Abstract
Tumor growth, which plays a central role in cancer evolution, depends on both the internal features of the cells, such as their ability for unlimited duplication, and the external conditions, e.g., supply of nutrients, as well as the dynamic interactions between the two. A stem cell theory of cancer has recently been developed that suggests the existence of a subpopulation of self-renewing tumor cells to be responsible for tumorigenesis, and is able to initiate metastatic spreading. The question of abundance of the cancer stem cells (CSCs) and its relation to tumor malignancy has, however, remained an unsolved problem and has been a subject of recent debates. In this paper we propose a novel model beyond the standard stochastic models of tumor development, in order to explore the effect of the density of the CSCs and oxygen on the tumor’s invasive behavior. The model identifies natural selection as the underlying process for complex morphology of tumors, which has been observed experimentally, and indicates that their invasive behavior depends on both the number of the CSCs and the oxygen density in the microenvironment. The interplay between the external and internal conditions may pave the way for a new cancer therapy.
Similar content being viewed by others
Introduction
Cancer usually begins with out-of-order duplication of a single cell that has stem cell-like behavior, referred to as the cancer stem cell (CSC)1. Based on the CSC hypothesis, a CSC can duplicate without limit and differentiate2. The classical CSC hypothesis proposes that, among all cancerous cells, only “a few” act as stem cells, but studies have reported3 that a relatively high proportion of the cells are tumorigenic, contradicting the general belief. The CSCs have been proposed as the driving force for tumorigenesis and the seeds for metastases4. Their decisive role in maintaining capacity for malignant proliferation, invasion, metastasis, and tumor recurrence has been reported frequently5. For example, CSCs of breast tumor are involved in spontaneous metastases in mouse models6. Moreover, CSCs promote the metastatic and invasive ability of melanoma7 and their prsence is correlated with invasive behavior at colorectal adenocarcinoma8. The effect of the number of CSCs on tumor morphology has been the subject of several experimental studies and simulation. Based on simulations9,10, the frequency of the CSCs smoothens the morphology of tumor, and based on an experimental study11, the number of CSCs is higher in tumors with medium invasiveness (the so-called Gleason grade) than tumors with lower (Gleason grade) and higher (Gleason grade) invasiveness. The relation between tumor malignancy and the frequency of the CSCs needs, however, more clarification4.
Cancerous cells use oxygen to produce metabolites for duplication and growth12. Experimental in-vivo13 and in-vitro14 studies, as well as computer simulations15,16, have reported that the density of oxygen regulates tumor morphology and its shortage drives morphological irregularities. Due to the apparent strong correlations between the tumors’ shape and their malignancy, fractal characterization of tumors has been used as a diagnostic assay for various types of tumors17,18,19. However, there is still no explanation as to why cellular structures at the scale of tumors display self-similar characteristics20. of well-known physical phenomena, including diffusion and reaction-diffusion, as well as percolation, surface growth, and models of phase transition21.
In this paper we propose a novel model to study the effect of the number of the CSCs and the oxygen’s density on the invasive behavior of a general type of cancer. As we show below, the development of irregular shapes and the respective tumor’s invasive behavior are correlated with the two factors. Unlike the previous studies, we present a quantitative measure by which one can understand better the effect of completion on the malignancy of tumors. We take the shape irregularity as the factor for identifying the invasive behavior of tumor and compare our results with experimental reports. The model that we present contains the essential features of the cells, such as symmetric/asymmetric division, metabolic state, cellular quiescence and movements, apoptosis, and existence of oxygen and its consumption. Our results explain, for the first time to our knowledge, the aforementioned experimentally-observed fractal behavior and contradict the predictions of recent models for the relation between the number of the CSCs and the growth rate and invasion. In addition, we believe that the results may cast doubt on the recent therapeutic approach based on oxygen deprivation.
Results
As the system evolves, the cells consume oxygen, enhance their metabolic state, and proliferate after reaching the energy level of u p , in order to create a clone - the tumor - see Fig. 1. The perimeter of the clone is the main object that we study in this paper.
Fractal structure of the tumors. Tumors are irregular, but exhibit self-similarity. The linearity of the plot indicates fractal behavior, with the slope being D f ≈ 1.99 ± 0.01 for p s = 0.1 (left), 1.76 ± 0.02 for p s = 0.5 (middle), and 1.47 ± 0.02 for p s = 1 (right), with (normalized) oxygen density, n = 1. Each contour line represents the borderline of the tumor with the corresponding gyration radius indicated by the dotted arrows. It should be noted that the figure on the left covers 5000 units (50 mm2) in 5000 time steps (30 days), while the ones in the middle and right cover the same area in 14000 (55 days) and 12000 (83 days) steps, respectively. The simulations were carried out in a 200 × 200 lattice
As Fig. 1 demonstrates, the cells take on irregular shapes during their growth whose complexity depends on the number of the CSCs (or probability p s ). One interesting approach is to study the structure of the perimeters in the context of interface instability22,23,24. The analogy with the instability of interfaces has been established for the case of melanoma25, and the instabilities were attributed to nutrient density. But, here, we quantify tumor behavior through classifying irregular morphology of the tumors. To quantify the irregularity of the tumor’s morphology and its evolution, we use fractal analysis. To this end, we measure the average distance r from the center of the mass, as well as the area of the tumor during its growth. Figure 1 indicates that log(area) versus log r is a linear plot so that, \({\rm{area}}\sim \,{r}^{{D}_{f}}\). Thus, the slope of the line in the logarithmic plot is the fractal dimension D f , implying self-similarity of the tumors of various sizes. The self-similarity of the tumors’ growth is the result of heterogeneous duplication on their perimeter, which itself is due to the oxygen gradient. Cells in the region with higher curvatures have better supply of oxygen, helping them increase their metabolic state, and proliferate faster. The proliferation also creates new perimeter curvature with the same behavior. As the number of oxygen consumers, which is proportional to p s , increases the competition between the cells for the limited oxygen supply intensifies and oxygen availability becomes more heterogeneous. Thus, the tumors take on more irregular shapes or lower fractal dimension D f , contradicting previous studies9,10 that proposed an adverse relation between the number of the CSCs and the invasive behavior.
We note that fractal scaling has been reported previously in the experimental studies17,18. Moreover, irregular shapes have been interpreted as an indication of invasive behavior of various tumors17,18,19. Tumors with more irregular shapes are more invasive, and in our model the more irregular tumors have smaller D f . There are several reports that confirm the correlation between D f and tumor malignancy (a malignant tumor possesses a lower fractal dimension than that of a benign mass)26,27,28,29.
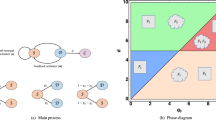
A study of the variations of D f with p s and the density n of the oxygen is useful to characterization of the tumor behavior. The computed D f for various values of p s and oxygen densities is shown in Fig. 2.
Interrelationship between malignancy, immortality and oxygen density. Fractal dimension D f as an indication of malignancy for various tumors. Our model reproduces some of the previously reported fractal dimensions: [i] \({D}_{f}\sim 1.338\pm 0.248\)30; [ii] 1.46 ¡ D f ¡ 1.6429; [iii] \({D}_{f}\sim 1.696\pm 0.009\); [iv] 1.74 ¡ D f ¡ 1.8531, and [v] \({D}_{f}\sim 1.887\pm 0.008\)32.
Figure 2 presents explicitly the value of D f and the corresponding malignancy of tumor as a result of both the internal feature and the external conditions. For a fixed density n of oxygen, the invasive behavior of tumor always increases with p s , implying that, regardless of the environmental conditions, higher numbers of CSCs always lead to a more invasive behavior; see Fig. 2 in the Supplementary Information (SI). This is in contradiction with the existing reports on the adverse effect of p s on the tumor’s invasive behavior9,10. On the other hand, the effect of the environmental stress on invasion is regulated by internal feature of the cells, p s . For p s = 1, the oxygen deprivation significantly increases the malignant behavior of tumors, while for p s = 0, the density of oxygen has negligible effect on tumor’s invasive behavior.
Relation to Superficial Spreading Melanoma
As presented here, our model explains a two-dimensional (2D) tumor growth. Early stages of Superficial Spreading melanoma has a 2D structure that might be a promising case to apply our findings to. Experiments indicate that there is no blood flow to the Superficial Spreading melanoma (SSM) with thickness less than 0.9 mm33. In addition, melanoma is, at least in its early stages, an approximately 2D phenomenon, so that a 2D model may properly produce its structure. The malignant cells in SSM stay within the original tissue - the epidermis - in an in-situ phase for a long time, which could be up to decades. Initially, the SSM grows horizontally on the skin surface, known as radial growth, with lesion indicated by a slowly-enlarging flat area of discolored skin. Then, part of the SSM becomes invasive, crossing the base membrane and entering the dermis, giving rise to a rapidly-growing nodular melanoma within the SSM that begins to proliferate more deeply within skin.
Discussion
The proposed model sheds new light on and provides new insight into the invasive behavior of tumors by deciphering the effect of both intrinsic and extrinsic features of cells. It also demonstrates that elimination of the oxygen in the previous models gives rise to such a relation. The fractal behavior that we identify and attribute to the growth limited to the perimeter is similar to surface growth17,34. Nevertheless, close inspection of the proliferation activity in the perimeter in the proposed model reveals larger parts of the cells as proliferative cells; see Fig. 1 of the SI. As the model demonstrates, a single biological parameter, namely p s , changes the cell’s features and results collectively in various self-similar states with distinct fractal dimensions. Previous models, which considered the CSCs9,10, obtained an inverse relation between the number of the CSCs and invasion, but our model indicates increased malignancy to be proportional to larger numbers of the CSCs. Compared to experimental data11 our model confirms increasing of morphological irregularities (Gleason grade), but complete consistency require more biological details in the model.
Tumors with low number of the CSCs that were proposed by the previous studies9,35 did not respond to oxygen deprivation, as was expected13,14. Hence, tumors that respond to oxygen deprivation must have larger number of the CSCs. In addition, models that do not consider the CSC evolution and endow the cells with unlimited proliferation capacity14,15 produce tumors corresponding to p s = 1. Such models consider the effect of oxygen and, as our model confirms, oxygen deprivation leads to higher irregularities. As p s decreases, the effect of oxygen vanishes. Thus, a lower number of the CSCs, which was proposed previously9,35, does not conform to the experimentally well-established oxygen effects. Our model, in addition to reproducing such result, provides quantitative and comparable results to classify the irregularities that can be used to analyze experimental data that have been reported for the fractal dimensions.
The conceptual results are applicable to the growth of other solid tumors that display the aforementioned behavior in response to oxygen tension and frequency of CSCs. For example, in the case of the SSM in which the number of CSCs is not small3,36, oxygen deprivation probably increases tumor malignancy. Contrary to the previous studies, the present model predicts invasion as the result of both the tumor and the microenvironment, demonstrating the effect of nutrient deprivation on the invasion. This implies that recent studies on such therapeutic approach37,38 must consider carefully the side effects that, based on our model for tumors with larger numbers of the CSCs, can increase tumor malignancy.
The Model
Similar to many other natural systems, biological media fluctuate due to the intrinsic randomness of the individual events39. Cells are involved in regulatory pathways that depend highly nonlinearly on the chemical species that are present in low copy numbers per cell40, as a result of which other factors, such as the forces between cells, fluctuate significantly41. Thus, statistical approaches are suitable for simulating cells’ behavior. We consider the 2D lattice shown in Fig. 3 in which each bond is 100 micrometer long, while each site has the capacity for 100 cancer cells that typically have 10 μm diameter42. The nutrient density is constant on the perimeter of a circle with a radius of 1 cm. It diffuses into the internal zones and is consumed by the living cells. In the SI we present the results for various other initial/boundary conditions for the oxygen supply, including smaller and larger radii of the circle, regular and random distribution of the oxygen source, as well as its uniform distribution in the medium, and show that the predictions of the model do not depend on the choice of the oxygen supply mechanism. Though we consider 2D structures, the results for a 3D structure for oxygen supply system (vessels and capillaries) would remain qualitatively the same, while the model can be extended to 3D.
Schematic of the model. (a) Various types of cells that are either proliferating or dying. Nutrient density in the milieu is constant and after diffusing from the surrounding is consumed by the cells. (b) An alternative mechanism for oxygen supply by the capillaries coming from the third dimension to feed the tumor at random sites. The results do not depend on the choice of the initial/boundary conditions for the nutrient; see the SI.
Keeping the oxygen density uniform in the milieu −0.15 mol/ml16 - a CSC is inserted at the center of the medium that consumes the oxygen and enhances its metabolic state. Although metabolic pathways are not fully understood, metabolic activity is a crucial factor in a cell’s decision to either proliferate or die43. In the former case a cell must increase its biomass and replicate its genome prior to division, in order to create two daughter cells. Thus, the cell must generate enough energy and acquire or synthesize biomolecules at a sufficient rate to meet the proliferation demand44. Given such biological facts, we choose metabolic state as the decisive factor for a cell’s decision to proliferate, and define an internal energy ucell for each cell as an indicator of its metabolic state. Physically, the cells acquire energy from the environment to accumulate internal energy45 - the energy of the absorbed molecules - which evolves according to the energy conservation law:
where n(x, y, t) is the oxygen density at position (x, y) and time t, with χ and γ being positive constants related to energy accumulation and consumption rate (for details of all the constants and their values see Table 1 in the SI). If a cell’s energy reaches a threshold u p , it will begin duplication. We set u p , χ and γ such that every cell in the appropriate situation will be in the duplication state after 15 hours46, which is about the time that tumor cells need to reach the so-called cell checkpoints eG1 (early G1), G1 and eS in the cell cycle for division. G1 is the primary point at which a cell must decide whether to divide. After it passes G1 and enters the S phase, the cell is committed to division46 (other checkpoints, such as G2 at which the cell is mostly concerned with the condition of its DNA, still remain to be completed in the next step). As we show below, Eq. (1) together with the limits imposed, reproduces cell plasticity and various proliferation activities under a variety of external conditions47 that were reported recently46. Time is measured in units of 10 minutes.
The evolution of the internal energy ucell of the cells depends on the local density of oxygen through a set of coupled differential equations, and if enough oxygen exists at the position of the first CSC, ucell increases to u p and the first CSC duplicates into two daughter cells. This relation between oxygen density, cell metabolic state and its duplication dynamics ensures the apparent role of the oxygen density in the tumor evolution. One may consider various scenarios for quantitative studies of the CSC proliferation48,49,50,51, but the probability of distinct kinds of divisions has yet to be assessed experimentally. Besides, some other studies52 have proposed the cells’ self-renewal ability as the prerequisite for tumor maintenance. Thus, we choose the simplest biologically-correct model that has the ability to generate the entire possible range of the CSC population percentage, from zero up to the values produced by the various mathematical48,49,50,51 and biological models52. In this model, during duplication of each CSC one daughter cell is assumed to be CSC, while the second one is either a CSC with probability p s - the probability of symmetric duplication of the CSCs - or a cancerous cell (CC) with probability (1 − p s ); see Fig. 4. Each CC duplicates into two CCs if it is allowed to10. Such a probabilistic approach is motivated by the fact stated earlier, that according to the classical CSC hypothesis, among all cancerous cells, only “a few” act as stem cells, whereas some studies3,53 have reported that the population of CSCs can be relatively high, which is why we take the population of the CSCs (with probability p s ) as a parameter of our model. For p s = 1 the model reduces to the stochastic model of tumor development54. Every CSC continues such a division for an unlimited frequency, but the CC can have only limited generations of duplication55, which we set it to be g = 51,10 after which it will die and produce dead cells (DCs); see Fig. 4. As the cells undergo apoptosis, they are recognized and removed from the body by phagocytes. Thus, we assume that the dead cells remain inactive in the medium, but even if we eliminate them after death, the main results remain the same; see the Fig. S15 in the SI.
Division of the cells. During division each CSC creates another CSC. The other daughter cell would either be a CSC with probability p s or a CC with probability 1 − p s . Each CC creates two CCs during duplication, if it is capable of division. The CSCs can continue the division process for a long time, whereas each CC loses its ability for duplicating after g divisions, and dies. Clearly, the first CC daughters could duplicate g − 1 times, where we set g = 510.
We define the density of cells of type i at location (x, y) at time t by,
with i ≡ CSCs, CCs, and DCs. Equation (2) is also valid for the total density of cells, C t = CCSC + CCC + CDC. Recall also that the capacity of each site is 100 cells42. The density of the CCs is denoted by CCC(x, y, t; j) in which j indicates their generation that varies from 1 to g (after g generations they produce the DCs). Healthy tissues contain healthy cells in which the distribution of the nutrients is in a steady state. We eliminate the healthy cells for all the tumors, as our results are based on comparison with and differences of tumors’ behavior that are the most important part of our study.
Local density gradients drive the stochastic motion of the cells56. Thus, one has,
where D is the diffusion coefficient. Equation (3) is applicable to the various kinds of cells, for which16,57 D ≈ 10−10 cm2/s. Population growth of biological groups depends on the species ability for proliferation and the environmental limitations. One important environmental limit is contact inhibition of cell division58, i.e., if after the energy rises to u p , the cells will duplicate if there is space; otherwise, they will stay quiescent until they find space for duplication59. Thus, proliferation at each site depends on the number of cells that can duplicate, and the effect of competition for space between all types of cells. The evolution of the CSCs that qualifies for the duplication metabolic threshold u p , is expressed by a diffusion-reaction equation,
where R m is the rate of passing the S, G2 and M phases in the cell cycle, which is fixed as a cell that has enough internal energy (has passed the aforementioned eG1, G1 and eS phases) will duplicate in 5 hours46, if there were no other cells. The last term on the right side of Eq. (5) that includes the term [1 − C t (x, y, t)] captures the effect of contact inhibition of proliferation in which C t (x, y, t) is the total density of all cells at (x, y, t). The entire cell cycle takes 20 h. The evolution of the jth generation of the CCs is governed by
where δ ij denotes the Kronecker delta, i.e., δ ij = 1 for i = j and 0 otherwise, with 1 ≤ i, j ≤ g. The first term on the right side of Eq. (5) represents diffusion of the cells due to the local concentration gradient;16,56 the second is the creation of the first generation of the CCS due to asymmetric duplication of the CSCs10, while the third term represents the creation of the jth generation (for j ≠ 1) of the CCs from duplication of the prior generation. The concentration of the CCs decreases due to duplication and creation of the next generation, which the 4th terms accounts for, while the last term takes into account the death of the final (gth) generation of the CCs. R a is the rate of apoptosis - the process of programmed cell death - and is fixed as the gth generation has a halflife equal to 1 day. Finally, the evolution of the oxygen density in the presence of the cells is governed by
with α being proportional to oxygen consumption rate by the cells, which is the same for both the CCs and cancerous stem cells. We varied the rates of oxygen consumption for every kind of cells, but the essential results remained the same; see the SI. α was fixed by setting the reported value for oxygen consumption16,60 to be 6.65 × 10−17 mol cell−1 s−1. β is the diffusion coefficient of oxygen in the medium, which we fixed it based on the calculations at room temperature, 10−5 cm2/s. We present in the SI the results for other values of β. For distances more than 1 cm from the medium’s center the oxygen density is constant (see the SI for the results for larger and smaller distances, as well as other ways of supplying the oxygen), and is equal to 0.15 mol/ml16. For simplicity, in all the calculations we normalize n to 1. From outside of the aforementioned circle, oxygen penetrates into the central area. Given the assumptions, the cells are active elastic species, consuming oxygen and proliferating.
As we show in the SI, other boundary conditions do not change the essential results. In addition, (i) we also varied both the proliferation activity and oxygen consumption rate for various kinds of cells, but the results remained qualitatively the same. (ii) The CSCs and CCs are assumed to have equal oxygen consumption rates, but when we changed them for every kind of cell, the results were qualitatively the same. (iii) The CSCs and CCs are assumed to have the same internal energy threshold u p for duplication, and equal rates of crossing the S, G2 and M phases in the cell cycle. But changing the proliferation activity of the cells did not change our main results. Let us also emphasize that our model is not the same as the classical models of diffusion-limited aggregation61, as such model did not deal with the effect of reaction and consumption.
References
Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. Stem cells, cancer, and cancer stem cells. nature 414, 105–111 (2001).
Beck, B. & Blanpain, C. Unravelling cancer stem cell potential. Nature Reviews Cancer 13, 727–738 (2013).
Quintana, E. et al. Efficient tumour formation by single human melanoma cells. Nature 456, 593–598 (2008).
Medema, J. P. Cancer stem cells: the challenges ahead. Nature cell biology 15, 338–344 (2013).
Li, S. & Li, Q. Cancer stem cells and tumor metastasis. International journal of oncology 44, 1806–1812 (2014).
Liu, H. et al. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proceedings of the National Academy of Sciences 107, 18115–18120 (2010).
Lin, X. et al. Notch4+ cancer stem-like cells promote the metastatic and invasive ability of melanoma. Cancer science 107, 1079–1091 (2016).
Choi, D. et al. Cancer stem cell markers cd133 and cd24 correlate with invasiveness and differentiation in colorectal adenocarcinoma. World journal of gastroenterology: WJG 15, 2258 (2009).
Enderling, H., Hlatky, L. & Hahnfeldt, P. Cancer stem cells: a minor cancer subpopulation that redefines global cancer features. Breast 11, 200 (2013).
Sottoriva, A. et al. Cancer stem cell tumor model reveals invasive morphology and increased phenotypical heterogeneity. Cancer research 70, 46–56 (2010).
Castellón, E. A. et al. Molecular signature of cancer stem cells isolated from prostate carcinoma and expression of stem markers in different gleason grades and metastasis. Biological research 45, 297–305 (2012).
Vaupel, P., Kallinowski, F. & Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer research 49, 6449–6465 (1989).
Höckel, M. et al. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer research 56, 4509–4515 (1996).
Cristini, V. et al. Morphologic instability and cancer invasion. Clinical Cancer Research 11, 6772–6779 (2005).
Anderson, A. R., Weaver, A. M., Cummings, P. T. & Quaranta, V. Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell 127, 905–915 (2006).
Anderson, A. R. A hybrid mathematical model of solid tumour invasion: the importance of cell adhesion. Mathematical Medicine and Biology 22, 163–186 (2005).
Brú, A. et al. Super-rough dynamics on tumor growth. Physical Review Letters 81, 4008 (1998).
Caldwell, C. B. et al. Characterisation of mammographic parenchymal pattern by fractal dimension. Physics in medicine and biology 35, 235 (1990).
Lee, T. K. & Claridge, E. Predictive power of irregular border shapes for malignant melanomas. Skin Research and Technology 11, 1–8 (2005).
Baish, J. W. & Jain, R. K. Fractals and cancer. Cancer research 60, 3683–3688 (2000).
Tracqui, P. Biophysical models of tumour growth. Reports on Progress in Physics 72, 056701 (2009).
Vasiev, B. N. Classification of patterns in excitable systems with lateral inhibition. Physics Letters A 323, 194–203 (2004).
Vasiev, B., Hogeweg, P. & Panfilov, A. Simulation of dictyostelium discoideum aggregation via reaction-diffusion model. Physical Review Letters 73, 3173 (1994).
Vasieva, O., Vasiev, B., Karpov, V. & Zaikin, A. A model of dictyostelium discoideum aggregation. Journal of theoretical biology 171, 361–367 (1994).
Amar, M. B., Chatelain, C. & Ciarletta, P. Contour instabilities in early tumor growth models. Physical review letters 106, 148101 (2011).
Tambasco, M., Eliasziw, M. & Magliocco, A. M. Morphologic complexity of epithelial architecture for predicting invasive breast cancer survival. Journal of translational medicine 8, 140 (2010).
Etehad Tavakol, M., Lucas, C., Sadri, S. & Ng, E. Analysis of breast thermography using fractal dimension to establish possible difference between malignant and benign patterns. Journal of Healthcare Engineering 1, 27–43 (2010).
Zook, J. M. & Iftekharuddin, K. M. Statistical analysis of fractal-based brain tumor detection algorithms. Magnetic Resonance Imaging 23, 671–678 (2005).
Pérez, J. L. et al. Relationship between tumor grade and geometrical complexity in prostate cancer. bioRxiv 015016 (2015).
Smitha, K., Gupta, A. & Jayasree, R. Fractal analysis: fractal dimension and lacunarity from mr images for differentiating the grades of glioma. Physics in medicine and biology 60, 6937 (2015).
Pribic, J. et al. Fractal dimension and lacunarity of tumor microscopic images as prognostic indicators of clinical outcome in early breast cancer. Biomarkers 9, 1279–1277 (2015).
Buczko, O. & Mikołajczak, P. Shape analysis of mr brain images based on the fractal dimension. Annales Universitatis Mariae Curie-Sklodowska, sectio AI–Informatica 3, 153–158 (2015).
Srivastava, A., Laidler, P., Hughes, L. E., Woodcock, J. & Shedden, E. J. Neovascularization in human cutaneous melanoma: a quantitative morphological and doppler ultrasound study. European Journal of Cancer and Clinical Oncology 22, 1205–1209 (1986).
Brú, A., Albertos, S., Subiza, J. L., Garca-Asenjo, J. L. & Brú, I. The universal dynamics of tumor growth. Biophysical journal 85, 2948–2961 (2003).
Hermann, P. C. et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell stem cell 1, 313–323 (2007).
Girouard, S. D. & Murphy, G. F. Melanoma stem cells: not rare, but well done. Laboratory investigation 91, 647–664 (2011).
Tang, X. et al. Cystine deprivation triggers programmed necrosis in vhl-deficient renal cell carcinomas. Cancer research 76, 1892–1903 (2016).
Li, H. et al. Dt-13, a saponin monomer of dwarf lilyturf tuber, induces autophagy and potentiates anti-cancer effect of nutrient deprivation. European Journal of Pharmacology (2016).
Hilfinger, A. & Paulsson, J. Separating intrinsic from extrinsic fluctuations in dynamic biological systems. Proceedings of the National Academy of Sciences 108, 12167–12172 (2011).
Berg, O. G., Paulsson, J. & Ehrenberg, M. Fluctuations and quality of control in biological cells: zero-order ultrasensitivity reinvestigated. Biophysical journal 79, 1228–1236 (2000).
Trepat, X. et al. Physical forces during collective cell migration. Nature physics 5, 426–430 (2009).
Wang, Y. et al. Fiber-laser-based photoacoustic microscopy and melanoma cell detection. Journal of biomedical optics 16, 011014–011014 (2011).
Buchakjian, M. R. & Kornbluth, S. The engine driving the ship: metabolic steering of cell proliferation and death. Nature reviews Molecular cell biology 11, 715–727 (2010).
Jones, R. G. & Thompson, C. B. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes & development 23, 537–548 (2009).
Scalerandi, M. & Sansone, B. C. Inhibition of vascularization in tumor growth. Physical review letters 89, 218101 (2002).
Haass, N. K. et al. Real-time cell cycle imaging during melanoma growth, invasion, and drug response. Pigment cell & melanoma research 27, 764–776 (2014).
Meacham, C. E. & Morrison, S. J. Tumour heterogeneity and cancer cell plasticity. Nature 501, 328–337 (2013).
Shahriyari, L. & Komarova, N. L. Symmetric vs. asymmetric stem cell divisions: an adaptation against cancer? PLoS One 8, e76195 (2013).
Dhawan, A., Kohandel, M., Hill, R. & Sivaloganathan, S. Tumour control probability in cancer stem cells hypothesis. PloS one 9, e96093 (2014).
Tomasetti, C. & Levy, D. Role of symmetric and asymmetric division of stem cells in developing drug resistance. Proceedings of the National Academy of Sciences 107, 16766–16771 (2010).
Cao, Y., Naveed, H., Liang, C. & Liang, J. Modeling spatial population dynamics of stem cell lineage in wound healing and cancerogenesis. In Engineering in Medicine and Biology Society (EMBC), 2013 35th Annual International Conference of the IEEE, 5550–5553 (IEEE, 2013).
Yoo, M.-H. & Hatfield, D. L. The cancer stem cell theory: is it correct? Molecules and cells 26, 514 (2008).
Gedye, C. et al. Cancer stem cells are underestimated by standard experimental methods in clear cell renal cell carcinoma. Scientific reports 6, 25220 (2016).
Nowell, P. C. The clonal evolution of tumor cell populations. Science 194, 23–28 (1976).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Experimental cell research 25, 585–621 (1961).
Ambrosi, D. & Preziosi, L. On the closure of mass balance models for tumor growth. Mathematical Models and Methods in Applied Sciences 12, 737–754 (2002).
Bray, D. Cell movements: from molecules to motility (Garland Science, 2001).
Martz, E. & Steinberg, M. S. The role of cell-cell contact in “contact” inhibition of cell division: A review and new evidence. Journal of cellular physiology 79, 189–210 (1972).
Montel, F. et al. Stress clamp experiments on multicellular tumor spheroids. Physical review letters 107, 188102 (2011).
Casciari, J. J., Sotirchos, S. V. & Sutherland, R. M. Variations in tumor cell growth rates and metabolism with oxygen concentration, glucose concentration, and extracellular ph. Journal of cellular physiology 151, 386–394 (1992).
Gerlee, P. & Anderson, A. R. Diffusion-limited tumour growth: simulations and analysis. Mathematical biosciences and engineering: MBE 7, 385 (2010).
Acknowledgements
A.A.S. would like to acknowledge support from the Alexander von Humboldt Foundation, and partial financial support from the research council of the University of Tehran. We also acknowledge the High Performance Computing center of the University of Tehran in its Department of Physics, where most of computations were carried out. We thank an anonymous referee for constructive criticisms that guided us to revise and improve the manuscript.
Author information
Authors and Affiliations
Contributions
A.A.S. proposed the project and computations. Y.A. did the simulations. Y.A., A.A.S. and M.S. analyzed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Azimzade, Y., Saberi, A.A. & Sahimi, M. Role of the Interplay Between the Internal and External Conditions in Invasive Behavior of Tumors. Sci Rep 8, 5968 (2018). https://doi.org/10.1038/s41598-018-24418-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24418-8
This article is cited by
-
Invasion front dynamics of interactive populations in environments with barriers
Scientific Reports (2022)
-
Invasion front dynamics in disordered environments
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.