. S. & Coresh, J. Chronic kidney disease. Lancet 379, 165–180, https://doi.org/10.1016/S0140-6736(11)60178-5 (2012).
Stevens, P. E. & Levin, A. & Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group, M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158, 825–830, https://doi.org/10.7326/0003-4819-158-11-201306040-00007 (2013).
Acknowledgements
This study was supported by grants from the Health Promotion Administration, Ministry of Health and Welfare, Institute for Biotechnology and Medicine Industry, Taiwan, ROC (MOHW104-HPA- H-114-134101).
Author information
Authors and Affiliations
Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Senyeong Kao and Cai-Mei Zheng conceived and designed the experiments; Cai-Mei Zheng, Hsin-Ting Lin, Jing-Quan Zheng, Nain-Feng Chu, Yu-Me Lin, Sui-Lung Su, Kuo-Cheng Lu, Jin-Shuen Chen, Fung-Chang Sung, Chien-Te Lee, Yu Yang, Shang-Jyh Hwang, Ming-Cheng Wang, Yung-Ho Hsu and Hung-Yi Chiou performed the experiments; Tian-Jong Chang, Mei-Yi Wu, Tzu-Ting Chen, Yun-Chun Wu and Yi-Lien Wu analyzed the data; Yuh-Feng Lin contributed reagents/materials/analysis tools and coordinate experiment performance; Tian-Jong Chang wrote the paper”. Authorship must be limited to those who have contributed substantially to the work reported.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chang, TJ., Zheng, CM., Wu, MY. et al. Relationship between body mass index and renal function deterioration among the Taiwanese chronic kidney disease population. Sci Rep 8, 6908 (2018). https://doi.org/10.1038/s41598-018-24757-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24757-6
This article is cited by
-
Long-term ozone exposure and mortality in patients with chronic kidney disease: a large cohort study
BMC Nephrology (2024)
-
Association between weight-adjusted-waist index and chronic kidney disease: a cross-sectional study
BMC Nephrology (2023)
-
Lipid accumulation product, visceral adiposity index and risk of chronic kidney disease
BMC Nephrology (2022)
-
Association between metabolic syndrome components and chronic kidney disease among 37,533 old Chinese individuals
International Urology and Nephrology (2022)
-
Population Pharmacokinetic Analysis and Dosing Optimization Based on Unbound Daptomycin Concentration and Cystatin C in Nonobese Elderly Patients with Hypoalbuminemia and Chronic Kidney Disease
Pharmaceutical Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.