Abstract
Increased red cell distribution width (RDW) is associated with poorer outcomes in various patient populations. We investigated the association between preoperative RDW and anaemia on 30-day postoperative mortality among elderly patients undergoing non-cardiac surgery. Medical records of 24,579 patients aged 65 and older who underwent surgery under anaesthesia between 1 January 2012 and 31 October 2016 were retrospectively analysed. Patients who died within 30 days had higher median RDW (15.0%) than those who were alive (13.4%). Based on multivariate logistic regression, in our cohort of elderly patients undergoing non-cardiac surgery, moderate/severe preoperative anaemia (aOR 1.61, p = 0.04) and high preoperative RDW levels in the 3rd quartile (>13.4% and ≤14.3%) and 4th quartile (>14.3%) were significantly associated with increased odds of 30-day mortality - (aOR 2.12, p = 0.02) and (aOR 2.85, p = 0.001) respectively, after adjusting for the effects of transfusion, surgical severity, priority of surgery, and comorbidities. Patients with high RDW, defined as >15.7% (90th centile), and preoperative anaemia have higher odds of 30-day mortality compared to patients with anaemia and normal RDW. Thus, preoperative RDW independently increases risk of 30-day postoperative mortality, and future risk stratification strategies should include RDW as a factor.
Similar content being viewed by others
Introduction
Red cell distribution width (RDW) is an automated measure of the heterogeneity in erythrocyte sizes and is routinely performed as part of a Full Blood Count1,2. An increase in RDW, termed anisocytosis, reflects increased variation in the sizes of red blood cells (RBC) attributable to the presence of small and large RBCs, or both. Anisocytosis can be acquired, from nutritional deficiency such as iron (small RBC), vitamin B-12 and folate deficiency (large RBC), or in anaemia of chronic disease. RDW can also reflect underlying diseases such as in haemolytic anaemia and clinically significant thalassemic syndromes3,4. Traditionally, RDW has been used with other red cell indices (such as Mean Corpuscular Volume), to provide clues for underlying causes of anaemia, such as nutritional deficiencies and clinically significant thalassemia. More recently however, there is increasing interest in the role of RDW as a biomarker for inflammatory states and as a prognostication tool, with accumulating studies demonstrating increased RDW as an independent predictor for poorer outcomes among patients with ischaemic heart disease5,6,7,8, cardiac failure9,10, cerebrovascular disease11,12, cancer13,14 and patients who are critically ill in the Intensive Care setting15. More importantly, a number of studies have demonstrated a strong correlation between RDW and mortality in the older population16,17,18. Although the exact mechanisms remains unclear, given that higher RDW is associated with advancing age3,10 and higher disease burden3,13, RDW may serve as a novel biomarker that reflects multiple physiological impairments related to aging.
In the perioperative setting, increased RDW has also been found to be associated with long term outcomes such as one-year mortality19,20. Its association with shorter term outcomes such as 30-day mortality remains to be elucidated. With increasing proportion of elderly patients presenting for surgery, postoperative complications and patient outcomes are a major concern21,22,23. Consequently, there is a need to identify significant perioperative risk factors that allow accurate risk stratification for short term outcomes such as mortality and morbidity. This could facilitate meaningful informed patient consent and shared decision-making as well as facilitate targeted perioperative risk mitigation strategies24,25. Given that the RDW is routinely reported as a component of the Full Blood Count and is readily available for most patients undergoing surgery, understanding its prognostic potential could be very valuable and cost effective.
We aim to investigate the association between preoperative RDW and 30-day mortality among the elderly patients undergoing non-cardiac surgery and hypothesize that there is a significant association between increased RDW and 30-day mortality. Additionally, as anisocytosis is closely related to the presence of anaemia, we aim to explore the impact of anaemia on the relationship between RDW and 30-day postoperative mortality.
Methods
Data source
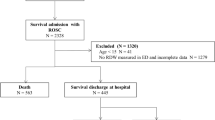
We retrospectively analysed the electronic medical records of 27053 patients aged 65 and older who underwent surgery under general or regional anaesthesia between 1 January 2012 and 31 October 2016 in Singapore General Hospital, a 1700-bedded tertiary academic hospital in Singapore. Institutional Review Board approval was obtained (Singhealth CIRB 2014/651/D) prior to the start of the study and the study was performed in accordance with relevant guidelines and regulations. Due to the retrospective nature of the review, waiver of informed consent from the patient was approved by the CIRB. Clinical records were sourced from our institution’s clinical information system (Sunrise Clinical Manager (SCM), Allscripts, IL, USA) and stored in our enterprise data repository and analytics system (SingHealth-IHiS Electronic Health Intelligence System - eHINTS), which integrates information from multiple healthcare transactional systems including administration, clinical and ancillary systems. Mortality data on the system was synchronized with the national death registry records, ensuring a complete follow-up. We excluded patients who underwent cardiac surgery, neurosurgery, transplant and burns surgery due to their categorically higher blood transfusion requirement and mortality rate. Only the outcome of the index surgery was evaluated if a patient underwent multiple surgeries during the data collection period. Our final dataset comprised of 24579 patients (Fig. 1).
Data collected include patient demographics as well as preoperative comorbidities - ASA-PS score, previous cerebrovascular accidents (CVA), ischemic heart disease (IHD), congestive cardiac failure (CCF), diabetes mellitus (DM), Revised Cardiac Risk Index (RCRI) score26, chronic kidney disease and preoperative anaemia. Priority of surgery (emergency or elective) and surgical risk classification based on the 2014 ESC/ESA guidelines27,28 were recorded as well. Operative data included type of anaesthesia received, perioperative blood transfusion and need for reoperation within 30 days of the index surgery. There was incomplete data in the clinical database on conditions associated with raised RDW, such as iron deficiency, vitamin B12 or folate deficiency and myelodysplastic syndrome, hence these were not included in the analysis.
Procedures and definitions
Full Blood Count were done in our institution’s College of American Pathologists accredited laboratory with Sysmex XN Automated Hematology Analyzer (Sysmex Corporation, Kobe, Japan) and Advia 2120i Hematology System (Siemens Healthcare Diagnostics Inc, USA). Preoperative laboratory results were taken as the latest blood results taken within 90 days before the surgery, and up to the day of surgery but before the start time of surgery. These results include the preoperative haemoglobin, red blood cell distribution width levels and serum creatinine levels. Anaemia was defined by the World Health Organisation (WHO)’s gender-based classification of anaemia severity29. Mild anaemia is defined as haemoglobin (Hb) 11–12.9 g/dL in males and 11–11.9 g/dL in females; moderate anaemia Hb 8–10.9 g/dL and severe anaemia Hb <8.0 g/dL. RDW is reported as a coefficient of variation (percentage) of red blood cell volume with the normal reference range for RDW in this hospital laboratory to be 10.9% to 15.7%.
Perioperative blood transfusion was defined as red blood cell (RBC) concentrate units given during the surgery and up to one month after the date of surgery. Pre-existing chronic kidney disease was defined based the estimated glomerular filtration rate (eGFR) that is derived from the latest preoperative serum creatinine levels by the MDRD equation according to KDIGO guidelines30. The individual components of RCRI were defined as per the original study by Lee et al.26.
Statistical analysis
Statistical analysis was done in IBM SPSS Statistics v21.0. We compared the demographic and perioperative variables between patients who are alive or dead within 30-days of surgery, and across RDW quartiles. We checked continuous variables such as age for normality. For continuous, non-parametric variables, the Mann-Whitney U test was used to test for a significant difference in median values between 2 groups, and the Kruskal Wallis for a significant difference in median values across more than 2 groups. For categorical variables, Chi-square test was used to compare the percentages between the groups.
Multivariate logistic regression was performed to determine independent predictors for 30-day mortality adjusted for demographic, perioperative clinical risk factors, surgical factors as well as RDW quartiles. We checked for multi-collinearity between surgical risk, ASA score and RCRI with the Spearman correlation analysis. We plotted the AUC for RDW in predicting 30-day mortality in our patient cohort as well as the test statistics for RDW at regular cut-off levels – 13%, 14%, 15%, 16% and 17%. In addition, to explore the interaction between RDW and anaemia, we repeated the logistic regression for 30-day mortality with anaemia stratified by the presence of elevated RDW. We took a RDW cut-off of 15.7%, with values above it considered to be elevated. An RDW value of 15.7% corresponds to 90th centile of our population, and is also our laboratory cut-off for the upper limit of normal. We performed bootstrapping to assess the influence of missing data on the stability of the results. The p values of both results are very consistent, and the p-values that were significant on the multivariate analysis remained significant at p < 0.046.
Results
Patient demographics and characteristics
The baseline characteristics of our study cohort is presented in Table 1. 348 (1.42%) patients died within 30 days of their index surgery. The median age of those who survived up to 30-days after surgery was 72.0, and is significantly lower (P < 0.001) compared to those who died within 30-days (77.0 years). A significantly (p < 0.001) higher proportion of patients who underwent emergency surgery died within 30-days (5.2%) compared to 0.6% in those who underwent elective surgery. The incidence of mortality increased with increasing units of perioperative blood transfusion from 0.9% in those who did not receive transfusion, to 10.6% in those who received 2 or more units. Similarly, incidence of mortality increased progressively with increasing severity of anaemia, from 0.5% in the non-anaemics to 4.6% in those with moderate/severe anaemia. Moreover, the median RDW levels were also significantly higher in those who died (15.0) compared to those who were alive (13.4), P < 0.001.
RDW and 30-day mortality
A comparison of the cohort across the various RDW quartiles is shown in Table 2. There were disproportionately more patients in the 4th RDW quartile with higher ASA PS (4&5), moderate/severe preoperative anaemia, received perioperative blood transfusion of 2 units or greater, higher RCRI scores (4&5), higher stages of chronic kidney disease (4&5) as well as those who died within 30 days after surgery.
Multivariate analysis
Based on multivariate logistic regression analysis, high preoperative RDW levels within 3rd quartile (OR 2.12, 1.13–3.99, p = 0.02) or 4th quartile (aOR 2.85, 1.57–5.17, p = 0.001) were independent risk factors for postoperative 30-day mortality in our cohort of elderly patients who underwent non-cardiac surgery. Other independent predictors of 30-day mortality were older age 76 years and above (aOR 2.41, 1.61–3.63, p < 0.001), Malay ethnicity (aOR 1.88, 1.17–3.02, p = 0.01) emergency surgery (aOR2.99, 2.15–4.14, p < 0.001), repeat surgeries within 30 days (aOR 2.29, 1.51–3.45, p < 0.001), perioperative blood transfusion of 1 unit (aOR 1.72, 1.15–2.58, p = 0.009) or 2 or more units (aOR 2.50, 1.51–4.14, p < 0.001), increasing ASA-PS score (3 or higher), presence of moderate/severe preoperative anaemia (aOR 1.61, 1.03–2.52, p = 0.04), history of ischaemic heart disease (aOR 1.70, 1.22–2.36, p = 0.002) and grade 4/5 chronic kidney disease (aOR 2.00, 1.29–3.10, p = 0.002). The results of univariate and multivariate analyses are shown in Table 3. Based on spearman correlation analysis, ASA and Surgical Risk category had high degree of correlation with a R of 0.742; ASA and RCRI had a weak correlation with R of 0.384; Surgical risk category and RCRI had negligible correlation with R of 0.079 (all P values <0.01). However, all 3 variables were included in our multivariate analysis as we feel that the degree of invasiveness and extent of the surgery is a very relevant clinical predictor.
Performance of RDW in predicting 30-day mortality
As patients with each progressive RDW quartiles showed progressively increasing aOR of 30-day mortality compared to patients with RDW levels in the first quartile, we plotted the ROC for RDW in predicting 30-day mortality is shown as Figs 2 and 3. The area under the curve (AUC) was 0.761 (95% CI; 0.736–0.787). Furthermore, we calculated the sensitivity, specificity, and predictive values for 30-day mortality with incremental RDW values as shown in Table 4. The higher the RDW value, the lower its sensitivity and negative predictive value. However, specificity and positive predictive value increased with increasing RDW. Our chosen cut-off value of 15.7%, based on the upper limit of the range of normal population values validated in our laboratory, had a sensitivity of 39.5%, specificity of 89.3%, positive predictive value of 5.3% and negative predictive value of 99.0%.
Adjusted Odds Ratio (aOR) for various degrees of anaemia stratified by red blood cell distribution width (RDW) levels and their 95% Confidence Intervals (CI) plotted on a log scale for the effect of anaemia stratified by RDW on 30-day mortality. Variables for adjustment are listed in Table 3, except anaemia and RDW.
Incremental effect of RDW and Anaemia on 30-day mortality
As anaemia and higher RDW quartiles were independently associated with 30-day mortality in the multivariate regression analysis, we repeated the multivariate logistic regression by stratifying anaemia based on RDW levels (≤15.7% vs >15.7%) to examine for any interactions between anaemia and normal or high RDW. We found an incremental effect of high RDW (>15.7%) on anaemia in increasing the odds of 30-day mortality (Fig. 3). Strikingly, patients with mild anaemia and high RDW had almost twice the odds of 30-day mortality - aOR 3.00 (1.50–6.03) compared to patients with moderate/severe anaemia and normal RDW - aOR 1.70 (1.05–2.77), and similar odds of mortality as moderate/severe anaemia and high RDW - aOR 3.11 (1.89–5.12). This shows that high RDW has a consistent effect on mortality that is independent of anaemia. Unfortunately, we were unable to demonstrate the effect of high RDW in patients with no anaemia due to the small patient numbers (n = 250), which contributed to the wide confidence interval of the effect size -aOR 1.51 (0.44–5.16), p = 0.10.
Discussion
In this study, we focused on older patients in the perioperative setting and demonstrated that a high RDW percentage is independently associated with 30-day all-cause postoperative mortality. This effect of high RDW on mortality is more pronounced in patients with anemia, and potentially could be used to improve the accuracy of clinical risk predictors. RDW is a simple parameter which reflects the degree of heterogeneity in erythrocyte volume and is routinely reported alongside other hematologic indices in the full blood count, which is a common blood investigation done prior to surgeries, and is thus available at no additional cost. Prior studies have demonstrated RDW to be a compelling independent risk factor for longer-term mortality in patients with cardiovascular diseases such as heart failure, acute myocardial infarction, coronary artery disease, pulmonary embolism, cardiac arrest, and stroke11,31,32,33,34,35,36,37,38. A meta-analysis revealed elevated RDW to be associated with aging and the progression of various types of disorders, and was also a strong independent predictor of long term survival in the older population17. Furthermore, multiple studies support the utilisation of RDW as a biomarker in risk stratification for mortality in general populations with or without cardiac diseases, cancer and other frequent comorbidities5,18,20,31,32,39,40,41. In the perioperative setting, elevated RDW has been associated with higher risks of one-year mortality after surgery19,42,43. Our study supports their finding by demonstrating that RDW also increases perioperative mortality in the short term (30-days). This suggests a role for RDW as a useful, cheap and convenient biomarker for predicting short term mortality in the perioperative setting.
While the exact biological mechanisms responsible for this association between increased RDW and mortality is currently unclear, previous studies suggested oxidative stress44,45,46, inflammation44,47,48, and malnutrition (such as iron, folate or vitamin B12 deficiency) as being the most plausible3. Oxidative stress, a common condition in most chronic human disorders, such as cancer, diabetes, heart diseases, liver failure and chronic kidney disease is associated with enhanced generation of reactive oxygen species and the consequent damage to nucleic acids, proteins and lipids has a profound influence on erythrocyte homeostasis and survival49. Inflammation, on the other hand, inhibits bone marrow function and iron metabolism48. Pro-inflammatory cytokines have also been determined to inhibit erythropoietin-induced erythrocyte maturation and proliferation and down regulate erythropoietin receptor expression, all of which increases RDW50,51. Hence, it is plausible that the stressful perioperative period may further increase the oxidative demands and inflammatory load beyond physiological tolerance, contributing to the poorer survival.
The presence of preoperative anaemia has been shown to be a strong predictor of postoperative mortality and morbidities52,53,54,55,56. Our findings add to current knowledge by demonstrating that high RDW (>15.7%) may further increase the odds ratio of 30-day mortality in those with anaemia, especially in patients with moderate/severe anaemia. This incremental effect suggests that the underlying reasons for increased RDW also have an impact on 30-day postoperative mortality. Admittedly, we were unable to show the impact of high RDW on mortality in patients with no anaemia, as we had few patients in our cohort with no anaemia and high RDW (n = 250), which may contribute to the wide confidence interval and statistical non-significance (aOR 1.51, 0.44–5.16), p = 0.102). Our choice of 15.7% as the cut-off for high versus normal RDW is in between the cut-offs of 14.5–16.0% chosen in other studies10,57,58 and was decided upon as it was the upper limit of normal population values validated in our laboratory. As shown in our cohort, as well as in other studies, mortality rate is higher in higher RDW quartiles5,39,59. In our study, despite having a study population of elderly patients who are at higher risk of adverse outcomes after surgery, the positive predictive value of the highest RDW cut-off of 17% is a mere 6.2%. Thus we feel at the choice of 15.7% is a reasonable trade-off between the desired sensitivity of a screening test for at-risk patients, yet balanced with a reasonable positive predictive value of 5.3%.
Apart from cost and the ability to standardize a test, an ideal biomarker for risk prediction in a population should include qualities such as the test’s independence from established risk factors and ability to extend predictability beyond current models and generalizability of the results60. Our study demonstrated that RDW was an independent risk factor for 30-day mortality in the multivariate analysis, however future studies can be done to investigate if the inclusion of RDW could improve the performance of currently available risk stratification models.
There is also a need for future studies to deepen the understanding of determinants for RDW, including identifying reversible factors such as nutritional deficiencies and oxidative stress, which may lead to improved outcomes after surgery. RDW could be a useful indicator of chronic health state and a practical addition to existing risk stratification strategy and shared decision making process.
Limitations
The single-centre, retrospective observational design with short-term follow up are among the main limitations of this study. We also did not classify the causes of death and therefore could not examine the associations more closely. Furthermore, we did not have comprehensive data that may be associated with both RDW and surgical patient outcomes, such as presence of malignancy, underlying haematological disorders, nutritional deficiencies, socioeconomic status, and other known or unknown confounders. Therefore, we cannot exclude the possibility of residual confounding.
In the absence of a concrete understanding of the mechanism between increased RDW and mortality, it could be said that at this point, detecting elevated RDW preoperatively may not necessarily be actionable, despite evidence of its association with short term mortality. However, the strength of using RDW as a prognostic biomarker lies with its ready availability at no extra cost, since it is routinely performed as part of a full blood count
Data Availability
Data from this study are available for download from the Dyrad Digital Repository at the following https://doi.org/10.5061/dryad.5772v.
Conclusion
Preoperative elevated RDW is an independent predictor of 30-day postoperative mortality among older patients undergoing non-cardiac surgery, and has an incremental effect on preoperative anaemia in predicting this risk. RDW could be used to improve existing risk stratification strategies and further studies are needed to explore this role and the biological process responsible for this association between RDW and mortality.
References
Buttarello, M. & Plebani, M. Automated blood cell counts: state of the art. Am. J. Clin. Pathol. 130, 104–116 (2008).
England, J. M. & Down, M. C. Red-cell-volume distribution curves and the measurement of anisocytosis. Lancet 1, 701–703 (1974).
Salvagno, G. L., Sanchis-Gomar, F., Picanza, A. & Lippi, G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 52, 86–105 (2015).
Karnad, A. & Poskitt, T. R. The automated complete blood cell count. Use of the red blood cell volume distribution width and mean platelet volume in evaluating anemia and thrombocytopenia. Arch. Intern. Med. 145, 1270–1272 (1985).
Skjelbakken, T. et al. Red cell distribution width is associated with incident myocardial infarction in a general population: the Tromsø Study. J. Am. Heart Assoc. 3 (2014).
Cavusoglu, E. et al. Relation between red blood cell distribution width (RDW) and all-cause mortality at two years in an unselected population referred for coronary angiography. Int. J. Cardiol. 141, 141–146 (2010).
Poludasu, S., Marmur, J. D., Weedon, J., Khan, W. & Cavusoglu, E. Red cell distribution width (RDW) as a predictor of long-term mortality in patients undergoing percutaneous coronary intervention. Thromb. Haemost. 102, 581–587 (2009).
Fatemi, O. et al. Red cell distribution width is a predictor of mortality in patients undergoing percutaneous coronary intervention. J. Thromb. Thrombolysis 35, 57–64 (2013).
Sotiropoulos, K. et al. Red cell distribution width and mortality in acute heart failure patients with preserved and reduced ejection fraction. ESC Heart Failure 3, 198–204 (2016).
Shah, N. et al. Red cell distribution width and risk of cardiovascular mortality: Insights from National Health and Nutrition Examination Survey (NHANES)-III. Int. J. Cardiol., https://doi.org/10.1016/j.ijcard.2017.01.045 (2017).
Ani, C. & Ovbiagele, B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J. Neurol. Sci. 277, 103–108 (2009).
Kara, H. et al. Red cell distribution width and neurological scoring systems in acute stroke patients. Neuropsychiatr. Dis. Treat. 11, 733–739 (2015).
Hu, L. et al. Prognostic value of RDW in cancers: a systematic review and meta-analysis. Oncotarget, https://doi.org/10.18632/oncotarget.13784 (2016).
Podhorecka, M. et al. Assessment of red blood cell distribution width as a prognostic marker in chronic lymphocytic leukemia. Oncotarget 7, 32846–32853 (2016).
Luo, R., Hu, J., Jiang, L. & Zhang, M. Prognostic Value of Red Blood Cell Distribution Width in Non-Cardiovascular Critically or Acutely Patients: A Systematic Review. PLoS One 11, e0167000 (2016).
Martínez-Velilla, N., Ibáñez, B., Cambra, K. & Alonso-Renedo, J. Red blood cell distribution width, multimorbidity, and the risk of death in hospitalized older patients. Age 34, 717–723 (2012).
Patel, K. V. et al. Red cell distribution width and mortality in older adults: a meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 65, 258–265 (2010).
Patel, K. V., Ferrucci, L., Ershler, W. B., Longo, D. L. & Guralnik, J. M. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch. Intern. Med. 169, 515–523 (2009).
Sim, Y. E. et al. Prevalence of preoperative anemia, abnormal mean corpuscular volume and red cell distribution width among surgical patients in Singapore, and their influence on one year mortality. PLoS One 12, e0182543 (2017).
Chan, D. X. H., Sim, Y. E., Chan, Y. H., Poopalalingam, R. & Abdullah, H. R. Development of the Combined Assessment of Risk Encountered in Surgery (CARES) surgical risk calculator for prediction of postsurgical mortality and need for intensive care unit admission risk: a single-center retrospective study. BMJ Open 8, e019427 (2018).
Polanczyk, C. A. et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann. Intern. Med. 134, 637–643 (2001).
Turrentine, F. E., Wang, H., Simpson, V. B. & Jones, R. S. Surgical risk factors, morbidity, and mortality in elderly patients. J. Am. Coll. Surg. 203, 865–877 (2006).
Ozturk, E. & Yilmazlar, T. Factors affecting the mortality risk in elderly patients undergoing surgery. ANZ J. Surg. 77, 156–159 (2007).
Protopapa, K. L., Simpson, J. C., Smith, N. C. E. & Moonesinghe, S. R. Development and validation of the Surgical Outcome Risk Tool (SORT). Br. J. Surg. 101, 1774–1783 (2014).
Yek, J. L. J. et al. Defining reasonable patient standard and preference for shared decision making among patients undergoing anaesthesia in Singapore. BMC Medical Ethics 18(1) (2017).
Lee, T. H. et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 100, 1043–1049 (1999).
Glance, L. G. et al. The Surgical Mortality Probability Model: derivation and validation of a simple risk prediction rule for noncardiac surgery. Ann. Surg. 255, 696–702 (2012).
Kristensen, S. D. et al. ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur. Heart J. 35, 2383–2431 (2014).
Health Organization, W. Nutritional anaemias: report of a WHO scientific group [meeting held in Geneva from 13 to 17 March 1967] (1968).
Levey, A. S. et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 67, 2089–2100 (2005).
Zalawadiya, S. K., Veeranna, V., Panaich, S. S., Afonso, L. & Ghali, J. K. Gender and ethnic differences in red cell distribution width and its association with mortality among low risk healthy United state adults. Am. J. Cardiol. 109, 1664–1670 (2012).
Horne, B. D., Anderson, J. L., Muhlestein, J. B., Ridker, P. M. & Paynter, N. P. Complete blood count risk score and its components, including RDW, are associated with mortality in the JUPITER trial. Eur. J. Prev. Cardiol. 22, 519–526 (2015).
Borné, Y., Smith, J. G., Melander, O. & Engström, G. Red cell distribution width in relation to incidence of coronary events and case fatality rates: a population-based cohort study. Heart 100, 1119–1124 (2014).
Dabbah, S., Hammerman, H., Markiewicz, W. & Aronson, D. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am. J. Cardiol. 105, 312–317 (2010).
Felker, G. M. et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J. Am. Coll. Cardiol. 50, 40–47 (2007).
Anderson, J. L. et al. Usefulness of a complete blood count-derived risk score to predict incident mortality in patients with suspected cardiovascular disease. Am. J. Cardiol. 99, 169–174 (2007).
Kim, J. et al. Red blood cell distribution width as an independent predictor of all-cause mortality in out of hospital cardiac arrest. Resuscitation 83, 1248–1252 (2012).
Nishizaki, Y. et al. Red blood cell distribution width as an effective tool for detecting fatal heart failure in super-elderly patients. Intern. Med. 51, 2271–2276 (2012).
Perlstein, T. S., Weuve, J., Pfeffer, M. A. & Beckman, J. A. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch. Intern. Med. 169, 588–594 (2009).
Chen, P.-C. et al. Red blood cell distribution width and risk of cardiovascular events and mortality in a community cohort in Taiwan. Am. J. Epidemiol. 171, 214–220 (2010).
Arbel, Y. et al. Red blood cell distribution width and the risk of cardiovascular morbidity and all-cause mortality. Thromb. Haemost. 111, 300–307 (2014).
Kim, C. H. et al. An increase in red blood cell distribution width from baseline predicts mortality in patients with severe sepsis or septic shock. Crit. Care 17, R282 (2013).
Kim, S., Lee, K., Kim, I., Jung, S. & Kim, M.-J. Red cell distribution width and early mortality in elderly patients with severe sepsis and septic shock. Clin Exp Emerg Med 2, 155–161 (2015).
Semba, R. D. et al. Serum antioxidants and inflammation predict red cell distribution width in older women: the Women’s Health and Aging Study I. Clin. Nutr. 29, 600–604 (2010).
Huerta, J. M., González, S., Fernández, S., Patterson, Á. M. & Lasheras, C. Lipid peroxidation, antioxidant status and survival in institutionalised elderly: A five-year longitudinal study. Free Radic. Res. 40, 571–578 (2006).
Friedman, J. S. et al. SOD2-deficiency anemia: protein oxidation and altered protein expression reveal targets of damage, stress response, and antioxidant responsiveness. Blood 104, 2565–2573 (2004).
Lippi, G. et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch. Pathol. Lab. Med. 133, 628–632 (2009).
Chiari, M. M. et al. Influence of acute inflammation on iron and nutritional status indexes in older inpatients. J. Am. Geriatr. Soc. 43, 767–771 (1995).
Kohen, R. & Nyska, A. Invited review: Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. (2002).
Macdougall, I. C. & Cooper, A. C. Erythropoietin resistance: the role of inflammation and pro‐inflammatory cytokines. Nephrol. Dial. Transplant (2002).
Faquin, W. C., Schneider, T. J. & Goldberg, M. A. Effect of inflammatory cytokines on hypoxia-induced erythropoietin production. Blood 79, 1987–1994 (1992).
Saager, L. et al. The association between preoperative anemia and 30-day mortality and morbidity in noncardiac surgical patients. Anesth. Analg. 117, 909–915 (2013).
Wu, W.-C. et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA 297, 2481–2488 (2007).
Shander, A., Javidroozi, M., Ozawa, S. & Hare, G. M. T. What is really dangerous: anaemia or transfusion? Br. J. Anaesth. 107, i41–i59 (2011).
Beattie, W. S., Karkouti, K., Wijeysundera, D. N. & Tait, G. Risk associated with preoperative anemia in noncardiac surgery: a single-center cohort study. Anesthesiology 110, 574–581 (2009).
Abdullah, H. R. et al. Association between preoperative anaemia with length of hospital stay among patients undergoing primary total knee arthroplasty in Singapore: a single-centre retrospective study. BMJ Open 7, e016403 (2017).
Zhou, X.-Y., Chen, H.-L. & Ni, S.-S. Red cell distribution width in predicting 30-day mortality in patients with pulmonary embolism. J. Crit. Care 37, 197–201 (2017).
Riedl, J. et al. Red Cell Distribution Width and Other Red Blood Cell Parameters in Patients with Cancer: Association with Risk of Venous Thromboembolism and Mortality. Blood 124, 2859–2859 (2014).
Warwick, R. et al. Red cell distribution width and coronary artery bypass surgery. Eur. J. Cardiothorac. Surg. 43, 1165–1169 (2013).
Pearson, T. A. et al. Markers of Inflammation and Cardiovascular Disease. Circulation 107, 499–511 (2003).
Acknowledgements
The authors acknowledge the contributions by Ms Sudha Harikrishnan (Executive, Department of Anaesthesiology, Singapore General Hospital) and Mr Koh Yee Jin (Principal Systems Specialist, Department of Health Insights, Integrated Health Information Systems Pte Ltd, Singapore) for their invaluable help in the data extraction process.
Author information
Authors and Affiliations
Contributions
A.H.R. and S.Y.E. contributed to the study conception, analysis, interpretation, drafting and final approval. S.Y.T. contributed to the acquisition, analysis, and interpretation, drafting and final approval. A.A.L., C.Y.H., R.T., O.B.C. contributed to the analysis, and interpretation, drafting and final approval.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdullah, H.R., Sim, Y.E., Sim, Y.T. et al. Preoperative Red Cell Distribution Width and 30-day mortality in older patients undergoing non-cardiac surgery: a retrospective cohort observational study. Sci Rep 8, 6226 (2018). https://doi.org/10.1038/s41598-018-24556-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24556-z
This article is cited by
-
Preoperative anemia in older individuals undergoing major abdominal surgery is associated with early postoperative morbidity: a prospective observational study
Canadian Journal of Anesthesia/Journal canadien d'anesthésie (2024)
-
Red cell distribution width and mortality in older patients with frailty in the emergency department
BMC Emergency Medicine (2023)
-
Low-dose CT from myocardial perfusion SPECT/CT allows the detection of anemia in preoperative patients
Journal of Nuclear Cardiology (2022)
-
Red blood cell distribution width and outcomes following coronary artery bypass grafting: looking back and forth
Indian Journal of Thoracic and Cardiovascular Surgery (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.