Abstract
Wind-mediated transport is an important mechanism in the dispersal of small metazoans. Yet, concrete dispersal rates have hardly been examined. Here we present the results of an one-year field experiment investigating the composition and dispersal rates of aeroplankton. To gain insights into the dynamics of dispersal at the species level, we focused on nematodes, worldwide the most common metazoan taxon. Among the six taxa collected in this study (nematodes, rotifers, collembolans, tardigrades, mites, and thrips), nematodes had the highest dispersal rates (up to >3000 individuals m−2 in 4 weeks, 27 species identified) and represented >44% of aeroplankton. Only living nematodes, and no propagules, were dispersed. All taxa had a higher dispersal potential in environments linked to the source habitat, evidenced by the much higher deposition of organisms in funnels placed on the ground than on the rooftop of a ten-story building. Nematodes under conditions of high humidity and wind speed had the highest dispersal rates, while increasing temperatures and dryness had a significantly positive impact on the wind drift of mites and thrips. The results indicated that wind dispersal over long distances is possible. The notable organismal input by wind dispersal may contribute to biodiversity and ecosystem functions.
Similar content being viewed by others
Introduction
Dispersal is a vital component of an organism’s life-history1, and the potential for dispersal determines the distribution, abundance, and thus, the community dynamics of species at different sites2,3,4. A new habitat must first be reached before filters such as organismal abilities and adaptations, the quality of a habitat, and the established biocoenosis determine the colonization efficiency of a species5. While larger animals can cover distances on their own and actively seek suitable habitats, small (<2 mm) organisms are often passively dispersed5, resulting in their more ubiquitous occurrence6. While active dispersal accounts for rather predictable distribution patterns, passive dispersal leads to a more randomized immigration of organisms2. Mechanisms for passive dispersal are the transport on (epizoochory) or in (endozoochory) larger animals (e.g., flying insects, birds, or mammals) and the erosion by wind5.
Often cited as important requirement for effective wind dispersal is the presence of propagules (e.g., resting eggs, cysts, ephippia, juvenile and adult resting stages)5,7,8, which also enables organisms to survive unfavorable environmental conditions until they enter a suitable habitat. These dispersal units can be blown from surfaces such as soil, moss, and the desiccated sediments of temporal waters. The passively dispersed organisms are typically pioneer colonizers9,10,11. However, wind-drifted species vary in their vagility (probability to be transported with the wind)12, with the weight and form of the propagules, and therefore, the wind speed required for their transport13, determining the dispersal distance. For example, in nematodes resting eggs are less effectively transported by wind than other life stages14, while organisms in anhydrobiosis are lighter and thus more readily transported than hydrated forms15,16. Because different organisms are, for the most part, not dispersed over the same distances, source habitats are also important, with the number of organisms contained in air declining with increasing distance from the source system9,17. The distances covered by small metazoans range from a few meters17, to several hundred meters9, and up to several kilometers14. While the wind dispersal of aquatic organisms is possible even during the wet phase of a transiently aquatic habitat5, during the dry stages a larger number of dormant propagules are exposed to wind and thus dispersed8,17,18. Freshwater organisms that must “cross the dry ocean”5 to enter new aquatic island systems will be passively dispersed more successfully than terrestrial taxa5. However, numerous taxa from both soil and freshwater systems have been captured from the air (e.g., bacteria, several algae, ciliates, flagellates, rotifers, crustaceans, mites, and tardigrades)9,17,18,19. While these have been qualitatively well studied, accurate estimates of their dispersal rates are lacking.
Few investigations of the aeroplankton specifically mention nematodes8,9,17, the most common metazoan taxon and an essential trophic link between unicellular organisms (e.g., bacteria) and larger organisms (e.g., tardigrades, copepods, flatworms, and fishes)20,21,22,23. For nematodes, anhydrobiosis is a widespread strategy allowing them to survive unfavorable conditions for months and even years24,25,26. Accordingly, nematodes can be readily dispersed by wind. However, as reported by Vanschoenwinkel et al.17, nematodes account for only ~1% of the wind-drifted metazoans. Among the habitats colonized by nematodes are those that are strongly exposed to wind erosion as e.g., littorals of permanent waters, soils, mosses, dead wood, and tree bark27,28,29,30. In addition, temporal waters, such as phytotelmata, were shown to be colonized by numerous nematode species already within a few days11,31.
The main goals of our study were to investigate the dispersal potential of small metazoan taxa (<2 mm), especially nematodes, their dispersal rate, and the impact of meteorological parameters on wind dispersal. These questions were addressed in an intensive one-year experimental field study documenting the seasonality of wind dispersal at two selected locations. A suitable experimental design for the determination of dispersal potential is the use of new, artificial habitats (e.g., artificial water bodies) that can be newly colonized5. Because of priority effects and other biotic and abiotic interactions, organisms entering a habitat can easily disappear5. Thus, for an effective measurement of the dispersal rate, incoming organisms should be captured directly from the air, before they are disrupted by local regulators9,17. For this reason, we conducted our study using both vessels filled with formaldehyde to collect wind-drifted organisms and vessels filled with water to document the viability of nematode propagules.
We expected that metazoan taxa, and especially nematodes, due to their frequent occurrence in wind affected habitats, are an important component of the aeroplankton (hypothesis H1). We also predicted that more organisms will be transported by wind at a location with close distance to potential immigration sources than at a location where the distance from those source habitats is larger (hypothesis H2.1) and that, for nematodes, a higher number of species would occur in this location with higher proximity to immigration sources (hypothesis H2.2). A further hypothesis (H3) was that the rate and extent of wind dispersal is affected by meteorological factors; thus, because drought is a crucial factor for wind dispersal, low humidity and high wind speeds will support organismal drift.
Focusing on nematodes, we documented their species composition, size classes, and sex distribution, anticipating that mainly nematodes with a short body length (<0.75 mm) would be able to reach habitats with less proximity to source habitats (hypothesis H4). Finally, as already shown for several other taxa, we predicted that (H5) nematodes are dispersed mainly as propagules (anhydrobiotic stages).
Material and Methods
Experimental setup
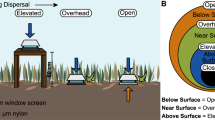
Plastic funnels with an opening of 20 cm in diameter were used to collect wind-dispersed organisms. Based on our initial hypotheses, two different treatments were implemented. For the first, the tube of each funnel was plugged at the bottom and the funnels then filled with 60 ml of 37% formaldehyde (Fig. 1). The funnels were also marked to indicate the liquid level when the formaldehyde was diluted to 4% by rain. For the second, the funnel tubes were plugged at the top and the funnels then filled with 60 g of sand (0.4–1.4 mm grain size; previously autoclaved at 121 °C for 20 min) and 500 ml of tap water to create a refuge for incoming aquatic organisms. Based on the results of preliminary investigation (unpublished data) we could exclude the presents of metazoan within the water.
Experimental setup of the two investigated treatments. One set of funnels was filled with formaldehyde and sampled every 2 weeks. A second set of funnels was filled with water and sediment and sampled and refilled after 4 weeks. Both the formaldehyde- and water-filled funnels were placed in a natural environment (meadow) and on the rooftop of a building at Bielefeld University.
One experimental setup (ground) was placed on a meadow at a distance of 8 m from a stand of trees (Acer platanoides, Carpinus betulus, Fagus sylvatica, and Quercus robur) located at Bielefeld University (Germany). The stand is surrounded by several ponds and natural vegetation units (e.g., hedges and old trees). The second experimental setup (roof) was placed on the roof of a Bielefeld University building (10th floor, 35 m above the ground). The roof is covered by a thin layer of stones (4–9 cm) to hinder the accumulation of organic particles and plants. The two study locations were located 200 m apart. The funnels of the two treatments (water and formaldehyde) were separated at a distance of 1–3 m from each other, with replicates (n = 3 per treatment) separated by 5–8 cm. The upper opening of the funnels was located 40 cm above the ground.
Sampling
The duration of the field trial was 56 weeks, beginning in April 2016 (Fig. 1). The formaldehyde-filled funnels were emptied every 2 weeks, and the water-filled funnels every 4 weeks. However, after strong precipitation, when the formaldehyde was diluted down to a concentration of <4%, the funnels filled with formaldehyde were emptied between sampling dates. In this case, the samplings of a 2-week interval were pooled. Conversely, after a long period of desiccation, the formaldehyde was diluted with water. In the water treatments, an overflow was not prevented, but water was added when the water in the funnels had nearly desiccated. This was necessary to enable the survival of the organisms and to avoid cross contamination between neighbored funnels. Especially the shape of the funnels makes the drift of organisms between the funnels of the same location unlikely.
At the sampling dates, the funnels of both treatments were thoroughly rinsed with water and the respective suspensions sieved through 5-µm meshes to retain the accumulated organisms. To prepare the emptied funnels for the next interval, new formaldehyde or a sediment-water mixture was added.
In addition, to qualitatively assess the nematodes that had colonized the roof of the university building, stones, organic material and plants were collected every 2 weeks (control).
All samples were fixed in 37% formaldehyde (final concentration >4%) and stained with Rose Bengal.
Counting and classification of organisms
The abundances of the dispersed metazoans were evaluated using a LEICA L2 stereomicroscope (40× magnification). The nematodes were assigned to specific size classes (<0.25 mm, 0.25–<0.5 mm, 0.5–<0.75 mm, 0.75–<1 mm, 1–<1.25 mm, 1.25–1.5 mm). Each sample contained a maximum of 50 nematodes, prepared as described by Seinhorst32. The nematodes were identified to the species level (Leica Dialux microscopy observations, 1250× magnification). The dispersal rates [individuals (Ind.) m−2 accumulated in 4 weeks] were calculated based on the results of the formaldehyde treatments (extrapolated from an area of 314.2 cm2).
Meteorological impact
Meteorological data were obtained from a meteorological station located in Bielefeld-Deppendorf (Germany). The relevant parameters were: mean temperature (°C), mean wind speed (km h−1), mean precipitation (mm−1), and mean humidity (%).
A generalized linear model (GLM) was applied to test whether the number of counted taxa in the formaldehyde funnels (all replicates per sampling date and the counts from the roof and ground were pooled) was influenced by these parameters. In this model, all predictors were treated as continuous variables. The GLM was based on a negative binomial distribution, as is typically done for count data33.
Since the ratio of the sample size and number of estimated parameters included in the models was <40, a second-order Akaike information criterion (AICc) was used to evaluate the models34. A forward-selection procedure using likelihood ratio tests for nested models was performed to determine the significances of the individual predictors in the model identified using χ2 tests35.
The GLMs were calculated using the ‘glm.nb’ function of the ‘MASS’ package36 in the R environment37.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Results
Collected organisms
In the formaldehyde treatments, 617 organisms belonging to six wind-dispersed taxa [nematodes, rotifers (mainly Bdelloidea and single Lecane species), tardigrades, mites, collembolans and thrips] were present in the funnels on the ground and 136 in those on the roof (Table 1). Numerous adult insects, especially dipterans and hymenopterans, were also detected but were not included in the aeroplankton. All counted organisms were tainted by rose Bengal, and therefore are contamination by metazoan during the counting process could be excluded. Nematodes and thrips made up the major part of the wind-drifted organisms (44.7% and 31.3% on the ground; 46.3% and 18.7% on the roof, respectively). Nematodes were detected in samples from 92.9% of the sampling dates, followed by mites and thrips (each 64.3%) (Fig. 2). Based on the formaldehyde treatments and a 4-weekly input, the highest dispersal rates at both sampling locations were calculated for nematodes: 3021 Ind. m−2 in 4 weeks on the ground; 445 Ind. m−2 in 4 weeks on the roof (Table 1).
Dispersal rates (Ind. m−2 in 4 weeks) of the wind-drifted taxa (nematodes, rotifers, tardigrades, mites, collembolans, and thrips), collected on the ground (filled circles) and on the roof (blank circles). Each data point represents the summed organism number of three replicates subsequently extrapolated to 1 m2.
The highest nematode dispersal rates in the formaldehyde treatments were measured on the ground between December 2016 and March 2017 (76% of the collected nematodes) and on the roof between May 2016 and November 2016 (87.2%) (Fig. 2). The tardigrades showed a similar seasonal progression but lower dispersal rates (up to 636 m−2 in 4 weeks). For the collected arthropods (mites, collembolans, and thrips) the highest dispersal rates also were measured between May 2016 and November 2016, and at both sampling locations. For rotifers, there was no clear trend. In July 2016, the nematode dispersal rates on the ground were equal to those on the roof but in May and September 2016 they were lower. At the other sampling dates, the dispersal rates on the roof were between 12.5% and 100% lower. All sampled nematodes were living individuals, with no indications of propagules or anhydrobiotic stages.
Nematode composition
Due to losses during the fixation process and to damaged individuals, of the 674 nematodes collected in the funnels only 594 could be classified, with 27 species thus identified (Table 2, Fig. 3). In the controls, collected from stones, organic material, and plants on the roof, 108 individuals were identified. More species were found at the natural location (17 in formaldehyde; 17 in water) than on the roof (13 in formaldehyde; 7 in water; 6 in the controls). Chiloplectus andrassyi was the most common species in all funnel treatments, accounting for 18.0% of the formaldehyde and 59.3% of the water funnels on the roof and for 52.1% of the formaldehyde and 49.8% of the water funnels on the ground (Fig. 3). Overall, the ten most frequent species made up 74.1–94.4% of the identified nematodes per treatment. The other taxa were mostly single finds, with the exception of an unknown parasite that occurred only on the roof; it represented 22.2% of the nematodes in the water treatment and 1.6% of those in the formaldehyde treatment. Three species (C. andrassyi, Plectus sp. and Plectus magadoni) identified on the roof were also collected from stones, organic material, and plants on the rooftop. Plectus parvus, Mesodorylaimus sp. Eudorylaimus sp. and Cephalobus sp. were collected only from the water treatments.
Percentage composition of nematode species from funnels filled with formaldehyde or water placed within a natural environment or on the roof of Bielefeld University. The number of identified individuals is also shown. The letters after the species names indicate their natural occurrence: terrestrial (T), semiaquatic (S), limnic (L). Underlined taxa are those that were also found between stones, organic material, and plants on the roof. The nematode taxa are sorted by frequency.
The largest nematode had a body length of 1.5 mm and was collected from a formaldehyde funnel placed in the natural environment. In general, 63.6% of the nematodes from the ground and 87.7% of those from the roof were <1 mm in size (Table 2).
In all treatments, the share of juvenile nematodes was the largest, comprising at least 56.1% of the collected nematodes (Table 2). Among the samples collected on the roof juveniles made up the largest share (70.4% in water and 73.8% in formaldehyde) whereas at the natural location their share was lower (61.2% in water and 56.1% in formaldehyde). Males (<6.6%) as well as gravid females (<4.6%) were underrepresented in all treatments and were only partly found in samples from the roof.
Meteorological impact
GLMs were calculated only for nematodes, mites, and thrips as the other taxa (rotifers, tardigrades and collembola) occurred only occasionally in the formaldehyde funnels such that statistical analysis of their abundance data was not possible. The GLMs that, according to the lowest AICc value and likelihood ratio tests, best fitted the count data of nematodes, mites, and thrips are summarized in Table 3 (for model selection see Table S1). In the interpretations of the estimated contrasts, a logarithmic link function was used; thus, an additive change in the predictor had multiple effects on the response. The model revealed that the number of nematodes in the funnels increased significantly with increasing relative humidity. It also predicted a significantly higher input of nematodes at high wind speeds, whereas this predictor was a less reliable estimate for nematode numbers because of the standard errors (Table 3). The model that predicted the abundance of mites indicated that increasing numbers were significantly related to higher temperatures. The number of thrips in the samples decreased significantly with increasing humidity.
Discussion
Our study is one of the first to provide clear information on the number of immigrating organisms caused by wind dispersal. The very large dispersion of nematodes in the complete absence of propagules, including anhydrobiotic stages, was unexpected.
Components of the aeroplankton
The results indicated that in Central Europe several metazoan taxa are transported by wind and that their dispersal is subject to a strong seasonal dynamic. Of the six groups (mites, tardigrades, collembolans, nematodes, rotifers, and thrips) examined in this study of aeroplankton, nematodes dominated, with dispersal rates as high as 3021 nematodes m−2 in 4 weeks. This value reflected the high abundances of nematodes in other habitats and the strong likelihood of wind dispersal, in line with hypothesis H1.
For organisms of all six taxa, the mean dispersal rates were higher on the ground, i.e., closer to potential source habitats, than on the roof, where dispersed organisms were deposited in funnels placed 35 m above ground. This result clearly confirmed our second hypothesis (H2.1), demonstrating both a dispersal limitation and sorting processes for wind-drifted taxa, as shown by Caceres and Soluk10 and by Jenkins12. It also demonstrated more efficient local wind dispersal within environments linked to several habitat types. The exception was thrips, whose input of individuals was almost as high on the roof as on the ground (Fig. 2), suggesting the higher potential of these organisms for long-distance transport. As this group was the only one of the six taxa that, with its wings, was at least partly able to actively reinforce dispersal38, this was a plausible result. However, also for the other groups, regional transport is to some extent possible, as shown by the organisms that over a period of 4 weeks entered the funnels on the roof with a dispersal rate from 2 (tardigrades) to 125 (nematodes) Ind. m−2.
The amount and type of wind-dispersed organisms collected in our experiment were substantially different from those reported in other experiments: In a South African study, 950 copepods, 203 cladoceres, and 45 ostracodes were captured within 1 month by windsocks with a surface area of 1 m2 17 whereas these groups were not present in any of our funnels. In that same study, 18 mites m−2 were detected, which is only a fifth to a tenth (roof and ground, respectively) of the dispersal rates of this group in our study. The dispersal rates of tardigrades and rotifers collected from Antarctica8 were also consistently lower (<100 Ind. m−2 per year) than those measured in the present work for nematodes (>10,000 Ind. m−2 per year), tardigrades (540 Ind. m−2 per year), and rotifers (480 Ind. m−2 per year) on the ground.
In contrast to our high nematode dispersal rates, those in previous studies were up to 600× lower: Thus, Nkem et al.8 detected only single nematodes (<5) m−2 in Antarctica within one year. Vanschoenwinkel et al.17 caught aeroplankton by windsocks placed for 1 month on an isolated mountain top in South Africa. Only propagules >100 µm were considered for this study. Based on the data, approximately 14 nematodes were sampled per m2. A longer testing period presumably would have led to higher values.
However, because of the substantially different environments (Antarctica, South Africa), a direct comparison of the results of this and previous studies is difficult.
Moreover, the different sampling methods used may have significantly affected the number of collected organisms8. In the study of Vanschoenwinkel et al.39, the composition of aeroplankton captured at the same site and time by windsocks vs. sticky traps strongly varied. It is also likely that most of the other studies considered the incoming organisms after they had interacted with the new habitat, whereas in our formaldehyde-filled funnels all organisms died immediately after their arrival. These two different approaches lead to different measures of dispersal rates. However, we are confident that our method realistically reflects the input of wind-dispersed organisms in a given habitat within the temperate zone.
Nematode dispersal was also considered strictly on a species level. Twenty-seven nematode species entered the experimental vessels, 21 in the natural environment and 15 on the roof, in accordance with H2.2, which predicted higher species richness in funnels with higher proximity to source habitats. Only three of the ten most frequent species on the roof were also found in the samples from the rooftop (controls); thus, the remaining two species most certainly drifted onto the roof by wind, implying their long-distance transport, as described by Carroll and Viglierchio14.
Previous studies reported three wind-drifted nematode species from Antarctica8, at least 14 species from Senegal40, and 10 genera from California41. At the species level none of the species in this study overlapped with any of those from other locations. However, many near-relative taxa are dispersed by wind, including those of the most common species of the present study. Rhabditida (in our study: Panagrolaimus rigidus, Panagrolaimus sp., Rhabditis sp., Diploscapter coronatus, Eucephalobus sp., Panagrellus sp., and Cephalobus sp.) and Dorylaimida (in our study: Mesodarylaimus sp., Eudorylaimus sp., and Dorylaimus stagnalis) are also frequently found in Senegal, California, and the Antarctic. Members of the genus Aphelechoides (in our study: Aphelenchoides helophilus, Aphelenchoides parietinus, Aphelenchoides sp., Aphelenchoides bicaudatus) were among the aeroplankton reported from California, while members of the Plectina (in our study: Chiloplectus andrassyi, Plectus sp., Plectus magadani, Plectus parvus, Anaplectus sp., and Plectus cirratus) were collected from the Antarctic. Thus, worldwide, certain taxonomic groups seem to be typically dispersed by wind.
The presence of one parasitic species indicated that nematodes are transported by larger organisms. This species was always collected from funnels containing (drowned) adult dipterans. However, we found no correlation between the numbers of insects and the parasite. Indeed, in the natural environment nematodes reached their highest number in winter, when no insects were collected from the funnels.
Dorylaimus stagnalis was the only species whose distribution is restricted to freshwater habitats. All of the other nematodes identified to the species level are mainly terrestrial28,29,30, but some are also found in semiaquatic or aquatic environments. Specifically, the main sources of the collected species were forest soils, leave litter, decomposed organic matter, moss, tree bark, plants, and phytotelmata11,27,31,42. Vanschoenwinkel et al.18 pointed out that the dispersal rates of aquatic invertebrates already drop off at 10–20 m distance to the nearest source pond, which might explain why so few freshwater species were collected in our study. Interestingly, other aquatic invertebrates, including ostracodes, copepods, and cladoceres were also not captured in the funnels (see above).
Also, molecular studies found evidence that dispersal and therefore the gene flow between populations may be more limited than originally thought. For example, Ristau et al.43 found genetic differentiation of a freshwater nematode species already in neighboring lakes. Also, the study of Suatoni et al.44 indicated dispersal limitations resulting in the presence of cryptic species of a rotifer species which was previously thought to have a broad geographic distribution and therefore high passive dispersal rates.
Influence of meteorological factors
For the three tested groups, nematodes, mites and thrips, different meteorological factors were evaluated for their significance as predictors of organismal dispersal rates within the model selection process. While, in general, meteorological parameters were significantly related to dispersal, hypothesis 3 (H3) was not confirmed, as neither dry conditions nor wind speed generally supported the input of all taxa. For mites the highest rate of dispersal occurred in summer (May–October), indicating the significant influenced of temperature on this process. A previous study showed that the density of mites in soil is strongly temperature restricted45. In another46, mites were present in abundance in the upper layers (0–2.5 cm) of soil ecosystems during the summer but moved deeper into the soil in winter. Thus, in summer more individuals would be vulnerable to wind erosion. Similarly, the dispersal of thrips was also restricted to the warmer months (May–October), consistent with their active phase occurring solely during the warmer months47. Although the model selection process identified humidity as the more crucial factor, temperature and humidity are inversely correlated (Pearson’s correlation: r = −0.56, p = 0.002) such that their individual influences are difficult to distinguish. However, in contrast to the other two groups, the dispersal rates of nematodes were highest in winter (December–March). Our study showed the importance of wind speed for nematode transport and supports the observation that only when the wind energy exceeds a specific threshold organisms can be lifted from the ground14. Accordingly, at higher wind speeds more nematodes were deposited in the funnels. Wind speed also regulates the distance of the transport.
Our results also identified moisture as a crucial factor in the wind dispersal of nematodes, as the highest dispersal rates were measured during periods of high humidity. Although it is widely assumed that small organisms are dispersed during dry periods5, most of the organisms collected in this study were those adapted to terrestrial environments, including soils. In forest soils, nematode abundances are highest during periods of high moisture48, which implies an increased potential for the erosion of these organisms.
Dispersal stages of nematodes
In terms of the sexual distribution, our results did not differ from those of previous studies of nematode communities in soil and freshwater habitats. However, there was a slight shift towards more juveniles31,49,50, mostly those with a body length <0.75 mm. As reported by Nkem et al.8 and by Carroll and Vigliechio14, especially juvenile nematodes can be drifted over long distances. Consistent with this finding, in our study there was a higher ratio of small juvenile nematodes on the roof than on the ground. Their longer transport distance lent support for H4.
Contradicting H5, however, was the observation that all nematodes collected from the formaldehyde treatments were dispersed in an active (living) condition, and only four species (Plectus parvus, Mesodorylaimus sp., Eudorylaimus sp. and Cephalobus sp.) were present solely in the water-filled funnels. These may have entered the funnels as propagules and subsequently developed into an active life stage. However, there were few such individuals (<1.5% of the total nematodes) and all other nematode species were found in both treatments, indicating the viability of incoming individuals.
For rotifers and tardigrades in this study, the input of propagules cannot be excluded. Like nematodes, these two groups of organisms can survive frost, heat, and desiccation for months and even years by adopting a state of anhydrobiosis24,26 (illustrated by40,49). Nematode genera able to outlast unfavorable environmental conditions by anhydrobiosis include those also detected in our study: Aphelenchoides, Panagrellus, Plectus, and Tylechus24,26,51. The factors that are crucial for a successful transition to anhydrobiosis and a subsequent re-emergence are temperature and moisture15,51, with the chances of survival increasing under conditions of slowly rising temperatures, high humidity, and therefore slow drying. Among these factors, our investigation showed that humidity is crucial for nematode dispersal. Previous studies identifying nematodes as part of the aeroplankton were mainly conducted in extreme habitats with harsh living conditions. Nkem et al.8 sampled wind-dispersed nematodes from Antarctica and found mainly “inactive” individuals. Baujard and Martiny40 as well as Vanschoenwinkel et al.17,18 documented similar forms in Africa during the dry season. By contrast, in their study in California under less harsh conditions, Vigliercho and Schmitt41 found living individuals. Thus, in temperate and more humid environments anhydrobiosis may not be necessary for wind dispersal, a conclusion supported by our investigation.
Conclusion
Our results well demonstrated the wind dispersal of several small metazoans and that their dispersal rates were related to seasonal or/and meteorological influences. A relationship between proximity to the source habitat (forest) and the number of drifted individuals and species was also determined. Based on the organisms deposited in funnels placed on the roof of a ten-story building, we concluded, that even transport on a larger scale is possible.
The wind dispersal of many organisms has been underestimated; this is especially true for nematodes, an ecologically essential group. In temperate zones, the annual immigration of nematodes of numerous species may exceed 10,000 per m2, which is much higher than measured for other wind-drifted taxa. Contrary to common assumptions, we demonstrated that neither propagules nor dry environmental conditions are a requirement for nematode dispersal by wind. Moreover, as the incoming nematodes were viable, the wind dispersal of nematodes enables gene flow between populations in very remote habitats and increases both the diversity and the stability of nematode populations. The colonization efficiency of nematodes with respect to other factors, such as landscape fragmentation, was beyond the scope of our study and should be the focus of further investigations.
References
Bonte, D. & Dahirel, M. Dispersal: a central and independent trait in life history. Oikos. 126, 472–479 (2017).
Rundle, S. D., Robertson, A. L. & Schmidt-Araya, J. M. Freshwater meiofauna: Biology and ecology. (Backhuys Publishers, 2002).
Kneitel, J. & Miller, T. E. Dispersal rates affect species composition in metacommunities of Sarracenia purpurea inquilines. Am. Naturalist. 162, 165–171 (2003).
Cottenie, K. Integrating environmental and spatial processes in ecological community dynamics. Ecology Lett. 8, 1175–1182 (2005).
Incagnone, G., Marrone, F. Barone R. Robba, L. & Naselli-Flores, L. How do freshwater organisms cross the “dry ocean”? A review on passive dispersal and colonization processes with a special focus on temporary ponds. Hydrobiologia. 750, 103–123 (2015).
Finlay, B. J. Global dispersal of free-living microbial eukaryote species. Science. 296, 1061–1063 (2002).
Panov, V. E., Krylov, P. I. & Riccardi, N. Role of diapause in dispersal and invasion success by aquatic invertebrates. J. Limnol. 63, 56–69 (2004).
Nkem, J. N. et al. Wind dispersal of soil invertebrates in the McMurdo Dry Valleys, Antarctica. Polar Biol. 29, 346–352 (2006).
Maguire, B. Jr. The passive dispersal of small aquatic organisms and their colonization of isolated bodies of water. Ecol. Monogr. 33, 161–185 (1963).
Cáceres, C. E. & Soluk, D. A. Blowing in the wind: A field test of overland dispersal and colonization by aquatic invertebrates. Oecologia. 131, 402–408 (2002).
Ptatscheck, C. & Traunspurger, W. The meiofauna of artificial water-filled tree holes: Colonization and bottom-up effects. Aquatic Ecol. 48, 285–295 (2014).
Jenkins, D. G. Dispersal-limited zooplankton distribution and community composition in new ponds. Hydrobiologia 313/314, 15–20 (1995).
Parekh, P. A., Paetkau, M. J. & Gosselin, A. Historical frequency of wind dispersal events and role of topography in the dispersal of anostracan cysts in a semi-arid environment. Hydrobiologia. 740, 51–59 (2014).
Carroll, J. J. & Viglierchio, D. R. On the transport of nematodes by the wind. Journal Nematol. 13, 476–483 (1981).
Van Gundy, S. D. Factors in survival of nematodes. Annu. Rev. Phytopathol. (1965).
Ricci, C. & Caprioli, M. Anhydrobiosis in bdelloid species, populations and individuals. Integr. Comp. Biol. 45, 759–763 (2005).
Vanschoenwinkel, B., Gielen, S., Seaman, M. & Brendonck, L. Any way the wind blows - frequent wind dispersal drives species sorting in ephemeral aquatic communities. Oikos. 117, 125–134 (2008).
Vanschoenwinkel, B., Gielen, S., Vandewaerde, H., Seaman, M. & Brendonck, L. Relative importance of different dispersal vectors for small aquatic invertebrates in a rock pool metacommunity. Ecography 31, 567–577 (2008).
Frankland, P. F. The distribution of micro-organisms in air. Proc. R. Soc. L. 40, 509–526 (1986).
Traunspurger, W., Bergtold, M. & Goedkoop, W. The effects of nematodes on bacterial activity and abundance in a freshwater sediment. Oecologia. 112, 118–122 (1997).
Beier, S., Bolley, M. & Traunspurger, W. Predator-prey interactions between Dugesia gonocephala and free-living nematodes. Freshwater Biol. 49, 77–86 (2004).
Hohberg, K. & Traunspurger, W. Predator-prey interaction in soil food web: Functional response, size-dependent foraging efficiency, and the influence of soil texture. Biol. Fertil. Soils. 41, 419–427 (2005).
Muschiol, D., Marković, M., Threis, I. & Traunspurger, W. Predator-prey relationship between the cyclopoid copepod Diacyclops bicuspidatus and a free-living bacterivorous nematode. Nematology. 10, 55–62 (2008).
Simons, W. R. Nematode survival in relation to soil moisture. Mededelingen Landbouwhogeschool Wageningen. 73, 1–85 (1973).
Hendriksen, N. B. Anhydrobiosis in nematodes: studies on Plectus sp. In New trends in soil biology (eds Lebrun, P. André, H. M., De Medts, A., Grégoire-Wibo, C. Wauthy, G. editors) 387–394 (Louvain-la-Neurve, Belgium, Dieu-Brichart 1982).
Watanabe, M. Anhydrobiosis in invertebrates. Appl. Entomol. Zool. 41, 15–31 (2006).
Pschorn-Walcher, H. & Gunhold, P. Zur Kenntnis der Tiergemeinschaften in Moos und Flechtenrasen an Park- und Waldbäumen. Z. Morphol. Ökol Tiere. 46, 342–354 (1957).
Andrássy, I. Free-living nematodes of Hungary I (Nematoda errantia). Pedozoologica Hungarica No. 3 (Budapest, Hungary, Hungarian Natural History Museum and Systematic Zoology Research Group of the Hungarian Academy of Sciences 2005).
Andrássy, I. Free-living nematodes of Hungary II (Nematoda errantia). Pedozoologica Hungarica No. 4 (Budapest, Hungary, Hungarian Natural History Museum and Systematic Zoology Research Group of the Hungarian Academy of Sciences 2007).
Andrássy, I. Free-living nematodes of Hungary III (Nematoda errantia). Pedozoologica Hungarica No. 5 (Budapest, Hungary, Hungarian Natural History Museum and Systematic Zoology Research Group of the Hungarian Academy of Sciences 2009).
Ptatscheck, C., Dümmer, B. & Traunspurger, W. Nematode colonisation of artificial water-filled tree holes. Nematology. 17, 911–921 (2015).
Seinhorst, J. W. On the killing, fixation and transferring to glycerine of nematodes. Nematologica 8, 195–202 (1962).
Greene, W. Functional forms for the negative binomial model for count data. Econ. Lett. 99, 585–590 (2008).
Burnham, K. P. & Anderson, D. R. Model selection and multimodel inference. A practical information-theoretic approach (Springer 2010).
McCulloch, C. E., Searle, S. R. & Neuhaus, J. M. Generalized, linear, and mixed models (John Wiley and Sons 2008).
Venables, W. N. & Ripley, B. D. Modern applied statistics with S. Fourth Edition (Springer, 2002).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2016).
Tipping, C. Thrips (Thysanoptera) in Encyclopedia of Entomology (ed. Capinera, J. L.) (Kluwer Academic Publishers 2004).
Vanschoenwinkel, B. et al. Community structure in temporary freshwater pools: Disentangling the effects of habitat size and hydroregime. Freshwater Biol. 54, 1487–1500 (2009).
Baujard, P. & Martiny, B. Transport of nematodes by wind in the peanut cropping area of Senegal, West Africa. Fundam. Appl. Nematol. 17, 543–550 (1994).
Vigliercho, D. R. & Schmitt, R. V. Background atmospheric burden of nematodes in California’s Sacramento Valley. Nematol. Mediterr. 9, 111–116 (1981).
Schenk, J., Traunspurger, W. & Ristau, K. Genetic diversity of widespread moss-dwelling nematode species in German beech forests. Eur. J. Soil Biol. 74, 23–31 (2016).
Ristau, K., Steinfartz, S. & Traunspurger, W. First evidence of cryptic species diversity and significant population structure in a widespread freshwater nematode morphospecies (Tobrilus gracilis). Mol. Ecol. 22, 4562–4575 (2013).
Suatoni, E., Vicario, S., Rice, S., Snell, T. & Caccone, A. An analysis of species boundaries and biogeographic patterns in a cryptic species complex: The rotifer. Brachionus plicatilis. Mol. Phylogenet. Evol. 41, 86–98 (2006).
Stamou, G. P. & Sgardelis, S. P. Seasonal distribution patterns of oribatid mites (Acari. Cryptostigmata) in a Forest Ecosystem. J. Anim. Ecol. 58, 893–904 (1989).
Mitchell, M. J. Vertical and Horizontal Distributions of Oribatid Mites (Acari. Cryptostigmata) in an Aspen Woodland Soil. Ecology 6, 655–669 (1978).
Capinera, J. L. Handbook of Vergetable Pests. (Academic Press 2001).
Bassus, W. Über die Vertikalverteilung und den Massenwechsel der Nematoden in Waldböden Mitteldeutschlands. Nematologica. 7, 281–293 (1962).
Yeates, G. W. Contribution of size classes to biovolume, with special reference to nematodes. Soil Biol. Biochem. 20, 771–773 (1988).
Michiels, I. & Traunspurger, W. A three year study of seasonal dynamics of a zoobenthos community in a eutrophic lake. Nematology. 6, 655–669 (2004).
Crowe, J. H. & Madin, K. A. Anhydrobiosis in tardigrades and nematodes. Trans. Am. Microsc. Soc. 93, 513–524 (1974).
Acknowledgements
We thank Stefanie Gehner for preparing the nematodes and Henrike Brüchner-Hüttemann for her helpful remarks. Our study was supported by the German Federal Institute of Hydrology (BfG). We acknowledge support for the article processing charge by the Deutsche Forschungsgemeinschaft and the Open Access Publication Fund of Bielefeld University.
Author information
Authors and Affiliations
Contributions
C.P., B.G. and W.T. conceived the ideas and designed methodology; C.P. and B.G. collected the data; C.P. and B.G. analysed the data; C.P. and B.G. led the writing of the manuscript. C.P., B.G. and W.T. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ptatscheck, C., Gansfort, B. & Traunspurger, W. The extent of wind-mediated dispersal of small metazoans, focusing nematodes. Sci Rep 8, 6814 (2018). https://doi.org/10.1038/s41598-018-24747-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24747-8
This article is cited by
-
Cryophilic Tardigrada have disjunct and bipolar distribution and establish long-term stable, low-density demes
Polar Biology (2023)
-
Wind dispersal differences between rotifer cryptic species: a proof of principle from a wind tunnel experiment
Hydrobiologia (2023)
-
Desiccation risk favours prevalence and diversity of tardigrade communities and influences their trophic structure in alpine ephemeral rock pools
Hydrobiologia (2022)
-
Microinvertebrate Colonization of New Zealand’s Thermally Extreme Environments
Evolutionary Biology (2022)
-
Five animal phyla in glacier ice reveal unprecedented biodiversity in New Zealand's Southern Alps
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.