Abstract
We conducted a systematic review and meta-analysis to investigate whether the use of statins could be associated with the risk of all-caused dementia, Alzheimer’s disease (AD), vascular dementia (VaD), and mild cognitive impairment (MCI). Major electronic databases were searched until December 27th, 2017 for studies investigating use of statins and incident cognitive decline in adults. Random-effects meta-analyses calculating relative risks (RRs) were conducted to synthesize effect sizes of individual studies. Twenty-five studies met eligibility criteria. Use of statins was significantly associated with a reduced risk of all-caused dementia (k = 16 studies, adjusted RR (aRR) = 0.849, 95% CI = 0.787–0.916, p = 0.000), AD (k = 14, aRR = 0.719, 95% CI = 0.576–0.899, p = 0.004), and MCI (k = 6, aRR = 0.737, 95% CI = 0.556–0.976, p = 0.033), but no meaningful effects on incident VaD (k = 3, aRR = 1.012, 95% CI = 0.620–1.652, p = 0.961). Subgroup analysis suggested that hydrophilic statins were associated with reduced risk of all-caused dementia (aRR = 0.877; CI = 0.818–0.940; p = 0.000) and possibly lower AD risk (aRR = 0.619; CI = 0.383–1.000; p = 0.050). Lipophilic statins were associated with reduced risk of AD (aRR = 0.639; CI = 0.449–0.908; p = 0.013) but not all-caused dementia (aRR = 0.738; CI = 0.475–1.146; p = 0.176). In conclusion, our meta-analysis suggests that the use of statins may reduce the risk of all-type dementia, AD, and MCI, but not of incident VaD.
Similar content being viewed by others
Introduction
As the life expectancy is getting longer worldwide, the number of people affected by cognitive decline and dementia is steadily increasing1. Dementia is an age-related neurodegenerative disease, characterized by a progressive decline in cognitive function often encompassing several domains (e.g. memory, attention, language, and problem solving)2. According to the World Health Organization (WHO) estimates, the number of individuals affected by dementia is expected to triple by 2050, with the rapid aging of populations globally [http://www.who.int/ageing/en/]. Meanwhile, the individual, societal and healthcare costs associated with dementia are steeply increased3. Unsurprisingly, the prevention of cognitive decline and dementia is a worldwide public health issue4.
Recently, interest has arisen in the potential for statins to delay cognitive decline in people with older age. Statins are drugs that inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase5. Statins not only lower serum cholesterol levels, but also inhibit pivotal enzymatic reactions (e.g. the isoprenylation of a subset of GTPases)6 that lead to amyloid deposition and plaque formation; both are considered cornerstone pathways underpinning the development of Alzheimer’s disease (AD)7. Epidemiological data have supported a possible association between the use of statins and the risk of dementia, but evidence appears controversial. Some observational studies have demonstrated that statins reduce the risk of dementia or incident AD8,9,10,11,12,13,14,15,16,17, whereas several others have failed to replicate those findings18,19,20,21,22,23,24,25. Several possible factors may contribute to discrepant findings across studies. First, statins might exhibit neuroprotective effects limited only to earliest stages of AD8,26. Some studies have shown that statins exert more robust protective effects upon cognition for subjects younger than 80 at baseline13,14. Second, the degree of exposure (i.e. time and dose of statins use) could influence outcomes14,15. Third, clinicians might not be willing to prescribe statins for patients with pre-existing poor cognitive status due to concerns about possible treatment-related side effects, and lesser benefit because of expected diminished life expectancy27,28. Fourth, ApoE genotype might alter the association between use of statins and incidence of dementia. One recent study has shown that statins may be more beneficial in AD patients with homozygous ApoE4 genotypes29. Finally, the effects of confounders may limit inferences from observational studies30, and hence may vary depending on both the specific population being examined and potential confounding variables that are controlled for in multivariable analysis.
Several meta-analyses of observational studies have previously been conducted to examine the potential role of statins on incident mild cognitive impairment (MCI) or dementia, but have provided conflicting results thus far31,32,33,34,35,36. A meta-analysis including case-control and cohort studies demonstrated statins use did not confer a protective effect on the risk of dementia or AD35. However, another meta-analysis of 8 prospective cohort studies found that the use of statins was associated with a significantly reduced relative risks (RRs) of dementia by 39%32. In the most recent study published in 2013, Richardson et al. found a reduced RR for all-caused dementia, AD, or MCI based on observational studies, while 16 cohort studies were available when this previous meta-analysis was conducted37. The majority of these meta-analyses have focused solely on statins use and the risk of all-caused dementia and AD. Only one meta-analysis had examined a possible effect in reducing the incidence of MCI37 and to our knowledge, no previous meta-analyses has separately assessed the risk of vascular dementia (VaD). Several new reports (including new studies which included different (i.e. Asian) populations38,39 have been published in more recent years36,38,39,40,41. In addition, possibly due tolimited evidence no previous meta-analysis has explored potential sources of heterogeneity across studies.
Therefore, the current systematic review and meta-analysis aims to reappraise available evidence from prospective studies, which investigated whether statins use could diminish incident all-caused dementia, AD, VaD, and MCI. In addition, due to the anticipated larger current evidence base, we aimed to explore potential sources of heterogeneity across studies.
Results
Studies Included in the Meta-analysis
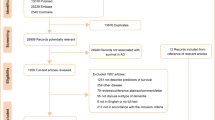
Overall, 3,824 unique references were identified after database searching, while 27 were identified from other sources. Of those, 2402 were excluded after title/abstract screening because they are not related to this meta-analysis; the main target of current meta-analysis aimed to discuss the association between statins and dementia. Total 238 articles were scrutinized, and 213 were excluded with reasons (see Supplementary material Tables S1 and S2). Finally, 25 articles met inclusion criteria. The flow chart of the search strategy and results is depicted in Fig. 1. The mean total numbers of covariates adjusted were 7.40 (Standard deviation [SD]: 3.38) and mean duration of follow-up periods were 6.95 (SD: 5.39) years.
In total, twenty-five articles met the inclusion criteria and were included in the current systematic review and meta-analysis (Table 1)8,9,10,13,16,18,19,21,22,23,25,36,38,40,41,42,43,44,45,46,47,48,49,50,51. Table 1 provides characteristics of included studies.
Among included studies, 16 studies investigated the association of statins use and incident all-caused dementia (N = 2,745,149, mean age 59.3 years, male = 67.3%, incident cases = 35,688), 14 studies assessed the association of statins use and incident AD (N = 52,218, mean age 71.3 years, male = 45.3%, incident cases = 3120), 3 studies investigated VaD (N = 5,987, mean age 77.2 years, male = 42.5%, incident cases = 422), and 6 studies investigated MCI (N = 6,808, mean age 68.4 years, male = 33.2%, incident cases of four studies9,36,42,48 = 359), respectively. Across those 25 studies, Supplementary material Table S3 provides a list of potential confounders considered in the multivariable models in each study, and the total number of covariates considered in the most fully adjusted risk estimate (e.g. aRRs) in each included study.
Methodological Quality Assessment
The average NOS were 7.72 with interquartile range (IQR) (7.00–8.00). The score in each domain of the NOS is provided in Supplementary material Table S4.
Statins and Incident All-caused Dementia
Across 16 studies8,9,18,21,22,25,38,40,41,42,43,44,46,47,49,50, participants who received statins were significantly less likely to develop all-caused dementia compared to those who were not treated with statins (aRR from 16 studies = 0.849, 95% CI = 0.787–0.916, p < 0.001) (Fig. 2). There was no evidence of publication bias according to Egger’s test (t value = 1.816, df = 14, p = 0.091). However, heterogeneity was verified (Q value = 29.778, df = 15, I2 = 49.627, p = 0.013).
Sources of Heterogeneity
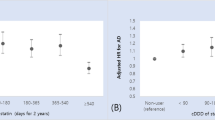
Subgroup analysis showed the use of hydrophilic statins were associated reduced risk of all-caused dementia (aRRs = 0.877; CI = 0.818–0.940; p < 0.001), whereas the use of lipophilic statins were not associated with this outcome (aRR = 0.738; CI = 0.475–1.146; p = 0.176) (Fig. 3A). Regarding status of statins use (current and former users), lower risk of all-caused dementia was found among current users (aRR = 0.828; CI = 0.692–0.990; p = 0.039) but not in former users (aRR = 1.125, CI = 0.818–1.547, p = 0.470) (Fig. 3B). In the meta-regression analyses, mean age, percentage of male, education in years, study duration, percentage of cardiovascular, cerebrovascular disease, DM, HTN, smoking, ApoE4 status, BMI > 25, cholesterol > 200 mg/dl, total Newcastle scores, and total number of covariates used in the multivariate analyses did not seem to contribute to heterogeneity. The percentage of white population (slope = 0.005; p = 0.006) and most notably the percentage of cholesterol > 200 mg/dl (slope = 0.018; p = 0.009) emerged as significant moderators of this outcome (Supplementary material Table S5).
Subgroup analyses. (A) The use of hydrophilic compared to lipophilic statins and incident all-caused dementia; (B) current versus former statins users and incident all-caused dementia; and (C)The use of hydrophilic compared to lipophilic statins and incident Alzheimer’s Disease. Squares depict individual studies and diamonds depict summary effect size estimates (aRRs).
Statins and Incident Alzheimer’s Disease
Across 14 studies8,10,13,16,18,19,21,22,23,25,38,41,42,51, participants who were treated with statins were significantly less likely to develop AD compared to those who were not treated with statins (aRR from 14 studies = 0.719, 95% CI = 0.576–0.899, p = 0.004). There was evidence of publication bias through Egger’s test was observed (t value = 2.307, df = 12, p = 0.039) (Fig. 4A). We used Duval and Tweedie’s trim and fill procedure toward the right to adjust effect size estimates to publication bias and the results remained significant (aRR = 0.814, 95% CI = 0.713–0.930). In addition, heterogeneity was large (Q value = 28.779, df = 13, I2 = 54.828, p = 0.007). Therefore, potential sources of heterogeneity were explored.
Sources of Heterogeneity
Subgroup analyses showed that the use of lipophilic statins was associated reduced risk of AD (aRR = 0.639; CI = 0.449–0.908; p = 0.013), while the use of hydrophilic statins reduced the risk of incident AD at the statistical trend level (aRRs = 0.619; CI = 0.383–1.000; p = 0.050) (Fig. 3C). We could not perform the association between statins use (current and former users) and the risk of AD because of recruited studies less than 3. In meta-regression analyses, the percentage of white participants (slope = 0.006; p = 0.047), study duration (slope = −0.063; p = 0.033), and percentage of apoE4 > or = 1 (apoE4 carriers) (slope = −0.042; p = 0.044) emerged as significant moderators of outcomes (Supplementary material Table S5).
Statins use and Incident Vascular Dementia
Across 3 studies8,22,23, no significant difference in the incidence of VaD was observed between participants with statins treatment and those without (aRR = 1.012, 95% CI = 0.620–1.652, p = 0.961). There was evidence of publication bias through Egger’s regression (t value = 17.932, df = 1, p = 0.035). We used Duval and Tweedie’s trim and fill procedure toward left to adjust effect size estimates to publication bias and the results remained non-significant (aRR = 0.720, 95% CI = 0.428–1.210). No significant heterogeneity was found (Q value = 3.256, df = 2, I2 = 38.581, p = 0.196) (Fig. 4B).
Statin use and Incident MCI
Across 6 studies9,16,36,42,45,48, participants who were using statins were significantly less likely to develop MCI compared with those who did not receive statins (aRR = 0.737, 95% CI = 0.556–0.976, p = 0.033). There was evidence of publication bias as indicated by Egger’s regression (t value = 4.051, df = 4, p = 0.015). We used Duval and Tweedie’s trim and fill procedure toward the right to adjust effect size estimates to publication bias and the results tend to insignificance (aRR = 0.923, 95% CI = 0.713–1.193). Additionally, there was significant heterogeneity was found (Q value = 12.330, df = 5, I2 = 59.449, p = 0.031). Therefore, potential sources of heterogeneity were explored.
Sources of Heterogeneity
In meta-regression analyses, the percentage of male proportion (slope = −0.008; p = 0.022) and number of covariables (slope = −0.114; p = 0.011) emerged as significant moderators of outcomes (Supplementary material Table S5).
Adverse Events
None of studies, except one43, reported information regarding side effects during follow-up period. Hippisley-Cox et al.43 reported that the use several statins including simvastatin, atrovastatin, fluvastatin, pravastatin, and rosuvastatin increased the risk of liver dysfunction (simvastatin 10–20 mg/day, for women, adjusted HR [aHR] = 1.47, 95% CI = 1.32–1.63, for men, aHR = 1.35, 95% CI = 1.25–1.54), myopathy (simvastatin 10–20 mg/day, for women, aHR = 2.91, 95% CI = 2.19–3.88, for men, aHR = 6.12, 95% CI = 4.97–7.55), acute renal failure (simvastatin 10–20 mg/day, for women, aHR = 1.38, 95% CI = 1.10–1.74, for men, aHRs = 1.39, 95% CI = 1.14–1.70), and cataract (simvastatin 10–20 mg/day, for women, aHR = 1.30, 95% CI = 1.24–1.36, for men, aHR = 1.32, 95% CI = 1.24–1.39).
Discussion
The current meta-analysis of 25 cohort studies provides the most comprehensive meta-analytic evidence regarding the effects of statins on incident all-caused dementia, AD, VaD, and MCI. We found that statins users, without baseline cognitive dysfunction, had a significantly reduced risk of developing all-caused dementia, AD, and MCI. Statins use was associated with a 15.1%, 28.1%, and 26.3% lowered risk of developing all-caused dementia, AD, and MCI respectively. Nevertheless, the few studies available provided no evidence that statins use could prevent the onset of VaD. In addition, the larger body of data included in the current meta-analysis compared to previous similar efforts37,52 allowed a more accurate exploration of potential sources of heterogeneity.
The current meta-analysis suggests that statins may offer a more significant preventative benefit for neurodegenerative dementing illness such as AD than for all-caused dementia31,32,33,34,37,53. Besides, both our study (aRR = 0.737, 95% CI = 0.556–0.976) and previous work by Richardson et al. found the statins appear to provide the stronger protective effects for incident MCI (aRR = 0.66, 95% CI: 0.51–0.86). However, compared to the Richardson study, we added two studies and enrolled more subjects in the present meta-analysis36,45. Nonetheless, the evidence base appears to consistently indicate that statins may protect against early stage cognitive decline, such as MCI8,9,36. A longitudinal study of 6,600 subjects with normal cognition or MCI even showed statins exert a cognitive protection among those with normal cognition at baseline but not MCI, indicating statins might exert benefit before MCI status54.
Importantly, this is the first meta-analysis to examine the association between statins use and risk of VaD, although no significant differences emerged (aRR = 1.012, 95% CI = 0.620–1.652, p = 0.961). Atherosclerosis is considered to be responsible for diffuse periventricular white matter abnormalities, which are one of major pathophysiological mechanisms of VaD55. We hypothesized that statins may reduce the development of VaD via alleviating or preventing cerebrovascular disease, which are the main risk factors for VaD56,57,58. However, the negative findings between VaD and statins from this meta-analysis might be due to the few number of included studies. Nevertheless, we considered adjust relative risks of individual studies. Therefore, it is possible that statins might not offer an independent preventative benefit for VaD. Besides, these studies did not account for important factors (such as plasma lipids or apolipoprotein E genotype)8,22,23, which might influence the results. Thus, clearly more research is required to consider if statins may confer a protective effect over future VaD.
The exact mechanism of statins on cognitive protection in older adults has not been elucidated but it might include pleiotropic effects on several mechanistic pathways. First, in animal models, statins could decrease cholesterol levels and attenuate formation of beta-amyloid (β-amyloid), which is main component of amyloid plaques in the brains of individuals with AD59. Besides, statins in preclinical models reduced brain cholesterol levels, leading to lower neurofibrillary tangles60. These effects could result in lower risk of dementia. The meta-regression analysis of present study could support this hypothesis, showing statins provide higher cognitive protective effect in studies with higher percentage of cholesterol >200 mg/dl (slope = 0.018, p = 0.009). Second, statins may exert anti-inflammatory effects in the brain. For example, statins significantly reduced β-amyloid-induced production of pro-inflammatory cytokines, such as interleukin (IL)-1beta, IL-6 and tumor necrosis factors-gamma (TNF-gamma) in the hippocampus61. This could be a relevant effect since accumulating evidence indicating that both peripheral immune activation and neuroinflammation are involved in the pathophysiology of AD. Third, statins exert cholesterol-independent effects (also referred to as pleiotropic effects), that showed antiproliferative and antithrombotic benefits and improved endothelial dysfunction, which plays import role in the initiation of inflammatory process-atherosclerosis62. Taken together, statins could attenuate the cognitive decline via, at least in partial, decreasing anti-inflammatory action, attenuate formation of beta-amyloid (β-amyloid) and neurofibrillary by lowering cholesterol levels, and pleiotropic effects.
Our subgroup analyses show that significant reduced risks of all-caused dementia and marginal significances of lower risk of AD were found for hydrophilic statins. In addition, lipophilic statins could reduce risks of AD but not all-caused dementia. The protective effects of statins on preventing the onset of dementia which can emerge as a function of their pharmacokinetic properties remains under debate, with some studies favoring lipophilic statins38 but other showing no difference between hydrophilic and lipophilic statins10. In the current meta-analysis, hydrophilic and lipophilic statins significantly decreased the incidence of all-caused dementia and AD, respectively. Studies had shown the lipophilic statins can more easily than hydrophilic statins cross the blood-brain barrier, thus potentially providing more robust benefits for the prevention of neurodegenerative disease63,64. Our results suggest that hydrophilic statins could exhibit more extensive protective effects than lipophilic statins in preventing all-caused dementia and possibly AD. However, these results should be interpreted cautiously. It is noteworthy that both forest plots of hydrophilic and lipophilic statins tended to the left, which means favor statins‘ preventive effect on all-caused dementia and AD. Although some results such as association between lipophilic statins and all-caused dementia and between hydrophilic statins and AD did not show significant differences, these make us not to reject the null hypothesis. Additionally, two recent epidemiological studies without non-statins comparator groups and therefore did not meet eligibility criteria for this study warrant mentioning. Zissimopoulos et al. used administrative claims data from Medicare beneficiaries to analyze the association between statin use with AD onset. They found lipophilic (simvastatin and atorvastatin) and hydrophilic (pravastatin and rosuvastatin) generally were associated with reduced risk of AD depend on different races and sex65. In contrast, Sinyavskaya et al. reported lipophic statins (simvastain, atorvastatin, fluvastatin) were not associated with decreased incident of AD compared to hydrophilic statins (pravastain, rosuvastatin), using data from the UK Clinical Practice Research Datalink cohort of patients aged 60 to 95 years who were followed for a median of 5.9 years66. Therefore, the association of statins and AD and all-caused dementia might vary across different properties of statins, sex, and race/ethnicity. Future intervention studies are needed to better study the differential effects of hydrophilic and lipophilic statins on preventing incidence of dementia or otherwise as novel neuroprotector agents for MCI and AD.
The meta-regression analysis showed several variables were associated with statins use and the relationship with the risk of all-caused dementia, AD and MCI. One of interesting findings was statins expressed lesser cognitive preventive effect in studies with higher percentage of white ethnicity in both all-caused dementia (slope = 0.005, p = 0.006) and AD (slope = 0.006, p = 0.047). Pharmacokinetic differences exist among different races67. Caucasian subjects tend to have lower plasma exposure to and its metabolites compared non-Caucasian subjects in several types of statins68,69, leading to lesser treatment effect. Nearly all of recruited studies are from Western countries. The sample sizes for other ethnicities are smaller, and the estimates might be less precisely measured. Future studies to address neuroprotective effects of statins in difference ethnicities are needed. In addition, apoE4 allele is a major genetic risk factor for both all-caused dementia and AD70. It is reasonable that apoE might alter the effects of statin on the cognition as apoE plays a critical role in the transport of cholesterol and fats to the brain71. Indeed, we did find significant negative association between presence of apoE4 and AD (slope = −0.042, p = 0.044), although not in all-caused dementia.
Some important cofounders should be considered. For example, studies have shown that different levels of social relationship72 and physical exercise73 might relate to the development of dementia. To determine the intensity of social involvement is challenge and lack of objective measures in the past. Currently, there is an increasing number of commercially available devices (wearable activity trackers), which provide researchers with secure and precise means to collect, and analysis data generated by health devices74. This wearable technology could be integrated into the future cohort studies to off real-time monitoring to improve dementia prevention and more person-centered care.
Several limitations should be addressed. First, the study is a pooled synthesis of observational studies which are susceptible to the effects of chance, bias and confounding and thus, results should be interpreted cautiously. Second, we excluded studies that considered cognitive function change as endpoint and did not discuss the clinical trajectory at every clinical assessment point until the last assessment. However, this is beyond the scope of current meta-analysis. Although the U.S. Food and Drug Administration (FDA) issued a statement regarding poor cognitive status associated with statin use in 2012, no convincing evidence so far support this warning75,76. Nevertheless, it would be potentially more informative to analysis all studies with investigating the effect of statins on the cognitive function change measured by objective tools. Future studies could be conducted to address this issue. Third, we only included peer-reviewed articles published in English language and thus it is possible that we might have missed some non-English studies. However, no publication bias was evident for almost all our outcomes. Fourth, meta-regression could not be performed for studies of VaD and MCI due to few datasets available. Fifth, none of the included studies used a propensity score or a matching criterion between statins users or not and so another potential bias could be present in our findings. In an attempt, to provide a better control of potential confounders we also performed meta-analyses considering the most fully adjusted outcome measures of individual studies. Finally, several diagnostic criteria for dementia (DSM, ICD, and NINCDS-ADRDA etc.) as well as different methods to assess the use of statins (electronic medical records, pharmacy records, and medication inspection etc.) across included studies may also limit the comparability and synthesis of studies included in this meta-analytic review.
Conclusions
The use of statins appears to have beneficial effects in reducing incidence of all-caused dementia, AD, and MCI, but not VaD. Subgroup analyses suggest that hydrophilic and lipophilic statins showed a beneficial effect in preventing all-caused dementia and AD, respectively. Thus, our data suggest that the use of statins may confer a potential benefit for the prevention of dementia.
Methods and Materials
The current systematic review and meta-analysis was in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines77 (Supplementary material Table S6). This systematic review and meta-analysis was approved by the Institutional Review Board of the Tri-Service General Hospital (TSGHIRB: B-105-12). Two investigators (CSC and PTT) have independently performed database searching, study selection, data extraction, and the rating of the methodological quality of included studies. Disagreements were resolved through consensus.
Database Searching
The PubMed, ScienceDirect, Psychology and Behavior Sciences Collection, ClinicalTrials.gov and Cochrane library were systematically searched from inception up until December 27th, 2017 (Supplementary material Table S7). The search string used for the electronic database search was provided in the Supplementary material. The search strategy was augmented through hand searching the reference lists of included articles, as well as previous reviews52,76 and meta-analysis37 on the topic.
Eligibility Criteria and Study Selection
The following eligibility criteria were applied: (1) prospective cohort studies investigating the effects of statins vs. participants not taking statins; (2) participants had to be cognitively healthy at baseline and without a history of cognitive dysfunction; (3) outcome measures had to include either incident all-caused dementia or otherwise specific-caused dementia (i.e. AD or VaD) or mild cognitive impairment (MCI) based on Diagnostic and Statistical Manual of Mental Disorders (DSM)2, International Classification of Diseases (ICD)78, National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA)79, diagnosis of MCI was based on Petersen’s, International Working Group, or National Institute on Aging-Alzheimer’s Association workgroups diagnostic systems80,81; (4) with a follow-up period longer than one year and (5) peer-reviewed article written in English language.
The exclusion criteria were: (1) cross-sectional studies; (2) retrospective case-control studies; (3) clinical trials (i.e. randomized controlled trial (RCT)); and (4) studies that did not assess incident dementia or MCI at endpoint; (5) conference abstracts and studies published in languages other than English.
Methodological Quality Assessment
We used the Newcastle-Ottawa Scale (NOS) to rate the methodological quality of included studies82. For prospective cohort studies, the NOS considers three domains: selection of participants (maximum of four stars), comparability of groups (maximum of two stars), and measurement (maximum of three stars). Scores were ranged from 0 (the lowest) to 9 (the highest), and a score higher or equal to 7 indicated high methodological quality.
Data Extraction
The primary outcomes were risk of all-caused dementia, AD, VaD, and MCI considering statins use as the exposure. Pooled estimates between statins use and dementia were calculated by using fully adjusted RRs (aRRs) provide in each publication (further details in meta-analysis section).
A pre-specified data extraction form was used to extract data for this meta-analysis. Data was extracted for each study included basic characteristics of participants (age, percentage of male, education in years, percentage of whites), study duration (follow-up time, in years), substance use (percentage of alcohol and smoking), prevalence of co-occurring medical conditions (cardiovascular, cerebrovascular, diabetes mellitus [DM], hypertension), percentage of > or = 1 Apolipoprotein E epsilon 4 (ApoE4) (ApoE4 carrier), cholesterol > 200 mg/dl and percentage of overweight/obese people (BMI > 25, (%)). Diagnosis of all-caused dementia, AD, and VaD were based on DSM-III-R10,25,42,50, DSM-IV9,13,18,21,38,39,41,51, NINCDS-ADRDA9,10,13,16,18,21,22,23,39,42,51, ICD-916,40, or ICD-10 41,49,50; some studies did not provide the criteria for interested outcome8,19,43,44,46,47; diagnosis of MCI was defined based on Petersen criteria42, probable prodromal AD16 or decline of specific cognitive decline (e.g. modified mini-mental state examination, Verbal Learning Test and Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) word list)9,36,45,48.
When data were not available in the articles, we electronically contacted the authors in at least two separate occasions to provide additional data.
Meta-analysis
All meta-analytic procedures were performed with the Comprehensive Meta-Analysis software, version 3 (Biostat, Englewood, NJ) software. For each meta-analysis, we considered the risk measure of each study which was most fully adjusted for potential confounders. We conducted the meta-analyses with random-effects models due to the anticipated heterogeneity across studies. Heterogeneity was assessed with the Cochran Q test and the I2 metric83. We changed ORs to RRs if data available in three recruited studies41,50,51 using the formula suggested by Cochrane Hanbook84. Therefore, the effect size (ES) and its 95% confidence interval [CI] was defined as RRs to indicate the difference of the incident rate of all-caused dementia (including AD, VaD, or MCI) as a function of statins use (exposure). A similar analysis was undertaken pooling the RR of each study, adjusted for the highest number of potential confounders available.
Subgroup analyses stratified by pharmacokinetic properties of statins (hydrophilic vs lipophilic), as previous studies, showed inconsistent results regarding the protective effect of hydrophilic and lipophilic statins on cognition10,38. Atovastatin, fluvastatin, pravastatin, and rosuvastatin are hydrophilic statins, whereas cerivastatin, lovastatin, and simvastatin are more lipophilic statins84,85. Besides, status of statins usage (current and former use) was undertaken. Subgroup analyses were performed when data from at least three datasets.
Publication bias was assessed via a visual inspection of funnel plots and by means of the Egger’s regression test86. Additionally, to account for publication bias, we used the trim-and-fill method, based on the assumption that the effect sizes of all the studies were normally distributed around the center of a funnel plot; in the event of asymmetry, this method adjusts for the potential effect of unpublished (trimmed) studies86. In addition, we conducted a meta-regression analysis using an unrestricted maximum likelihood method to explore potential moderators. The following variables were considered for meta-regression analyses: mean age, male gender (%), education (years), percentage of ethnicity, study duration (follow-up duration, in years), and prevalence comorbidities (cardiovascular, cerebrovascular, DM, and hypertension), percentage of presence of ApoE4 (ApoE4 carriers), total NOS scores, BMI > 25, cholesterol > 200 mg/dl and numbers of covariates adjusted. The meta-regression procedure was only undertaken when moderator variables were available from more than 5 individual studies. Statistical significance was set as two-tailed P value less than 0.05.
References
Prince, M. et al. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther 8, 23, https://doi.org/10.1186/s13195-016-0188-8 (2016).
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed (1994).
Wimo, A. et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 13, 1–7, https://doi.org/10.1016/j.jalz.2016.07.150 (2017).
Shah, H. et al. Research priorities to reduce the global burden of dementia by 2025. Lancet Neurol 15, 1285–1294, https://doi.org/10.1016/S1474-4422(16)30235-6 (2016).
McGuinness, B. & Passmore, P. Can statins prevent or help treat Alzheimer’s disease? J Alzheimers Dis 20, 925–933, https://doi.org/10.3233/JAD-2010-091570 (2010).
Ostrowski, S. M. et al. Simvastatin inhibits protein isoprenylation in the brain. Neuroscience 329, 264–274, https://doi.org/10.1016/j.neuroscience.2016.04.053 (2016).
Pedrini, S. et al. Modulation of statin-activated shedding of Alzheimer APP ectodomain by ROCK. PLoS Med 2, e18, https://doi.org/10.1371/journal.pmed.0020018 (2005).
Bettermann, K. et al. Statins, risk of dementia, and cognitive function: secondary analysis of the ginkgo evaluation of memory study. J Stroke Cerebrovasc Dis 21, 436–444, https://doi.org/10.1016/j.jstrokecerebrovasdis.2010.11.002 (2012).
Cramer, C., Haan, M. N., Galea, S., Langa, K. M. & Kalbfleisch, J. D. Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology 71, 344–350, https://doi.org/10.1212/01.wnl.0000319647.15752.7b (2008).
Haag, M. D., Hofman, A., Koudstaal, P. J., Stricker, B. H. & Breteler, M. M. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry 80, 13–17, https://doi.org/10.1136/jnnp.2008.150433 (2009).
Hajjar, I., Schumpert, J., Hirth, V., Wieland, D. & Eleazer, G. P. The impact of the use of statins on the prevalence of dementia and the progression of cognitive impairment. J Gerontol A Biol Sci Med Sci 57, M414–418 (2002).
Jick, H., Zornberg, G. L., Jick, S. S., Seshadri, S. & Drachman, D. A. Statins and the risk of dementia. Lancet 356, 1627–1631 (2000).
Li, G. et al. Age-varying association between statin use and incident Alzheimer’s disease. J Am Geriatr Soc 58, 1311–1317, https://doi.org/10.1111/j.1532-5415.2010.02906.x (2010).
Rockwood, K. et al. Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch Neurol 59, 223–227 (2002).
Rodriguez, E. G., Dodge, H. H., Birzescu, M. A., Stoehr, G. P. & Ganguli, M. Use of lipid-lowering drugs in older adults with and without dementia: a community-based epidemiological study. J Am Geriatr Soc 50, 1852–1856 (2002).
Sparks, D. L. et al. Reduced risk of incident AD with elective statin use in a clinical trial cohort. Curr Alzheimer Res 5, 416–421 (2008).
Zamrini, E., McGwin, G. & Roseman, J. M. Association between statin use and Alzheimer’s disease. Neuroepidemiology 23, 94–98, https://doi.org/10.1159/000073981 (2004).
Ancelin, M. L. et al. Lipid lowering agents, cognitive decline, and dementia: the three-city study. J Alzheimers Dis 30, 629–637, https://doi.org/10.3233/JAD-2012-120064 (2012).
Arvanitakis, Z. et al. Statins, incident Alzheimer disease, change in cognitive function, and neuropathology. Neurology 70, 1795–1802, https://doi.org/10.1212/01.wnl.0000288181.00826.63 (2008).
Benito-Leon, J., Louis, E. D., Vega, S. & Bermejo-Pareja, F. Statins and cognitive functioning in the elderly: a population-based study. J Alzheimers Dis 21, 95–102, https://doi.org/10.3233/JAD-2010-100180 (2010).
Li, G. et al. Statin therapy and risk of dementia in the elderly: a community-based prospective cohort study. Neurology 63, 1624–1628 (2004).
Rea, T. D. et al. Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch Neurol 62, 1047–1051, https://doi.org/10.1001/archneur.62.7.1047 (2005).
Reitz, C., Tang, M. X., Luchsinger, J. & Mayeux, R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol 61, 705–714, https://doi.org/10.1001/archneur.61.5.705 (2004).
Smeeth, L., Douglas, I., Hall, A. J., Hubbard, R. & Evans, S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol 67, 99–109, https://doi.org/10.1111/j.1365-2125.2008.03308.x (2009).
Zandi, P. P. et al. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry 62, 217–224, https://doi.org/10.1001/archpsyc.62.2.217 (2005).
Sparks, D. L. et al. Statin therapy in Alzheimer’s disease. Acta Neurol Scand Suppl 185, 78–86, https://doi.org/10.1111/j.1600-0404.2006.00689.x (2006).
Brauner, D. J., Muir, J. C. & Sachs, G. A. Treating nondementia illnesses in patients with dementia. JAMA 283, 3230–3235 (2000).
Glynn, R. J., Schneeweiss, S., Wang, P. S., Levin, R. & Avorn, J. Selective prescribing led to overestimation of the benefits of lipid-lowering drugs. J Clin Epidemiol 59, 819–828, https://doi.org/10.1016/j.jclinepi.2005.12.012 (2006).
Geifman, N., Brinton, R. D., Kennedy, R. E., Schneider, L. S. & Butte, A. J. Evidence for benefit of statins to modify cognitive decline and risk in Alzheimer’s disease. Alzheimers Res Ther 9, 10, https://doi.org/10.1186/s13195-017-0237-y (2017).
Hemkens, L. G. et al. Interpretation of epidemiologic studies very often lacked adequate consideration of confounding. J Clin Epidemiol 93, 94–102, https://doi.org/10.1016/j.jclinepi.2017.09.013 (2018).
Etminan, M., Gill, S. & Samii, A. The role of lipid-lowering drugs in cognitive function: a meta-analysis of observational studies. Pharmacotherapy 23, 726–730 (2003).
Song, Y., Nie, H., Xu, Y., Zhang, L. & Wu, Y. Association of statin use with risk of dementia: a meta-analysis of prospective cohort studies. Geriatr Gerontol Int 13, 817–824, https://doi.org/10.1111/ggi.12044 (2013).
Swiger, K. J., Manalac, R. J., Blumenthal, R. S., Blaha, M. J. & Martin, S. S. Statins and cognition: a systematic review and meta-analysis of short- and long-term cognitive effects. Mayo Clin Proc 88, 1213–1221, https://doi.org/10.1016/j.mayocp.2013.07.013 (2013).
Wong, W. B., Lin, V. W., Boudreau, D. & Devine, E. B. Statins in the prevention of dementia and Alzheimer’s disease: a meta-analysis of observational studies and an assessment of confounding. Pharmacoepidemiol Drug Saf 22, 345–358, https://doi.org/10.1002/pds.3381 (2013).
Zhou, B., Teramukai, S. & Fukushima, M. Prevention and treatment of dementia or Alzheimer’s disease by statins: a meta-analysis. Dement Geriatr Cogn Disord 23, 194–201, https://doi.org/10.1159/000099037 (2007).
Harding AC, S. C. Impact of statin use on cognitive decline in healthy women from a long-term longitudinal sample. Alzheimer’s & Dementia 13, 736–737 (2017).
Richardson, K. et al. Statins and cognitive function: a systematic review. Ann Intern Med 159, 688–697, https://doi.org/10.7326/0003-4819-159-10-201311190-00007 (2013).
Chen, J. M. et al. Effects of statins on incident dementia in patients with type 2 DM: a population-based retrospective cohort study in Taiwan. PLoS One 9, e88434, https://doi.org/10.1371/journal.pone.0088434 (2014).
Gnjidic, D. et al. Statin Therapy and Dementia in Older Adults: Role of Disease Severity and Multimorbidity. J Am Geriatr Soc 64, 223–224, https://doi.org/10.1111/jgs.13907 (2016).
Chitnis, A. S. et al. Use of Statins and Risk of Dementia in Heart Failure: A Retrospective Cohort Study. Drugs Aging 32, 743–754, https://doi.org/10.1007/s40266-015-0295-4 (2015).
Hendrie, H. C. et al. Statin Use, Incident Dementia and Alzheimer Disease in Elderly African Americans. Ethn Dis 25, 345–354, https://doi.org/10.18865/ed.25.3.345 (2015).
Beydoun, M. A. et al. Statins and serum cholesterol’s associations with incident dementia and mild cognitive impairment. J Epidemiol Community Health 65, 949–957, https://doi.org/10.1136/jech.2009.100826 (2011).
Hippisley-Cox, J. & Coupland, C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ 340, c2197, https://doi.org/10.1136/bmj.c2197 (2010).
Parikh, N. M. et al. Risk factors for dementia in patients over 65 with diabetes. Int J Geriatr Psychiatry 26, 749–757, https://doi.org/10.1002/gps.2604 (2011).
Schneider, E. B., Mielke, M. M., Yasar, S. & Carlson, M. C. Statin use is not associated with cognitive impairment in a cohort of older women. Alzheimer’s & Dementia 5, e13–e14 (2009).
Solomon, A., Soininen, H., Laatikainen, T., Tuomilehto, J. & Kivipelto, M. Statins and dementia prevention: A population-based study (FINRISK). Alzheimer’s & Dementia 5, 292 (2009).
Zigman, W. B. et al. Cholesterol level, statin use and Alzheimer’s disease in adults with Down syndrome. Neurosci Lett 416, 279–284, https://doi.org/10.1016/j.neulet.2007.02.023 (2007).
Yaffe, K., Barrett-Connor, E., Lin, F. & Grady, D. Serum lipoprotein levels, statin use, and cognitive function in older women. Arch Neurol 59, 378–384 (2002).
Wolozin, B. et al. Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med 5, 20, https://doi.org/10.1186/1741-7015-5-20 (2007).
Szwast, S. J. et al. Association of statin use with cognitive decline in elderly African Americans. Neurology 69, 1873–1880, https://doi.org/10.1212/01.wnl.0000279333.77404.d7 (2007).
Li, G. et al. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology 69, 878–885, https://doi.org/10.1212/01.wnl.0000277657.95487.1c (2007).
Ott, B. R. et al. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med 30, 348–358, https://doi.org/10.1007/s11606-014-3115-3 (2015).
Macedo, A. F. et al. Unintended effects of statins from observational studies in the general population: systematic review and meta-analysis. BMC Med 12, 51, https://doi.org/10.1186/1741-7015-12-51 (2014).
Steenland, K., Zhao, L., Goldstein, F. C. & Levey, A. I. Statins and cognitive decline in older adults with normal cognition or mild cognitive impairment. J Am Geriatr Soc 61, 1449–1455, https://doi.org/10.1111/jgs.12414 (2013).
Ryglewicz, D. et al. Plasma antioxidant activity and vascular dementia. J Neurol Sci 203-204, 195–197 (2002).
Heart Protection Study Collaborative. G. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360, 7–22, https://doi.org/10.1016/S0140-6736(02)09327-3 (2002).
Shepherd, J. et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 360, 1623–1630 (2002).
Sever, P. S. et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 361, 1149–1158, https://doi.org/10.1016/S0140-6736(03)12948-0 (2003).
Refolo, L. M. et al. A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Neurobiol Dis 8, 890–899, https://doi.org/10.1006/nbdi.2001.0422 (2001).
Kandiah, N. & Feldman, H. H. Therapeutic potential of statins in Alzheimer’s disease. J Neurol Sci 283, 230–234, https://doi.org/10.1016/j.jns.2009.02.352 (2009).
Zhang, Y. Y., Fan, Y. C., Wang, M., Wang, D. & Li, X. H. Atorvastatin attenuates the production of IL-1beta, IL-6, and TNF-alpha in the hippocampus of an amyloid beta1-42-induced rat model of Alzheimer’s disease. Clin Interv Aging 8, 103–110, https://doi.org/10.2147/CIA.S40405 (2013).
Oesterle, A., Laufs, U. & Liao, J. K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ Res 120, 229–243, https://doi.org/10.1161/CIRCRESAHA.116.308537 (2017).
Hawkins, B. T. & Davis, T. P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57, 173–185, https://doi.org/10.1124/pr.57.2.4 (2005).
Vuletic, S. et al. Statins of different brain penetrability differentially affect CSF PLTP activity. Dement Geriatr Cogn Disord 22, 392–398, https://doi.org/10.1159/000095679 (2006).
Zissimopoulos, J. M., Barthold, D., Brinton, R. D. & Joyce, G. Sex and Race Differences in the Association Between Statin Use and the Incidence of Alzheimer Disease. JAMA Neurol 74, 225–232, https://doi.org/10.1001/jamaneurol.2016.3783 (2017).
Sinyavskaya, L. et al. Comparative effect of statins on the risk of incident Alzheimer disease. Neurology. https://doi.org/10.1212/WNL.0000000000004818 (2017).
Mangravite, L. M., Thorn, C. F. & Krauss, R. M. Clinical implications of pharmacogenomics of statin treatment. Pharmacogenomics J 6, 360–374, https://doi.org/10.1038/sj.tpj.6500384 (2006).
Birmingham, B. K. et al. Rosuvastatin pharmacokinetics and pharmacogenetics in Caucasian and Asian subjects residing in the United States. Eur J Clin Pharmacol 71, 329–340, https://doi.org/10.1007/s00228-014-1800-0 (2015).
Birmingham, B. K. et al. Impact of ABCG2 and SLCO1B1 polymorphisms on pharmacokinetics of rosuvastatin, atorvastatin and simvastatin acid in Caucasian and Asian subjects: a class effect? Eur J Clin Pharmacol 71, 341–355, https://doi.org/10.1007/s00228-014-1801-z (2015).
Poirier, J. et al. Apolipoprotein E and lipid homeostasis in the etiology and treatment of sporadic Alzheimer’s disease. Neurobiol Aging 35(Suppl 2), S3–10, https://doi.org/10.1016/j.neurobiolaging.2014.03.037 (2014).
Chouinard-Watkins, R. & Plourde, M. Fatty acid metabolism in carriers of apolipoprotein E epsilon 4 allele: is it contributing to higher risk of cognitive decline and coronary heart disease? Nutrients 6, 4452–4471, https://doi.org/10.3390/nu6104452 (2014).
Kuiper, J. S. et al. Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev 22, 39–57, https://doi.org/10.1016/j.arr.2015.04.006 (2015).
Barreto, P. S., Demougeot, L., Vellas, B. & Rolland, Y. Exercise training for preventing dementia, mild cognitive impairment, and clinically meaningful cognitive decline: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. https://doi.org/10.1093/gerona/glx234 (2017).
Murphy, J., Holmes, J. & Brooks, C. Measurements of Daily Energy Intake and Total Energy Expenditure in People with Dementia in Care Homes: The Use of Wearable Technology. J Nutr Health Aging 21, 927–932, https://doi.org/10.1007/s12603-017-0870-y (2017).
Bitzur, R. Remembering Statins: Do Statins Have Adverse Cognitive Effects? Diabetes Care 39, S253–259 (2016).
Samaras, K. et al. Metabolic Burden and Disease and Mortality Risk Associated with Impaired Fasting Glucose in Elderly Adults. J Am Geriatr Soc 63, 1435–1442, https://doi.org/10.1111/jgs.13482 (2015).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6, e1000100, https://doi.org/10.1371/journal.pmed.1000100 (2009).
International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). https://www.cdc.gov/nchs/icd/icd9cm.htm.
McKhann, G. et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944 (1984).
Petersen, R. C. et al. Aging, memory, and mild cognitive impairment. Int Psychogeriatr 9(Suppl 1), 65–69 (1997).
Albert, M. S. et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 270–279, https://doi.org/10.1016/j.jalz.2011.03.008 (2011).
Wells G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Available: www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2011).
Huedo-Medina, T. B., Sanchez-Meca, J., Marin-Martinez, F. & Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 11, 193–206, https://doi.org/10.1037/1082-989X.11.2.193 (2006).
Dale, K. M., White, C. M., Henyan, N. N., Kluger, J. & Coleman, C. I. Impact of statin dosing intensity on transaminase and creatine kinase. Am J Med 120, 706–712, https://doi.org/10.1016/j.amjmed.2006.07.033 (2007).
Tan, P. et al. Effect of statins type on incident prostate cancer risk: a meta-analysis and systematic review. Asian J Androl 19, 666–671, https://doi.org/10.4103/1008-682X.190327 (2017).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Acknowledgements
The review was based on the work supported by the grants: MOST 105–2314-B-182A-057 - from the Ministry of Science and Technology in Taiwan.
Author information
Authors and Affiliations
Contributions
C.S.C. and P.T.T. prepared the manuscript. T.Y.C., C.H.T., D.J.L., W.C.Y., Y.W.C. and C.K.W. conceived and designed the study. P.T.T. and P.Y.L. analyzed the data. B.S., N.V., A.F.C., B.S.F. and N.H. critically read the manuscript and made important suggestions. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chu, CS., Tseng, PT., Stubbs, B. et al. Use of statins and the risk of dementia and mild cognitive impairment: A systematic review and meta-analysis. Sci Rep 8, 5804 (2018). https://doi.org/10.1038/s41598-018-24248-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24248-8
This article is cited by
-
Statins and cognitive decline in patients with Alzheimer’s and mixed dementia: a longitudinal registry-based cohort study
Alzheimer's Research & Therapy (2023)
-
Re: Yang et al. Statins and post-stroke dementia
European Journal of Epidemiology (2023)
-
Simvastatin rescues memory and granule cell maturation through the Wnt/β-catenin signaling pathway in a mouse model of Alzheimer’s disease
Cell Death & Disease (2022)
-
Association study identifies genetic determinants and non-genetic factors on steady-state plasma and therapeutic outcome of galantamine in mixed dementia
European Journal of Clinical Pharmacology (2022)
-
Nanomedicine-based technologies and novel biomarkers for the diagnosis and treatment of Alzheimer’s disease: from current to future challenges
Journal of Nanobiotechnology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.