Abstract
We have previously described that the NFκB pathway is upregulated during differentiation of glioblastoma stem-like cells (GSCs) which keeps differentiating GSCs in a proliferative astrocytic precursor state. However, extracellular signals and cellular mediators of this pathway are not clear yet. Here, we show that TLR4 is a key factor to promote NFκB activation in differentiating GSCs. TLR4 is upregulated during differentiation of GSCs and promotes transcriptional activation of NFκB as determined by luciferase-reporter assays and expression of NFκB target genes. Downregulation of TLR4 by shRNAs or blockade with anti-TLR4 specific antibodies drastically inhibited NFκB activity which promoted further differentiation and reduced proliferation of GSCs. We found that hyaluronic acid (HA), a main component of brain extracellular matrix, triggers the TLR4-NFκB pathway in differentiating GSCs. Moreover, HA is synthesized and released by GSCs undergoing differentiation and leads to transcriptional activation of NFκB, which is inhibited following downregulation of TLR4 or blockade of HA synthesis. Thus, we have demonstrated that during the process of differentiation, GSCs upregulate TLR4 and release the TLR4 ligand HA, which activates the TLR4-NFκB signaling pathway. This strategy may efficiently be used by differentiating GSCs to maintain their proliferative potential and consequently their tumorigenic capacity.
Similar content being viewed by others
Introduction
Glioblastoma is a highly aggressive tumor with poor survival rates and treatment remains a challenge. Work in different types of cancer, including glioblastoma, suggest that cancer stem-like cells are resistant against radiotherapy and chemotherapy, which facilitates tumor recurrence1. A number of antitumor strategies induce DNA damage, or inhibit cell division and angiogenesis, mainly by small molecular inhibitors or blocking antibodies. Activation of DNA damaging mechanisms by genotoxic agents may induce apoptotic or senescent cell death2. Differentiation-inducing agents offer an alternative antitumor strategy and some compounds are able to increase the efficacy of chemotherapy or radiotherapy3,4. Based on this, strategies that promote differentiation and senescence provide therapeutic opportunities that need to be further explored.
There are many signaling pathways that promote oncogenic transformation or maintain the aggressiveness of tumor cells. Some of these pathways induce activation of NFκB, a transcription factor involved in many cellular processes including cell survival, proliferation and migration5. It has also been shown that inhibition of NFκB facilitates stemness6, and triggers proliferation of neural stem cells7,8. Proinflammatory signals like cytokines and pathogen-associated molecular patterns (PAMP) trigger an intracellular mechanism that leads to the activation of NFκB9. Several of these signaling pathways are initiated by activating cell surface receptors, including tumor necrosis factor receptor and Toll-like receptor (TLR) superfamilies. At present, 11 mammalian TLRs have been described. TLR proteins recognize PAMPS that include bacterial lipopolysaccharides and peptidoglycans or viral RNAs among others. Upon binding of the ligand, TLR proteins recruit a signaling adaptor protein, mainly MyD88, that activates a kinase cascade which ultimately promotes activation of the NFκB protein complex that is translocated to the nucleus to induce the expression of target genes. TLR4 is one of the most studied members of the TLR family and it has been involved in inflammation and resistance to virus, as well as in tumor microenvironment. TLRs, including TLR4, have been shown to be overexpressed in breast cancer10 and tumor cells from a wide variety of tissues11 suggesting that TLR activation may be an important event in tumor cell immune evasion. Activation of TLR4 in tumor cells promotes the synthesis of NFκB target genes, including IL-6 and IL1β, which results in resistance of tumor cells against cytotoxic lymphocytes. However, the role of the TLR4-NFκB signaling pathway during differentiation of cancer stem-like cells has been poorly studied.
Here we show that TLR4 is upregulated during differentiation of GSCs, which triggers the NFκB transcriptional pathway, avoiding terminal differentiation and maintaining proliferation. This cell behaviour is reversed following downregulation or inactivation of TLR4. We also show that TLR4 is activated by hyaluronic acid (HA) that is synthesized and secreted by differentiating GSCs.
Materials and Methods
Reagents
Blocking anti-TLR4 antibodies and control IgG2a (both from eBioscience-ThermoFisher, Waltham, MA) were mostly used at 0.5 μg/ml in cell cultures. HA fragments with a size distribution of 15–40 kDa (R&D Systems, Minneapolis, MN) were added at a concentration of 100 μg/ml when indicated. HA synthesis inhibitor 4-Methylumbelliferone (4-ME) (Sigma-Aldrich, St Louis, MO) was added to cell cultures at 2 mM. HA levels in cell culture supernatants were determined by an ELISA kit from Echelon Biosciences (Salt Lake City, UT).
Primary tumor neurosphere cultures
GSCs were derived from patients with primary glioblastoma with wild-type IDH1 and EGFR amplification. All the results shown in figures have been obtained from a single cell culture, but in all cases similar data were replicated in one or two more GSC cultures. Neurosphere cultures were established as previously described12. Briefly, tumor cells were obtained following digestion of tissue samples and cultured in serum-free DMEM/F12 medium (Invitrogen, Carlsbad, CA) with growth factors containing human recombinant EGF (20 ng/ml EGF and 20 ng/ml bFGF, both from Sigma, St. Louis, MO). To induce differentiation, neurospheres were disaggregated into single cells that were cultured as a monolayer in the same medium but containing 10% FCS for the indicated time intervals as previously described12,13. For cell proliferation assays, neurospheres were disaggegated into single cell suspensions before cell growth was analysed at the indicated time intervals by using Alamar blue reagent (Life Technologies, Paisley, UK).
Immunofluorescence
Briefly, differentiating GSCs were grown on 10 × 10 mm coverslips. Cells were then fixed in 3.7% formaldehyde and permeabilized with 0.5% Triton X-100. Then, cells were incubated with rabbit anti-GFAP (DAKO, Glostrup, Denmark) and anti-Ki67 (SP6, Thermo Scientific, Waltham, MA) or mouse anti-phospho histone H2AX (05–636, Millipore, Billerica, MA) antibodies. Texas red-conjugated or FITC-conjugated goat anti–rabbit or anti-mouse secondary antibodies (Jackson ImmunoResearch, Cambridgeshire, UK) were used for detection. For fluorescence quantification, markers were analyzed by using the AxioVision software (Carl Zeiss Inc.). Nuclear area was quantified using ImageJ software (NIH, Bethesda) on at least 100 DAPI-positive nuclei.
Gene expression analyses
Gene expression analysis comparing neurosphere-forming GSCs and GSCs differentiated in the presence of 10% FCS for 4 days, was performed with the Human Genome U133 Plus 2.0 array (Affymetrix, Santa Clara, CA) as described12. Differential expression of all TLR family members included in the array was analyzed using the fold-change value. Gene expression was assessed by using primers for TLR4 (5′AGTTTCCTGCAATGGATCAAGG3) and (5′CTGCTTATCTGAAGGTGTTGCAC3), Map2 (5′TTCGTTGTGTCGTGTTCTCA3′) and (5′AACCGAGGAAGCATTGATTG3′), TNFα (5′CGGGACGTGGAGCTGGCCGAGGAG3′) and (5CACCAGCTGGTTATCTCTCAGCTC3′), IL-10 (5′CCGAGATGCCTTCAGCAGAG3′) and (5′CACATGCGCCTTGATGTCTG3′), IL-6 (5′AGTGAGGAACAAGCCAGAGC3′) and (5′GAGATGAGTTGTCATGTCCTGC3′), and β-Actin (5′GCGGGAAATCGTGCGTGACATT3′) and (5′GATGGAGTTGAAGGTAGTTTCGTG3′). Quantitative PCR was performed as previously described14.
TLR protein expression analysis
Detection of the levels of TLR protein was achieved by using a TLR cell surface screening set (NOVUS, Littleton, CO) that included specific antibodies against TLR1, TLR2, TLR4, TLR5, TLR6 and TLR10. For each individual analysis, an isotype antibody was used for non-specific staining. PE-labeled secondary antibodies were then used and labelled cells were analysed by flow cytometry (FACSCalibur, BD Biosciences) using the ModFit software (Verity, Topsham, ME). TLR expression levels are shown as mean fluorescence intensity subtracting the corresponding isotype control.
Transfection experiments and gene reporter assays
GSCs were co-transfected with 1 μg of pGL2-MAP2 promoter construct12 and 50 ng of pRSV-β-gal by nucleofection (Amaxa, Cologne, Germany). Cells were also transfected with 1 μg of pBVIx-Luc, a plasmid containing NFκB consensus sequences linked to the luciferase reporter gene. Luciferase activity was analysed by a dual-light reporter gene assay (Applied Biosystems). Results were normalized by cotransfection with pRSV-β-gal.
Gene silencing
GSCs were dissociated from neurospheres and cultured at a density of 2 × 104 cells per well in a 96-well plate. Five non-overlapping TLR4-specific or control scrambled shRNA-containing lentiviral particles were used (MISSION shRNA library, SIGMA) as indicated by the manufacturer. As a control of transduction efficiency, GSCs were infected with lentivirus containing shRNA to EGFP.
Statistical analysis
Statistics were analyzed with the SPSS package (version 13.0). The Student t test was used to compare continuous variables as means ± SD, between two groups. The significance level was set at p < 0.05.
Results
TLR4 is upregulated during differentiation of GSCs and mediates activation of NFκB
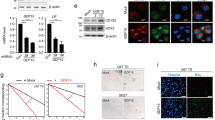
We have previously shown that NFκB pathway is activated in differentiating GSCs (cultured in the presence of 10% FCS) avoiding terminal differentiation and promoting proliferation12. Consistently, GBM is characterized by poorly differentiated cancer cells with high mitotic activity15. The terms differentiating cells or cells undergoing differentiation, refer to a population of proliferative astrocyte precursors without stellate morphologies and with low levels of stem cell markers and increased expression of GFAP astrocytic marker. It is also well known that TLR signaling pathways promote activation of NFκB16. Our first goal was to study the expression of TLR family members in differentiating GSCs. Based on our microarray gene expression analyses of GSCs before and after differentiation for 4 days12, TLR4 was the only TLR member that increased its levels in all five cell lines tested (Fig. 1a). Flow cytometry further confirmed that TLR4 protein reached the highest levels following differentiation compared with the expression of TLR1, TLR2, TLR5, TLR6 and TLR10 (Fig. 1b). Downregulation of stem cell markers and increased levels of the astrocyte-specific marker GFAP confirmed the differentiation process (Fig. 1c). Then, TLR4 protein levels were studied in seven different GBM cell lines before (GSCs) and after differentiation and all of them upregulated TLR4 following differentiation (Fig. 1d). A well known ligand of TLR4 is the bacterial lipopolysaccharide (LPS), which has been shown to transduce proliferation signals in GBM cells17,18. As expected, TLR4 expressed in differentiated cells was functional, responding to LPS by increasing the proliferation of these cells (Fig. 1e and f). In order to study the contribution of TLR4 to NFκB activation, we transfected GSCs with five different TLR4-specific shRNAs. Three of these shRNAs significantly reduced the levels of TLR4 protein (between 40% and 60%) in differentiating cells as analyzed by flow cytometry (Fig. 2a). Downregulation of TLR4 dramatically decreased (about 5-fold with shRNA-1) the transcriptional activity of NFκB after differentiation as determined by using NFκB -luciferase reporter assays (Fig. 2b) and a similar result was obtained when we treated GSCs with a TLR4 neutralizing antibody that blocked signaling (Fig. 2c). NFκB pathway is able to promote the expression of a number of genes, including cytokines. As shown in Fig. 2d, downregulation of TLR4 reduced the expression of NFκB target genes TNFα, IL-10 and IL-6. Interestingly, low levels of TLR4 were associated with morphological changes in differentiating GSCs giving rise to a population of cells (more than 60 out of 100 cells analyzed as compared with 15% in control cells) with elongated morphologies and some stellate-like projections suggestive of a more differentiated state (Fig. 2e). This result is consistent with that we previously described by treating GSCs undergoing differentiation with an inhibitor of NFκB12.
TLR4 is upregulated in differentiating GSCs. (a) Fold changes in the levels of TLR genes in differentiating compared with stem-like cells as assessed by gene expression microarray. (b) Flow cytometry analyses of TLRs. The expression in differentiating cells relative to stem-like cells is represented. (c) GSCs were induced to differentiate in the presence of serum for 4 days. The expression of progenitor (Nestin, Sox2, Nanog) and lineage (GFAP) markers was analyzed by qPCR. The expression levels were represented as fold changes of differentiating cells compared with GSCs. All markers showed significant differences (p < 0.01). (d) Expression levels of TLR4 protein in different GBM cell lines as determined by flow cytometry. Asterisks represent significant differences (*p < 0.05, **p < 0.01) compared to stem-like cells. (e) Proliferation and (f) Morphology of GSCs after 4 days of differentiation in the presence of LPS from E. coli strains O111:B4 (S1) and O55:B5 (S2). *p < 0.05. Histograms represent the mean ± SD of three independent experiments. Scale bar: 50 μm.
Downregulation of TLR4 blocks differentiation-induced activation of NFκB. (a) Protein levels of TLR4 are significantly reduced (**p < 0.01) in GBM cells with three out of five non-overlapping TLR4-specific shRNAs as determined by flow cytometry. (b,c) NFκB transcriptional activity following differentiation of GSCs transfected with TLR4-specific shRNAs (b) or cultured in the presence of TLR4 blocking antibodies (c). (d) Downregulation of TLR4 levels produced a decrease in the expression of NFκB target cytokines as determined by real-time PCR. (e) Morphological changes in GSCs with reduced levels of TLR4 after 4 days of differentiation. Scale bar: 50 μm. Histograms represent the mean ± SD of three independent experiments. **p < 0.01. a.u., arbitrary units.
Blockade of TLR4 promotes differentiation and reduces proliferation of GSCs
GSCs have the capacity to undergo differentiation to different neural lineages, mainly acquiring astrocytic (48–66%) and neuronal (22–45%) phenotypes12. In order to assess whether downregulation of TLR4 modified the differentiation pattern of GSCs, we first analyzed the expression of GFAP, an astrocytic differentiation marker, and found that low levels of TLR4 correlated with increased expression of GFAP (about 3-fold higher intensity with shRNA-1 relative to control) as determined by immunofluorescence (Fig. 3a and b). Transfection with the most efficient TLR4-specific shRNA reduced the levels of stem cell markers Sox2, Nanog and Nestin and increased the expression of GFAP in cells cultured under differentiation conditions (Fig. 3c). Additionally, we transfected GBM cells with the promoter region of the neuron-specific marker MAP2 linked to a luciferase reporter gene. Downregulation of TLR4 by shRNAs or functional blockade by specific anti-TLR4 antibodies highly increased the transcriptional activation of MAP2 in GSCs undergoing differentiation (Fig. 3d and e). In line with these results, the mRNA levels of MAP2 were also upregulated in differentiating cells following treatment with neutralizing anti-TL4 antibodies (Fig. 3f). Consistent with the acquisition of a mature phenotype following downregulation of TLR4, the proliferation capacity of GSCs was reduced more than 5-fold after 8 days of differentiation (Fig. 4a) as determined by the Alamar bioassay, a marker of metabolic activity frequently used to analyze cell proliferation. Another marker of proliferation, Ki67, was also significantly reduced in cells transfected with TLR4-specific shRNAs by counting the proportion of Ki67-positive cells (Fig. 4b and c). Two features of terminal maturation and senescence are the presence of megalonuclei19 and focal accumulation of phospho-histone H2AX at the sites of DNA double-strand breaks20. Consistently, when GSCs with low levels of TLR4 were differentiated for 8 days, the nuclear area was clearly increased (from about 30 μm2 to 60 μm2 with shRNA-1) and nuclei showed an accumulation of phospho-H2AX in approximately 24% of cells (Fig. 5a and b). Similar results but with more pronounced differences were obtained in cells treated with blocking anti-TLR4 antibodies for the same period of differentiation (Fig. 5c and d).
Downregulation of TLR4 accelerates differentiation of GSCs. (a,b) GSCs were transfected with the indicated TLR4-specific shRNAs and expression of GFAP after 8 days of differentiation was assessed by immunofluorescence. Fluorescence intensity was measured using ImageJ software. Scale bar: 20 μm. (c) Cells were transfected with control or TLR4-specific shRNAs and differentiated for 8 days. The expression of progenitor (Nestin, Sox2, Nanog) and lineage (GFAP) markers was analyzed by qPCR. The expression levels were represented as fold changes of cells transfected with TLR4 shRNA compared with control cells. All markers showed significant differences (p < 0.01). (d) Transcriptional activity of the MAP2 promoter in stem and differentiating cells following transfection with three TLR4-specific shRNAs. (e) Transcriptional activity of the MAP2 promoter in cells treated with blocking anti-TLR4 antibodies. (f) Expression levels of MAP2 mRNA in stem and differentiating cells treated with blocking antibody. Histograms represent the mean ± SD of three independent experiments. Asterisks in grey bars represent significant differences (*p < 0.05, **p < 0.01) compared to empty (control) bars. a.u., arbitrary units.
Proliferation of differentiating GSCs decreases following downregulation of TLR4. (a) GSCs were transfected with TLR4-specific shRNAs and proliferation was assessed at different time points of differentiation by using Alamar Blue reagent. a.u., arbitrary units. (b) Expression of the proliferation marker Ki67 and (c) quantification of Ki67-positive cells in GSCs with low levels of TLR4 following 5 days of differentiation. Cells were counterstained with DAPI. Histograms represent the mean ± SD of three independent experiments. Asterisks in grey bars represent significant differences (**p < 0.01) compared to empty (control) bars. Scale bar: 10 μm.
Differentiating GSCs acquire a senescent phenotype after downregulation of TLR4. (a) Detection of histone H2AX phosphorylation by immunofluorescence staining of GSCs transfected with TLR4-specific shRNAs or (c) treated with blocking anti-TLR4 antibodies after 5 days of differentiation. Histograms shown in (b) and (d) represent the nuclear area of DAPI-stained cells treated as described in (a) and (b) respectively. At least 20 nuclei were counted. Asterisks in grey bars represent significant differences (*p < 0.05, **p < 0.01) compared to empty (control) bars. Scale bar: 5 μm.
TLR4 signaling is triggered by hyaluronic acid released from differentiating GSCs
Once we have established that TLR4 is a main activator of the NFκB pathway during differentiation of GSCs, we asked about the ligand that triggers this signaling mechanism. Hyaluronic acid (HA) is a main component of the extracellular matrix in the brain21, and has been shown to signal through TLR422. We showed that exogenous HA increases the NFκB transcriptional activity in differentiating GSCs and this activation was lost following downregulation of TLR4 by shRNAs (Fig. 6a). We have previously described that activation of NFκB in differentiating GSCs promotes proliferation and avoids terminal maturation12. In support of this, it has been shown in a number of cell lines that GSCs cultured under differentiation conditions are fast-growing23. Additionally, the absence of fully differentiated morphology of tumor cells in GSC xenografts24 and the high proliferation rate of astrocytic precursors25, indicate that GBM tumor cell populations comprise of proliferative progenitor cells that have not completed pathways toward astrocytic differentiation. We showed that incubation of GSCs undergoing differentiation in the presence of HA increased proliferation, which was drastically reduced when TLR4 was downregulated (Fig. 6b). Moreover, GSCs synthesized and secreted HA to culture medium and these levels were increased (about 2.5-fold) after 4 days of differentiation (Fig. 6c). We have also induced differentiation by growth factor withdrawal, which tipically results in spontaneous multi-lineage differentiation of GSCs26. Although differentiating cells exhibited less pronounced changes of differentiation markers, HA levels increased near 2-fold when compared with GSCs (1.7 ± 0.2 mean ± SD, p < 0.05) (data not shown). However, if we block the production of HA with 4-Methylumbelliferone (4-MU), a specific HA synthesis inhibitor used in many in vitro studies27, the secretion of HA by differentiating cells was reduced to levels similar to those of GSCs (Fig. 6c). Consistently, 4-MU reduced the proliferation capacity (Fig. 6d) and the NFκB transcriptional activity (Fig. 6e) of differentiating GSCs. To further confirm this result, we showed that the NFκB target gene IL-6 was upregulated in cells undergoing differentiation but its levels were drastically reduced in the presence of the HA synthesis inhibitor (Fig. 6f). Thus, HA produced and secreted during differentiation of GSCs triggers the TLR4-NFκB signaling pathway either in a paracrine or an autocrine manner.
Hyaluronic acid triggers TLR4 signaling in differentiating GSCs. (a) Cells were cotransfected with the NFκB-luciferase reporter vector and TLR4-specific or control shRNAs in the presence or in the absence of hyaluronic acid (HA). Luciferase activity was determined after 48 h of incubation under stem or differentiation conditions. (b) Proliferation of cells in the presence of HA following downregulation of TLR4 as determined by Alamar blue. (c) HA levels present in the culture medium of stem and differentiating cells treated with the HA synthesis inhibitor, 4-MU by ELISA. (d) Proliferation of stem and differentiating cells following treatment with 4-MU. (e) Luciferase activity of cells transfected with the NFκB-reporter vector and treated with 4-MU. (f) Expression levels of IL-6 mRNA in differentiating GSCs treated with 4-MU by quantitative RT-PCR. Histograms represent the mean ± SD of three independent experiments. Asterisks represent significant differences (**p < 0.01). a.u., arbitrary units.
Discussion
GSCs have been identified as key cells for tumor initiation and recurrence, providing therapeutic opportunities28. We have previously demonstrated that canonical NFκB signaling, that is poorly activated in GSCs, is upregulated during differentiation of GSCs which allows cells to proliferate and avoid terminal differentiation and senescence12. This is consistent with previous work showing that canonical NFκB pathway has no or very little effect on GSCs29. In addition, activation of canonical NFκB has also been associated with mesenchymal transdifferentiation and radioresistance of a subset of GSCs30,31. However, the stimuli that trigger this activation in differentiating GSCs are not well defined. We showed that TLR4 was the only TLR member consistently upregulated in all differentiating GSC cultures established from surgical specimens of GBM patients. In line with this, it has been recently described that cancer stem cells isolated from patient-derived GBM xenografts showed a gradual increase in the expression of TLR4 under differentiation conditions13. TLR4 signaling results in activation of the NFκB pathway in astrocytes, microglia, neurons and neural progenitors32. Consistently, we demonstrated that TLR4 upregulation in differentiating GSCs was associated with increased NFκB transcriptional activity that was blocked by downregulating the levels of TLR4 or treatment with neutralizing TLR4 antibodies. Moreover, activation of the TLR4-NFκB axis in differentiating GSCs promoted proliferation of these cells and avoided terminal differentiation and senescence, a phenotype that characterizes GBM tumors. Experimental support of this phenotype comes from GSC xenograft models, that showed a clear absence of fully differentiated morphology indicating that tumor cell populations comprise of progenitor cells that have not completed astrocytic differentiation24. Previous data also showed that astrocytic precursors have a relatively high proliferation rate25. Consistent with our data, activation of the NFκB pathway is low in embryonic stem cells, but increases after differentiation6,33. However, it is not known whether this activation relies on a TLR4-mediated pathway. The low activation of canonical NFκB signaling in GSCs indicates that NFκB may be dispensable for survival and proliferation of stem cells, and this is consistent with a predominant accumulation of NFκB proteins in the cytoplasm of cells within neurospheres from embryonic mouse brain7. Recent data have shown that low TLR4 expression in GBM cancer stem cells allows these cells to survive by disregarding inflammatory signals13. Differentiated tumor cells would not have this adaptive advantage. However, on the other hand, a sustained activation of TLR4-NFκB will provide proliferation signals and some of the newly generated cells may develop biological mechanisms that enable them to migrate to more favorable microenvironments or survive to the immune attack. To this end, tumor heterogeneity is one of the most important hallmarks of GBM. Different cell subclones have been established from a GBM patient and all of them behave differently in terms of morphology, proliferation, response to treatment and aggressiveness in animal models34. NFκB has been shown to induce and also to inhibit differentiation indicating a broad spectrum of activities depending on the cell type35,36,37,38. We showed that blockade of TLR4-NFκB signaling by using genetic strategies or blocking antibodies accelerates maturation of differentiating GSCs. Previous data indicate a role of NFκB in terminal differentiation. For instance, activation of NFκB pathway induces cell division and inhibits differentiation of mammary epithelial cells39. Moreover, in vivo inhibition of NFκB signaling by using a small molecule induced sensescence of tumor cells in mice bearing GSC-derived tumors12.
At this point a key issue is how do GSCs undergoing differentiation activate the TLR4-NFκB pathway. It has been shown that hyaluronic acid (HA) is among the key organizers of the brain extracellular matrix21,40, and there are several examples showing that HA signals through TLR422,41. We found that HA promotes activation of NFκB through TLR4 in differentiating GSCs when added to cell cultures. However, we observed an increase in NFκB activity in GSCs maintained under differentiation conditions without exogenous HA. Interestingly, we demonstrated that GSCs produce and secrete their own HA and this production is highly increased in differentiating cells. Blockade of HA synthesis abrogated the TLR4-NFκB pathway indicating that GSCs develop their own mechanism to assure activation of NFκB during the differentiation process. This is consistent with previous work showing that a human GBM cell line synthesized and secreted HA into the medium42. These authors also found that HA synthesis by glioma cells occurred during the proliferative phase, which is in line with our model of differentiating GSCs where upregulation of TLR4 and secretion of HA by intermediate precursor cells activate the NFκB transcriptional pathway. NFκB promotes proliferation by inducing the expression of growth factors such as IL1β or IL643 and we showed that differentiating GSCs increased the levels of IL6 which were abrogated in the presence of a HA synthesis inhibitor. Thus, immature GBM cells may use this HA-TLR4-NFκB pathway as a mechanism to allow proliferation and avoid terminal differentiation and senescence. Although there are a number of studies demonstrating that HA signals via TLR441,44 and even suggesting HA-TLR4 interactions22, another study claimed that HA was not a ligand but a regulator of TLRs45. Two relevant issues may help explain these conflicting results. First, those studies that suggest a ligand-receptor scenario used HA fragments with molecular size above 100 kDa, whereas the study arguing that HA is a TLR4 regulator used HA fragments below 3 kDa and this is an important issue as HA biological activities differ widely depending on the fragment sizes46. Additionally, all these works used different cell models, including glomerular mesangial cells, macrophages, dendritic cells and endothelial cells which may have different responses to HA. Further biochemical studies will be needed to undoubtedly identify HA as a cognate TLR4 ligand. It would also be interesting to study the relevance of the HA-TLR4 pathway in other in vitro or in vivo models that provide a more physiological context. In this regard, 3D cultures such as the spheroid model have been used for evaluating drug efficay or response to antiangiogenic conditions47,48,49. However, the cellular heterogeneity of GBM may need more complex in vitro systems or in vivo xenograft models to more closely mimic the native tumor microenvironment.
Overall, we have demonstrated that TLR4 is upregulated during the differentiation process of GSCs and that TLR4 promotes activation of the canonical NFκB pathway in these cells. TLR4-NFκB signaling is mainly orchestrated by HA that is synthesized and secreted by differentiating GSCs. Our results provide new findings for therapeutic opportunities aimed to promote terminal differentiation of GSCs through inhibition of the NFκB signaling pathway.
References
Eyler, C. E. & Rich, J. N. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol 26, 2839–2845 (2008).
Shay, J. W. & Roninson, I. B. Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene 23, 2919–2933 (2004).
Kawamata, H., Tachibana, M., Fujimori, T. & Imai, Y. Differentiation-inducing therapy for solid tumors. Curr Pharm Des 12, 379–385 (2006).
Campos, B. et al. Differentiation therapy exerts antitumor effects on stem-like glioma cells. Clin Cancer Res 16, 2715–2728 (2010).
Naugler, W. E. & Karin, M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev 18, 19–26 (2008).
Torres, J. & Watt, F. M. Nanog maintains pluripotency of mouse embryonic stem cells by inhibiting NFkappaB and cooperating with Stat3. Nat Cell Biol 10, 194–201 (2008).
Young, K. M., Bartlett, P. F. & Coulson, E. J. Neural progenitor number is regulated by nuclear factor-kappaB p65 and p50 subunit-dependent proliferation rather than cell survival. J Neurosci Res 83, 39–49 (2006).
Widera, D., Mikenberg, I., Elvers, M., Kaltschmidt, C. & Kaltschmidt, B. Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-kappaB signaling. BMC Neurosci 7, 1–18 (2006).
Napetschnig, J. & Wu, H. Molecular basis of NF-kappaB signaling. Annual review of biophysics 42, 443–468 (2013).
Chen, X. et al. Significance of TLR4/MyD88 expression in breast cancer. Int J Clin Exp Pathol 8, 7034–7039 (2015).
Huang, B. et al. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res 65, 5009–5014 (2005).
Nogueira, L. et al. Blockade of the NFkappaB pathway drives differentiating glioblastoma-initiating cells into senescence both in vitro and in vivo. Oncogene 30, 3537–3548 (2011).
Alvarado, A. G. et al. Glioblastoma Cancer Stem Cells Evade Innate Immune Suppression of Self-Renewal through Reduced TLR4 Expression. Cell Stem Cell 20, 450–461 (2017). e454.
Gutierrez, O. et al. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-kappa B activation. J Biol Chem 277, 41701–41705 (2002).
Ohgaki, H. et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res 64, 6892–6899 (2004).
Kawai, T. & Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med 13, 460–469 (2007).
Che, F. et al. TLR4 interaction with LPS in glioma CD133+ cancer stem cells induces cell proliferation, resistance to chemotherapy and evasion from cytotoxic T lymphocyte-induced cytolysis. Oncotarget 8, 53495–53507 (2017).
Han, S., Wang, C., Qin, X., Xia, J. & Wu, A. LPS alters the immuno-phenotype of glioma and glioma stem-like cells and induces in vivo antitumor immunity via TLR4. J Exp Clin Cancer Res 36, 83 (2017).
Gupta, S. Hepatic polyploidy and liver growth control. Semin Cancer Biol 10, 161–171 (2000).
Gire, V., Roux, P., Wynford-Thomas, D., Brondello, J. M. & Dulic, V. DNA damage checkpoint kinase Chk2 triggers replicative senescence. EMBO J 23, 2554–2563 (2004).
Ruoslahti, E. Brain extracellular matrix. Glycobiology 6, 489–492 (1996).
Jiang, D. et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11, 1173–1179 (2005).
Galli, R. et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res 64, 7011–7021 (2004).
Ehtesham, M. et al. Glioma tropic neural stem cells consist of astrocytic precursors and their migratory capacity is mediated by CXCR4. Neoplasia 6, 287–293 (2004).
Kleiderman, S. et al. Functional and phenotypic differences of pure populations of stem cell-derived astrocytes and neuronal precursor cells. Glia 64, 695–715 (2016).
Pollard, S. M. et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell 4, 568–580 (2009).
Kultti, A. et al. 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp Cell Res 315, 1914–1923 (2009).
Cheng, L., Bao, S. & Rich, J. N. Potential therapeutic implications of cancer stem cells in glioblastoma. Biochem Pharmacol 80, 654–665 (2010).
Sun, S. C. The noncanonical NF-kappaB pathway. Immunol Rev 246, 125–140 (2012).
Kim, S. H. et al. Serine/Threonine Kinase MLK4 Determines Mesenchymal Identity in Glioma Stem Cells in an NF-kappaB-dependent Manner. Cancer Cell 29, 201–213 (2016).
Bhat, K. P. L. et al. Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell 24, 331–346 (2013).
Okun, E., Griffioen, K. J. & Mattson, M. P. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci 34, 269–281 (2011).
Kang, H. B., Kim, Y. E., Kwon, H. J., Sok, D. E. & Lee, Y. Enhancement of NF-kappaB expression and activity upon differentiation of human embryonic stem cell line SNUhES3. Stem Cells Dev 16, 615–623 (2007).
Soeda, A. et al. The evidence of glioblastoma heterogeneity. Sci Rep 5, 7979 (2015).
Pevsner-Fischer, M. et al. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood 109, 1422–1432 (2007).
Li, X. & Makarov, S. S. An essential role of NF-kappaB in the “tumor-like” phenotype of arthritic synoviocytes. Proc Natl Acad Sci USA 103, 17432–17437 (2006).
Zhang, M. Y., Sun, S. C., Bell, L. & Miller, B. A. NF-kappaB transcription factors are involved in normal erythropoiesis. Blood 91, 4136–4144 (1998).
Vaira, S. et al. RelA/p65 promotes osteoclast differentiation by blocking a RANKL-induced apoptotic JNK pathway in mice. J Clin Invest 118, 2088–2097 (2008).
Gonzalez-Suarez, E. et al. RANK overexpression in transgenic mice with mouse mammary tumor virus promoter-controlled RANK increases proliferation and impairs alveolar differentiation in the mammary epithelia and disrupts lumen formation in cultured epithelial acini. Mol Cell Biol 27, 1442–1454 (2007).
Oohashi, T., Edamatsu, M., Bekku, Y. & Carulli, D. The hyaluronan and proteoglycan link proteins: Organizers of the brain extracellular matrix and key molecules for neuronal function and plasticity. Exp Neurol 274, 134–144 (2015).
Taylor, K. R. et al. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem 279, 17079–17084 (2004).
Wiranowska, M. et al. Modulation of hyaluronan production by CD44 positive glioma cells. Int J Cancer 127, 532–542 (2010).
Chen, F., Castranova, V. & Shi, X. New insights into the role of nuclear factor-kappaB in cell growth regulation. Am J Pathol 159, 387–397 (2001).
Termeer, C. et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med 195, 99–111 (2002).
Ebid, R., Lichtnekert, J. & Anders, H. J. Hyaluronan is not a ligand but a regulator of toll-like receptor signaling in mesangial cells: role of extracellular matrix in innate immunity. ISRN Nephrol 2014, 714081 (2014).
Stern, R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol 83, 317–325 (2004).
Gong, X. et al. Generation of Multicellular Tumor Spheroids with Microwell-Based Agarose Scaffolds for Drug Testing. PLoS One 10, e0130348 (2015).
Carbonell, W. S., DeLay, M., Jahangiri, A., Park, C. C. & Aghi, M. K. beta1 integrin targeting potentiates antiangiogenic therapy and inhibits the growth of bevacizumab-resistant glioblastoma. Cancer Res 73, 3145–3154 (2013).
Pozo, N. et al. Inhibition of DYRK1A destabilizes EGFR and reduces EGFR-dependent glioblastoma growth. J Clin Invest 123, 2475–2487 (2013).
Acknowledgements
This work was supported by Instituto de Salud Carlos III (Spanish Ministry of Science and Innovation) grants RD12/0036/0022 (Red Temática de Investigación Cooperativa en Cáncer), and PI1301760, and grant APG/03 from Fundacion Instituto de Investigacion Valdecilla (IDIVAL).
Author information
Authors and Affiliations
Contributions
J.L.F.-L. conceived the idea, designed experiments and wrote the manuscript. E.F., O.G. and D.S.S. did the experiments. All authors have reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ferrandez, E., Gutierrez, O., Segundo, D.S. et al. NFκB activation in differentiating glioblastoma stem-like cells is promoted by hyaluronic acid signaling through TLR4. Sci Rep 8, 6341 (2018). https://doi.org/10.1038/s41598-018-24444-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24444-6
This article is cited by
-
Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments
Molecular Cancer (2023)
-
Toll-like receptors and toll-like receptor-targeted immunotherapy against glioma
Journal of Hematology & Oncology (2021)
-
ADO/hypotaurine: a novel metabolic pathway contributing to glioblastoma development
Cell Death Discovery (2021)
-
Late p65 nuclear translocation in glioblastoma cells indicates non-canonical TLR4 signaling and activation of DNA repair genes
Scientific Reports (2021)
-
Dissecting and rebuilding the glioblastoma microenvironment with engineered materials
Nature Reviews Materials (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.