Abstract
Obese patients could be more susceptible to mechanical ventilation (MV)-induced lung injury than non-obese patients due to weight-dependent changes in lung properties. The aim of this study was therefore to evaluate the pulmonary effects of 2 hours low VT MV in a diet-induced obese mice model, with VT calculated on either the actual body weight (VTaw) or the ideal body weight (VTiw) . First, we hypothesized that a MV with VTaw would be associated with altered lung mechanics and an increased lung inflammation. Second, we hypothesised that a MV with a VTiw would preserve lung mechanics and limit lung inflammation. We analyzed lung mechanics and inflammation using bronchoalveolar lavage (BAL) cell counts, flow cytometry tissue analysis and histology. Lung mechanics and inflammation were comparable in control and obese mice receiving VTiw. By contrast, obese mice receiving VTaw had significantly more alterations in lung mechanics, BAL cellularity and lung influx of monocytes as compared to control mice. Their monocyte expression of Gr1 and CD62L was also increased. Alveolar neutrophil infiltration was significantly increased in all obese mice as compared to controls. In conclusion, our findings suggest that protective MV with a VTaw is deleterious, with a marked alteration in lung mechanics and associated lung inflammation as compared to lean mice. With VTiw, lung mechanics and inflammation were close to that of control mice, except for an increased alveolar infiltrate of polymorphonuclear neutrophils. This inflammation might be attenuated by a blunted recruitment of inflammatory cells associated with obesity.
Similar content being viewed by others
Introduction
Over the last several decades, the worldwide prevalence of obesity has steadily risen, reaching 36% in the United States in 20101. Consequently, the number of obese inpatients requiring mechanical ventilation (MV), either in intensive care units or in the operating room, is increasing.
MV with excessive lung stress and strain may induce pulmonary adverse effects, called “ventilator-induced lung injury (VILI)”, due to barotrauma, volutrauma, atelectotrauma and biotrauma2,3,4, leading to increased mortality in patients with the acute respiratory distress syndrome (ARDS)5. Some evidence has suggested that high tidal volume (VT) may also be deleterious for patients with a healthy lung6,7, even for short periods of ventilation, as during general anesthesia8,9,10. Recent guidelines recommend limiting VT to 6 to 8 mL/kg of ideal body weight (IBW) in patients with ARDS11, which tend to be extended to all critically ill patients12 and a VT < 10 mL/kg of IBW has also been recommended during anesthesia13,14.
Despite the increasing number of ventilated obese patients, no specific study has been published concerning these characteristics of MV. Guidelines advocate the use of the IBW to calculate the VT, but the formulas are unfamiliar to medical doctors and are not fully integrated into current practice. Indeed, it has been recently demonstrated that obesity is a risk factor for being ventilated with a large VT (>10 mL/kg of IBW)15,16, with an increased risk of secondary ARDS in obese ICU patients17.
Moreover, obese patients might be more susceptible to VILI, even when ventilated with a low VT, because of both mechanical and biological characteristics of this condition. Firstly, obese individuals have a lower thoraco-pulmonary compliance because of fatty infiltration of the chest, which induces increased airway pressures, and their lungs may be not uniformly inflated during MV because of greater basal atelectasis18. Secondly, obesity is associated with a baseline low-grade inflammatory status, which might increase vulnerability to biotrauma19,20,21. Thus, low serum adiponectin levels have been shown to promote acute lung injury in obese mice22. It can therefore be hypothesized that obese subjects may be more prone to MV-associated lung injuries than non-obese subjects, with low VT ventilation calculated on actual weight or even calculated on IBW. However, to the best of our knowledge, this hypothesis has not been previously investigated despite the important implications in terms of public health given the continuously growing prevalence of obesity.
The aim of this study was therefore to evaluate the pulmonary effects of low VT MV in a diet-induced obese mice model, with VT calculated on either the actual body weight or the ideal body weight. First, we hypothesized that a MV with a VT calculated on the actual body weight would be associated with altered lung mechanics and an increased lung inflammation. Second, we hypothesized that a MV with a VT calculated on the ideal body weight would preserve lung mechanics and limit lung inflammation as compared to a VT calculated on the actual body weight. Some of our results have been previously reported in abstract form23.
Results
Diet-induced obesity model
After the 12-week feeding period, the weight and BMI of all mice fed a high-fat diet (non-ventilated obese (NVO) + obese mice ventilated with VT based on actual weight (OVTaw) + obese mice ventilated with VT based on ideal weight (OVTiw)) was higher than that of mice fed a low-fat diet (non-ventilated controls (NVC) + ventilated controls (VC)): 41.1 [38.6–45.9]g vs 30.9 [29.5–32;1]g, p < 0.0001. Their body mass index (BMI) was also increased (0.40 [0.38–0.44] vs 0.31[0.30–0.32] g/cm2, p < 0.0001) (Table 1).
Baseline characteristics of obese and control mice
Pulmonary inflammation, assessed by BAL cell counts, histologic score and inflammatory cells in lung tissue quantified by flow cytometry, did not differ between NVO and NVC mice (Table 2). Alveolar permeability, assessed by the BAL protein concentration, was also similar in the two groups (196 [183–228] in NVO mice vs 198 [188–226] µg/mL in NVC mice, p = 0.84). Lung mechanics measured at the beginning of the ventilation period showed that obese (OVTiw) and control (VC group) mice displayed similar compliance (125 [115–131] vs 123 [111–127] µL/cmH2O, p = 0.49). After recruitment maneuvers, obese mive (OVTiw and OVTaw) had higher peak and mean airway pressures than VC mice and OVTaw mice had higher peak and mean airway pressures than OVTiw mice (Fig. 1).
Variations of peak pressure, mean pressure and static compliance during the two hours of ventilation. H0: On connection of the ventilator, H0’: after recruitment maneuver, H1: after one hour of ventilation, H2: after 2 hours of ventilation. a, b and c denote a p value (Mann-Withney pairwise comparisons after Kruskal-Wallis test) <0.05 for the following pairwise comparisons: for VC vs OVTiw, VC vs OVTaw and OVTaw vs OVTiw, respectively. *Denotes p value (Mann-Withney pairwise comparison) <0.05 between H2 and H0’ in each group (n = 19–25 per group).
Lung mechanics during MV
Because the mean weight of VC mice was used to calculate the VT applied in OVTiw mice, the two groups had an identical VT of 0.241 [0.230–0.252] mL, which was significantly lower than the VT received by OVTaw mice (0.331 [0.304–0.376]mL, p < 0.0001). Lung mechanics slightly deteriorated during the 2-hour MV period in the 3 ventilated groups, with a increase in airway pressures and a decrease in respiratory system compliance (Fig. 1). Deterioration of lung mechanics during the MV period was more pronounced in the OVTaw group, with a ΔH2-H0’ higher for peak and mean airway pressures (p = 0.00015 vs VC and OVTiw) and lower for the respiratory system compliance (p = 0.04 vs VC and p = 0.02 vs OVTiw after corrections for multiple testing).
BAL fluid cell counts and protein concentration
Inflammatory cells in BAL fluid were almost exclusively macrophages (Fig. 2). The global cell count and the differential cell count of macrophages did not differ between VC and OVTiw groups. There was a a higher global cell count in the OVTaw group as compared to the VC and NVO groups (p = 0.0287 and 0.0238 after correction for multiple testing, respectively). These cells were macrophages and neutrophils, as macrophages and neutrophils cell counts were higher in the OVTaw group as compared to the NVO group (p = 0.0455 and 0.0434, respectively) and tend to be higher as compared to the VC group (p = 0.0959 and 0.0602 after correction for multiple testing, respectively). The BAL protein concentration did not differ between the three groups (Fig. 2).
Inflammatory cell subpopulations and their activation by flow cytometry in lung tissue
Both obese and control mice exposed to MV displayed higher monocyte and neutrophil counts in lung tissue, compared to non-ventilated mice (VC vs NVC, OVTaw vs NVO and OVTiw vs NVO comparisons). Similar macrophage counts were observed in the ventilated and non-ventilated groups. Within ventilated mice, we observed a higher monocyte count in lung tissue of OVTaw group (but not OVTiw animals) as compared to the VC group. Macrophage and neutrophil counts in lung tissue were not significantly different between the three ventilated groups (OVTaw, OVTiw and VC groups), but neutrophils also tend to be higher in OVTaw as compared to VC (p = 0,0602 after correction for multiple testing) (Fig. 3).
Concerning the degree of activation of the inflammatory cells, no difference in neutrophil CD11b and CD62L expression was observed between the 3 ventilated groups (Fig. 4d,e). In contrast, high monocyte activation was observed in OVTaw mice, as the expression of Gr-1 on monocytes was higher in the OVTaw group than in both the NVO and OVTiw groups. Moreover, the expression of CD62L on monocytes was higher in the OVTaw group than in the NVO group, whereas no difference was observed between the VC and NVC groups on one hand and between the OVTiw and NVO groups on the other hand (Fig. 4a,b). Interestingly, Gr-1 expression on monocytes was less intense in OVTiw mice than in VC mice (Fig. 4a).
Quantification of lung tissue neutrophil infiltration
Ventilated mice displayed higher neutrophil infiltration in the lungs compared to non-ventilated mice (VC vs NVC, OVTaw vs NVO and OVTiw vs NVO) (Fig. 5). This lung injury was particularly marked in obese mice, as the histologic score was higher in both the OVTaw and OVTiw groups compared to the VC group. No significant difference was observed between the OVTaw and OVTiw groups. Figure 5 represents the values of the histologic score and Fig. 6 shows representative sections of alveolar neutrophil infiltration.
Discussion
This study shows that ventilating obese mice with a VT calculated on their actual weight is deleterious, with a broader alteration of ventilatory mechanics, BAL total cell count, monocyte cell count and activation in lung tissue.
When VT was calculated using the actual weight, ventilatory mechanics were considerably impaired compared to ventilated controls, achieving the highest airway pressures and lowest compliance at H2. This cannot be explained a priori by more atelectasis, as these obese mice were subjected to the same level of PEEP. The most likely hypothesis is the presence of interstitial and alveolar edema, thereby inducing alveolar rigidity and decreased compliance, which may correspond to an early manifestation of ventilator-induced lung injury24 in OVTaw mice. Although we did not demonstrate increased alveolar-capillary permeability in this group, it displayed increased BAL total cell count. Analysis on flow cytometry revealed an increase in recruited monocytes as compared to VC, and the number of neutrophils also tends to be higher. Moreover, monocytes of OVTaw displayed more intense L-selectin expression as compared to their non-ventilated counterparts, whereas no difference was observed between VC and OVTiw mice on one hand and VC and NVC on the other hand. Wilson et al.25 have previously shown that aggressive ventilation (35 mL/kg of VT in their model) and, to a lesser extent, protective ventilation, induced an influx of immature Gr-1high monocytes from the bone marrow. These monocytes also overexpressed L-selectin, allowing them to migrate out of the bloodstream into the lung. Similar results were observed in our study, highlighting the harmful effect of a supposed “protective” VT calculated using the actual weight of obese mice. On histologic examination, we also demonstrated increased alveolar inflammation in OVTaw mice, with a higher neutrophil infiltration score compared to VC mice. The lack of significant increase in neutrophil by cytometry could be due to the short duration of the experiment (2 hours) that dit not allow monocytes time to differentiate into neutrophils and/or the lack of infectious challenge.
Obese mice ventilated with a VT calculated using the ideal body weight had lung mechanics and inflammation parameters close to control mice except for a higher alveolar infiltration of neutrophils on histological score, which was similar to OVTaw, and a lower Gr-1 expression on monocyte. The decrease in most inflammatory parameters in the OVTiw group as compared to OVTaw may be ascribed to the reduction in VT with the use of ideal weight, but the higher neutrophilic alveolar infiltration on histological score do not corroborate this hypothesis. An alternative hypothesis, supported by some publications, could be a role of obese phenotype in this decreased monocyte activation, as obese individuals appear to present a degree of immune deficiency, with delayed secretion of cytokines and recruitment of inflammatory cells in response to stimulation by a pathogen26,27, which could explain decreased activation of monocytes in OVTiw mice compared to VC mice, while alveolar infiltration by inflammatory cells was increased. Recent reports have highlighted abnormal diapedesis in obese mice exposed to inhalation of LPS28 or ovalbumin29 with increased influx of neutrophils and eosinophils in blood and lung tissue, but decreased BAL cell counts and cytokines. This phenomenon could also explain our finding of excess neutrophils observed in the alveolar walls, but not in the BAL of OVTiw mice. Overall, our findings may suggest that protective ventilation in obese subjects, even when VT is calculated using the IBW, induces an increased lung inflammation as compared to that observed in lean subjects, but this inflammation could be attenuated by a blunted recruitment of inflammatory cells associated with obesity.
This study presents several limitations. First, we used a short (2 hours) MV. However, a short period (2 to 3 hours) of low VT MV was shown to cause lung inflammation and edema in uninjured mice30, whereas a short period of high VT MV was shown to provide much more lung injury31,32,33. Second, we used a minimal PEEP (1.5 cmH2O). This might be critized since numerous investigations encourage to use higher PEEP in the operating room, especially in obese patients34. However, other teams applied a similar low PEEP (below 2 cmH2O) during short MV in mice32,35,36. Here, with a PEEP settled at 1.5 cmH2O, we observed a slight deterioration of lung mechanics over the MV period. Indeed, the static compliance after 2 hours of MV was closed to that one recorded at time of connexion to the ventilator in both the VC and OVTiw groups. In another work by our group, we showed that the dynamic compliance decreased below the H0 value after 3 hours of mechanical ventilation in uninjured mice37. Therefore, a 1.5 cmH2O PEEP looked suitable in such a low-VT short MV applied to uninjured mice. Third, our model of obesity was induced by diet, with a moderate weight gain, as compared to some genetic models38. This moderate weight gain may have precluded hightlighting meaningful differences between obese and control groups. However, this model is much closer to clinical reality in humans and genetic models like ob/ob and db/db type could not be used in this study, as these animals present early weight gain that induces an abnormal development of the lungs, leading to a twofold lower lung volume than in control mice39. This discrepancy would have induced a major bias in the interpretation of the ventilatory mechanics. Fourth, we used the mean weight of control mice to calculate the ideal weight of obese mice. However, no formula is available to calculate IBW in mice. In addition, the naso-anal length of obese and control mice was similar in our study (data not shown). Fifth, some of our results (BAL cell count and flow cytometry neutrophils comparisons) were no longer statistically significant after correction for multiple testing, due to the low number of animals and the number of study groups. However, the corrected p values were “marginally significant”; overall, our results suggested that, with a short-term ventilation, the conjunction of obesity and a VT calculated on the actual weight induce a significant alveolar inflammation. Finally, we did not use an infectious challenge like lipopolysaccharide, that could mimic the clinical scenario in intensive care where pneumonia is a major cause of mechanical ventilation. This lack of infectious challenge could in part explain the absence of enhanced inflammatory state in diet-induced obese mice at baseline before starting mechanical ventilation, like previously described in this model40. However, other studies have demonstrated the absence of overt lung inflammation in unchallenged obese mice41,42. Further experimental studies are needed to assess the exact impact of mechanical ventilation using the actual vs ideal body weight in terms of recruitment and migration of inflammatory cells (which could be blunted in obese individuals) and when using an infectious challenge like lipopolysaccharide.
Our study has some clinical implications. These data suggest systematically calculating the IWB of obese patients, both in intensive care and in the operative room, even for short periods of ventilation. However, further studies are required to assess the exact impact of protective mechanical ventilation on the IBW of obese subjects, as it appears to induce more marked pulmonary inflammation compared to controls, but the presence of secondary lesions must be determined. A deficiency in the recruitment and migration of inflammatory cells in obese subjects could also play a protective role against lung injury induced by inflammation secondary to mechanical ventilation. At present, clinical studies investigating the morbidity and mortality of ventilated obese subjects, often retrospective and comprising numerous biases, especially disparities between the management of obese and non-obese patients, have reported contradictory results5,43,44,45,46. Acute lung injury or ARDS may also have been over-diagnosed due to poor quality radiographs or undiagnosed atelectasis.
In conclusion, we herein demonstrate, for the first time, the harmful effect of a two hours ventilation strategy with tidal volume of 8 mL/kg calculated on the actual weight in obese mice. When the tidal volume was calculated using the ideal weight, lung mechanics and inflammation were close to that of control mice, except for an increased alveolar infiltrate of neutrophils on histology. Further studies are needed to determine the effect of protective ventilation based on IBW on the morbidity and mortality of these patients.
Material and Methods
Additional details on the methods are provided online (see supplementary information file, SIF).
Animal protocol
Five-week-old male C57/Bl6 mice were purchased from the Janvier laboratory (Le Genest-Saint-Isle, France) and were housed in our “small animal” facility. They received one week of adaptation with their usual food. At the age of 6 weeks, they were divided into two groups. One group (control group) received a diet in which 10% of the calories were derived from fat in the form of lard (D12450B, SSNIFF, Soest, Germany), while the other group (obese group) was fed a diet in which 60% of the calories were derived from fat in the from of lard (D12492, SSNIFF). Both groups were given food and water ad libitum and were exposed to a 12:12 h light-dark cycle. At the age of 18 weeks and after 12 weeks of the diet, animals were weighed and then fasted for 12 hours with free access to water before the experimentation. The body mass index (BMI) was calculated as previously described formula: BMI = weight (g)/(naso-anal length (cm))2 47. Mice were then anesthetized with intraperitoneal pentobarbital (30 µg/g of body weight, Hospira, Meudon La Forêt, France) followed by continuous 1.5% isoflurane (Abbott, Rungis, France). The larynx was surgically exposed and the trachea was intubated orally under direct vision with a metal cannula (inner diameter of 1 mm, Harvard Apparatus, Les Ulis, France). The tracheal cannula was properly secured with surgical thread (Ethicon 3–0, Ethicon, Auneau, France) before connection to mechanical ventilator in order to avoid leaks.
Mechanical ventilation
After intubation, animals were left for 5 min in spontaneous breathing and half of the mice (obese and control) were sacrificed. The other half of were ventilated by means of a small-animal ventilator (flexiVent, Scireq, Montreal, Canada) as follows: VT = 8 mL/kg of body weight, respiratory rate = 180/min, end-expiratory pressure = 1.5 cmH2O, and inspired fraction of oxygen = 0.4–0.6. This combination of VT (8 ml/kg) and respiratory rate (180/min) has been shown to produce adequate minute ventilation and PaCO2 in mice48. Mechanical ventilation lasted 2 hours with continuous anesthesia maintained by 1.5% isoflurane and muscle paralysis using intraperitoneal pancuronium at the onset of the experiment (0.8 mg/kg of body weight, Organon, Puteaux, France) to ensure passive mechanical conditions. Body temperature was monitored using a rectal probe and was maintained at 36.5 °C with a blanket connected to a homeothermic regulator (Homeo-blanket system 50–7221 F, Harvard Apparatus, Les Ulis, France). Mice received one intraperitoneal warm fluid bolus (physiological serum) at the onset of the experiment (20 mL/kg of body weight). The dosage of all medications administered was calculated on actual weight. In ventilated obese mice, tidal volume was calculated either on actual weight or ideal weight. As no formula is available to calculate predicted body weight in mice, ideal weight was based on the mean weight of control mice (namely 31.2 g) (see SIF for details).
Experimental design
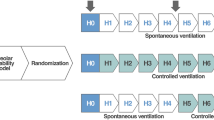
The experimental design included five groups: NVC (non-ventilated control mice), VC (ventilated control mice), NVO (non-ventilated obese mice), OVTaw (ventilated obese mice with VT calculated on the actual weight) and OVTiw (ventilated obese mice with VT calculated on an ideal weight) (Fig. 7). The number of animals used in each experiment is detailed in the legend of all result table or figure.
Design of experimental groups. (a) Mice were fed either a diet containing 10% fat (control mice) or 60% fat (obese mice) for 12 weeks. Non-ventilated control or obese mice (NVC and NVO) underwent general anesthesia, were intubated and were sacrificed after 5 min of spontaneous ventilation. (b) Mice were fed the same diet (10% or 60% fat). Ventilated control and obese mice underwent general anesthesia, were intubated and were sacrificed after 2 hours of mechanical ventilation, with a tidal volume calculated on actual weight for control mice (VC) or on either actual weight (OVTaw) or ideal weight (OVTiw) for obese mice.
Respiratory mechanics
Mean and peak airway pressures were recorded at initiation of mechanical ventilation and then hourly throughout the experimental period. Quasi-static compliance of the respiratory system was determined from a continuous pressure-volume curve performed at initiation of mechanical ventilation and at the end of MV.
Specimen collection
After 2 hours of MV, mice were sacrificed by exsanguination. Two different specimen collection procedures were used: For one half of the mice, immediately following thoracotomy, the pulmonary circulation was flushed by injection of 2 mL of saline into the right ventricle, and the lungs were then inflated by tracheal injection of 4% formaldehyde at a pressure of 20 cmH2O, fixed in 4% formaldehyde and finally paraffin-embedded. For the other half of the mice, bronchoalveolar lavage was performed with two tracheal injections of 1 mL of saline via the tracheal tube. A pulmonary circulation flush was then performed as previously described and both lungs were mechanically disrupted for flow cytometry analysis.
Histologic examination
After formalin fixation and paraffin embedding, 5 µm thick sections of the left lung were cut and stained with hematoxylin, eosin and saffron (HES). An optical microscope (BX51 Olympus) with camera (Camedia 5060, Olympus) was used for histologic examination and photographs. An inflammatory score, derived from the VILI score49,50, assessing the degree of neutrophil infiltration in alveolar spaces, was determined on each lung (see SIF for details). In practice, HES-stained lung sections were examined at high power field (x40) and only alveolar spaces, located at a distance of more than one alveolus from bronchial spaces, were analyzed. Ten random fields were examined and the neutrophil count was scored from 0 to 3: 0 for <5 neutrophils per field, 1 for 5 to 10 neutrophils per field, 2 for 10 to 20 neutrophils per field, and 3 for >20 neutrophils per field. All lung sections were examined under blinded conditions, without knowledge of the mouse status (obese or not, ventilated or not).
Bronchoalveolar lavage
The total cell count was determined for a fresh fluid specimen using slides with counting grids (Hycor biomedical, Indianapolis, IN, USA). The cell pellet was diluted in saline, and differential cell counts were performed on cytospin preparations (Cytospin 3; Shandon Scientific, Cheshire, UK) stained with Diff-Quick stain (Baxter Diagnostics, McGaw Park, IL, USA). Bronchoalveolar lavage fluid was centrifuged (1500 rpm, 13 min at 4 °C), and cell-free supernatants were stored at −80 °C for subsequent assessment of protein content (using a colorimetric BCA assay, Thermo Scientific, Rockford, IL, USA).
Flow cytometric analysis
After performing BAL, a pulmonary circulation flush was performed as previously described and both lungs were removed and placed in 2 mL of cell culture medium (RPMI 16/40 supplemented with 0.1% L-glutamine and 0.1% of nonessential amino acids) and then in ice. At the end of the experiment, lungs were placed in the same medium with 5% fetal bovine serum (FBS), 0.35 mg/mL collagenase XI (Sigma-Aldrich, Saint Louis, MO, USA) and 5 mg/mL type IV bovine pancreatic DNAse (Sigma-Aldrich), mechanically disrupted with a scalpel, and placed in an incubator at 37 °C with 5% CO2 for 35 min. The action of the enzymes was stopped by adding 9 mL of RPMI medium containing 10% FBS, and digested lungs were further disrupted by gently pushing the tissue through a nylon screen (70 µm). The single-cell suspension was then washed and centrifuged at 900 rpm. To lyze contaminating red blood cells, the cell pellet was incubated for 5 min at room temperature with 5 mL of Gey’s solution (NH4Cl and KHCO3). Cells were then washed with PBS, recentrifuged and the pellet was resuspended in 4 mL of PBS. Cell count was then performed using slides with counting grids (Hycor biomedical) after Trypan blue staining.
In cytometry tubes, 500,000 cells in 50 µL were incubated with 50 µL of Fc Block (Anti-CD16/CD32 antibody, BD Biosciences, San Jose, CA, USA) at 4 °C for 15 min and then stained with fluorophore-conjugated anti-mouse antibodies for CD11b, CD11c, Gr-1 (Ly6C/G), F4/80 and CD62L (L-selectin) or appropriate isotype-matched controls.
Samples were analyzed using a CyAn cytometer and Summit software (Beckman Coulter, Brea, USA). Macrophages were identified as high CD11c and low CD11b cells. In order to differentiate and quantify monocytes, low side-scatter, high CD11b and low CD11c events were gated. Neutrophils were differentiated from monocytes as high side-scatter, high CD11b and low CD11c cells. F4/80 was used to facilitate differentiation between monocytes and neutrophils. Cell activation was assessed based on L-selectin and CD11b adhesion molecule expression for neutrophils and L-selectin, CD11b and Gr-1 expression for monocytes. Details of the leukocyte identification procedure are represented in Supplementary Fig. S1.
Statistical analysis
Data were analyzed using Prism software version 5.0 (San Diego, CA, USA). Continuous data were expressed as median [IQ25-IQ75]. Statistical comparisons between two groups used the Mann-Whitney or the Wilcoxon test. Statistical comparisons between more than two groups used the Kruskal-Wallis test followed by a pairwise Mann-Whitney test with Benjamini-Hochberg correction for multiple testing. Two-tailed p values less than 0.05 were considered significant.
Ethical approval
All the experiments were performed in accordance with the official regulations of the French Ministry of Agriculture and the US National Institute of Health guidelines for the experimental use of animals, were approved by the animal institutional review board of the Paris-Est Creteil Val de Marne University, and were conducted in a specific “little animal” plateform (Plateforme Exploration Fonctionnelle du Petit Animal, INSERM U955, Créteil, France).
References
Flegal, K. M., Carroll, M. D., Kit, B. K. & Ogden, C. L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 307, 491–497 (2012).
Dreyfuss, D. & Saumon, G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 157, 294–323 (1998).
Ranieri, V. M. et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 282, 54–61 (1999).
Slutsky, A. S. & Ranieri, V. M. Ventilator-induced lung injury. The New England journal of medicine 369, 2126–2136 (2013).
Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. The New England journal of medicine 342, 1301–1308 (2000).
Gajic, O. et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med 32, 1817–1824 (2004).
Gajic, O., Frutos-Vivar, F., Esteban, A., Hubmayr, R. D. & Anzueto, A. Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Med 31, 922–926 (2005).
Fernandez-Perez, E. R., Keegan, M. T., Brown, D. R., Hubmayr, R. D. & Gajic, O. Intraoperative tidal volume as a risk factor for respiratory failure after pneumonectomy. Anesthesiology 105, 14–18 (2006).
Wolthuis, E. K. et al. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anesthesiology 108, 46–54 (2008).
Lellouche, F., Dionne, S., Simard, S., Bussieres, J. & Dagenais, F. High tidal volumes in mechanically ventilated patients increase organ dysfunction after cardiac surgery. Anesthesiology 116, 1072–1082 (2012).
Rhodes, A. et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 43, 304–377 (2017).
Determann, R. M. et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care 14, R1 (2010).
Schultz, M. J., Haitsma, J. J., Slutsky, A. S. & Gajic, O. What tidal volumes should be used in patients without acute lung injury? Anesthesiology 106, 1226–1231 (2007).
Futier, E., Constantin, J. M. & Jaber, S. Protective lung ventilation in operating room: a systematic review. Minerva Anestesiol 80, 726–735 (2014).
Fernandez-Bustamante, A., Wood, C. L., Tran, Z. V. & Moine, P. Intraoperative ventilation: incidence and risk factors for receiving large tidal volumes during general anesthesia. BMC Anesthesiol 11, 22 (2011).
Jaber, S. et al. A multicentre observational study of intra-operative ventilatory management during general anaesthesia: tidal volumes and relation to body weight. Anaesthesia 67, 999–1008 (2012).
Gong, M. N., Bajwa, E. K., Thompson, B. T. & Christiani, D. C. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax 65, 44–50 (2010).
Delay, J. M. et al. The effectiveness of noninvasive positive pressure ventilation to enhance preoxygenation in morbidly obese patients: a randomized controlled study. Anesth Analg 107, 1707–1713 (2008).
Mancuso, P. Obesity and lung inflammation. J Appl Physiol 108, 722–728 (2010).
McCallister, J. W., Adkins, E. J. & O’Brien, J. M. Jr. Obesity and acute lung injury. Clin Chest Med 30, 495–508, viii (2009).
Ferrante, A. W. Jr. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med 262, 408–414 (2007).
Shah, D. et al. Obesity-induced adipokine imbalance impairs mouse pulmonary vascular endothelial function and primes the lung for injury. Scientific reports 5, 11362 (2015).
Guivarch, E. et al. Effects Of Adjusting Tidal Volume On Actual Or Ideal Body Weight In Ventilated Obese Mice [abstract]. Am J Respir Crit Care Med 185, A5457 (2012).
de Prost, N., Ricard, J. D., Saumon, G. & Dreyfuss, D. Ventilator-induced lung injury: historical perspectives and clinical implications. Annals of intensive care 1, 28 (2011).
Wilson, M. R. et al. Role of lung-marginated monocytes in an in vivo mouse model of ventilator-induced lung injury. Am J Respir Crit Care Med 179, 914–922 (2009).
Smith, A. G., Sheridan, P. A., Harp, J. B. & Beck, M. A. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr 137, 1236–1243 (2007).
Smith, A. G., Sheridan, P. A., Tseng, R. J., Sheridan, J. F. & Beck, M. A. Selective impairment in dendritic cell function and altered antigen-specific CD8+ T-cell responses in diet-induced obese mice infected with influenza virus. Immunology 126, 268–279 (2009).
Kordonowy, L. L. et al. Obesity is associated with neutrophil dysfunction and attenuation of murine acute lung injury. Am J Respir Cell Mol Biol 47, 120–127 (2012).
Calixto, M. C. et al. Obesity enhances eosinophilic inflammation in a murine model of allergic asthma. Br J Pharmacol 159, 617–625 (2010).
Voiriot, G. et al. Middle age exacerbates acute respiratory distress syndrome in a double hit murine model. Experimental gerontology 96, 146–154 (2017).
Papaiahgari, S. et al. Genetic and pharmacologic evidence links oxidative stress to ventilator-induced lung injury in mice. Am J Respir Crit Care Med 176, 1222–1235 (2007).
Finigan, J. H. et al. Activated protein C protects against ventilator-induced pulmonary capillary leak. Am J Physiol Lung Cell Mol Physiol 296, L1002–1011 (2009).
Peng, X. et al. Inducible nitric oxide synthase contributes to ventilator-induced lung injury. Am J Respir Crit Care Med 172, 470–479 (2005).
Fernandez-Bustamante, A. et al. Perioperative lung protective ventilation in obese patients. BMC Anesthesiol 15, 56 (2015).
Allen, G. B., Suratt, B. T., Rinaldi, L., Petty, J. M. & Bates, J. H. Choosing the frequency of deep inflation in mice: balancing recruitment against ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 291, L710–717 (2006).
Vaporidi, K. et al. Effects of respiratory rate on ventilator-induced lung injury at a constant PaCO2 in a mouse model of normal lung. Crit Care Med 36, 1277–1283 (2008).
Voiriot, G. et al. Interleukin-6 displays lung anti-inflammatory properties and exerts protective hemodynamic effects in a double-hit murine acute lung injury. Respiratory research 18, 64 (2017).
Shore, S. A. Obesity and asthma: lessons from animal models. J Appl Physiol 102, 516–528 (2007).
Tankersley, C. G. et al. Leptin attenuates respiratory complications associated with the obese phenotype. J Appl Physiol 85, 2261–2269 (1998).
Rausch, M. E., Weisberg, S., Vardhana, P. & Tortoriello, D. V. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 32, 451–463 (2008).
Johnston, R. A. et al. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol 104, 1727–1735 (2008).
Lang, J. E., Williams, E. S., Mizgerd, J. P. & Shore, S. A. Effect of obesity on pulmonary inflammation induced by acute ozone exposure: role of interleukin-6. Am J Physiol Lung Cell Mol Physiol 294, L1013–1020 (2008).
O’Brien, J. M. Jr. et al. Excess body weight is not independently associated with outcome in mechanically ventilated patients with acute lung injury. Ann Intern Med 140, 338–345 (2004).
O’Brien, J. M. Jr. et al. Body mass index is independently associated with hospital mortality in mechanically ventilated adults with acute lung injury. Crit Care Med 34, 738–744 (2006).
Morris, A. E. et al. The association between body mass index and clinical outcomes in acute lung injury. Chest 131, 342–348 (2007).
Martino, J. L. et al. Extreme obesity and outcomes in critically ill patients. Chest 140, 1198–1206 (2011).
Warden, C. H. et al. Identification of four chromosomal loci determining obesity in a multifactorial mouse model. J Clin Invest 95, 1545–1552 (1995).
Mekontso Dessap, A. et al. Conflicting physiological and genomic cardiopulmonary effects of recruitment maneuvers in murine acute lung injury. Am J Respir Cell Mol Biol 46, 541–550 (2012).
Belperio, J. A. et al. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest 110, 1703–1716 (2002).
Imanaka, H. et al. Ventilator-induced lung injury is associated with neutrophil infiltration, macrophage activation, and TGF-beta 1 mRNA upregulation in rat lungs. Anesth Analg 92, 428–436 (2001).
Acknowledgements
We are very grateful to the following members of U955 INSERM unit for their invaluable help, and priceless scientific and technical assistance: Dr. Sabine Le Gouvello, Dr. Laurent Boyer, Dr. Maeva Zysman, Marie-Laure Franco-Montoya (Equipe 4, INSERM U955, Créteil), Rachid Souktani (Plateforme Exploration Fonctionnelle du Petit Animal, INSERM U955, Créteil), Aurélie Guguin and Adeline Henri (Plateforme Cytométrie en Flux, INSERM U955, Créteil), Xavier Decrouy (Plateforme Imagerie, INSERM U955 Créteil).
Author information
Authors and Affiliations
Contributions
E.G., G.V., A.R., M.D. and J.B. designed the study. E.G., A.R., S.K. and J.T.V.N. acquired the data. E.G., G.V., A.R., J.T.V.N., P.M., B.M., A.M.D., M.D. and J.B. analyzed and interpreted the data. All authors drafted the article, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guivarch, E., Voiriot, G., Rouzé, A. et al. Pulmonary Effects of Adjusting Tidal Volume to Actual or Ideal Body Weight in Ventilated Obese Mice. Sci Rep 8, 6439 (2018). https://doi.org/10.1038/s41598-018-24615-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24615-5
This article is cited by
-
Ventilator-induced lung-injury in mouse models: Is there a trap?
Laboratory Animal Research (2021)
-
Relationship of respiratory mechanics parameters acquired by forced oscillation technique (FOT) and morphological measurements in BALB/c mice
Research on Biomedical Engineering (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.