Abstract
Population replenishment of marine life largely depends on successful dispersal of larvae to suitable adult habitat. Ocean acidification alters behavioural responses to physical and chemical cues in marine animals, including the maladaptive deterrence of settlement-stage larval fish to odours of preferred habitat and attraction to odours of non-preferred habitat. However, sensory compensation may allow fish to use alternative settlement cues such as sound. We show that future ocean acidification reverses the attraction of larval fish (barramundi) to their preferred settlement sounds (tropical estuarine mangroves). Instead, acidification instigates an attraction to unfamiliar sounds (temperate rocky reefs) as well as artificially generated sounds (white noise), both of which were ignored by fish living in current day conditions. This finding suggests that by the end of the century, following a business as usual CO2 emission scenario, these animals might avoid functional environmental cues and become attracted to cues that provide no adaptive advantage or are potentially deleterious. This maladaptation could disrupt population replenishment of this and other economically important species if animals fail to adapt to elevated CO2 conditions.
Similar content being viewed by others
Introduction
The process of larval settlement represents a bottleneck in the population dynamics of many marine species. This process is not a simple function of stochastic processes and passive passage via ocean currents. Larval stages often possess sophisticated behaviours, imprinting, and competencies that enable them to identify and navigate towards suitable settlement habitats1. Failure to do so would reduce the chance of survival via increased risk of starvation and predation, or settlement in suboptimal habitats. During this critical life history transition, larvae depend on multiple cues such as sounds and odour plumes to orient themselves and progress from a planktonic to a demersal lifestyle2,3.
Ocean acidification alters or even reverses many behaviours and cue preferences4, by interfering with the processing of sensory information5. For example, under future ocean acidification scenarios, clownfish larvae are less attracted to the odour of their settlement habitat and can be attracted to odours of undesirable settlement habitats that are normally ignored or avoided6. By far the majority of studies on behaviour and ocean acidification have focussed on vision and olfaction, with relatively little known about its effects on the processing of auditory information. Sound is increasingly recognized as a cue used by dispersing propagules of tropical species because it propagates with little attenuation in water, is independent of currents and also carries information about the habitat type and its resident species community2,7. As a result of ocean acidification, larvae of the catadromous barramundi (Lates calcarifer) are deterred rather than attracted to the sound of their estuarine settlement habitat at settlement stage8, settlement-stage larvae of temperate gobies are deterred by reef soundscapes9, whilst post-settlement clownfish lose their avoidance response to reef sounds10.
Ocean acidification has the potential to detrimentally impact a range of species by either leading to avoidance of relevant cues, or attraction to irrelevant or misleading cues6. Here we test whether ocean acidification can stimulate auditory preferences of fish towards irrelevant or suboptimal cues that would provide no adaptive advantage at settlement stage. In choice experiments, we determined the response of larval barramundi towards soundscapes of habitats located outside of its distribution range, and towards artificially generated white noise as a proxy for irrelevant anthropogenic sounds.
Results
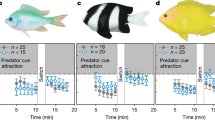
Under current-day CO2 concentrations, larval barramundi showed a significant (Wilcoxon Signed Rank Test, p = 0.017) attraction to tropical estuarine soundscapes at 16 days post-hatching (dph) (Fig. 1a). As expected, contemporary tropical barramundi larvae showed no response towards either of the two ecologically irrelevant sounds of temperate reefs or artificial white noise (Fig. 1b,c).
Effect of elevated CO2 on preferences for sound cues by barramundi larvae. Average (±SE) percentage of time the larvae were found in the section closest to the active speaker when sound cues were broadcast from: (a) settlement habitat (tropical mangrove estuary), (b) irrelevant soundscape (temperate reef), and (c) artificial noise (white noise). The horizontal lines at 50% indicate lack of any response by larvae (neither deterred nor attracted by the cue). Asterisks indicate a statistically significant difference from a 50% response (Wilcoxon signed rank test). Horizontal lines above bars indicate significant differences (within dph) between control and elevated CO2 larvae (2-way ANOVA). N = 10 for control as well as elevated CO2 for each day post hatching in Fig. 1a and c. N = 16 for control as well as elevated CO2 for each day post hatching in Fig. 1b. Data in (a) are a subset of the data from a previous study8 in which larval responses were measured during 13–28 dph but pooled in blocks of 3 days, whilst in the present study only responses for 16–21 dph are used but shown for single days.
However, under projected end-of-century elevated CO2 conditions for coastal and estuarine environments, larval barramundi not only showed avoidance towards the soundscape of its natural estuarine settlement habitat (Wilcoxon Signed Rank Test, p = 0.007 on 16 dph and p = 0.028 on 18 dph), but also attraction towards sounds that are ecologically irrelevant and are ignored by present-day larvae. Barramundi raised under elevated CO2 showed significant attraction towards temperate rocky reef soundscapes and white noise (Wilcoxon Signed Rank Test, temperate rocky reef: p = 0.043 on 16 dph; white noise: p = 0.047 on 13 dph and p = 0.015 on 21 dph).
Aside from showing a significant attraction or deterrence towards natural and/or irrelevant noises (i.e. signification deviation from a 50% random choice response), larvae raised under present-day conditions also differed in their response from those raised under elevated CO2 conditions (Fig. 1): on 16 and 18 dph for natural sounds (2-way ANOVA, CO2 treatment × dph interaction: p = 0.006) and on 13 and 21 dph for white noise (CO2 treatment × dph interaction: p = 0.037) the % time spent close to the speaker broadcasting sounds differed significantly within dph between larvae raised under the two CO2 treatments.
Discussion
We reveal that ocean acidification not only reduces attraction towards natural habitat cues, but also attracts animals towards ecologically irrelevant auditory cues. We compared the response of larvae towards the sounds of their natural settlement habitat to their response towards sounds from habitats outside of their biogeographic range and to artificially generated white noise. Larvae exposed to ocean acidification were not only deterred to the acoustic cues of their natural settlement habitat, but puzzlingly they were attracted to ecologically irrelevant and potentially misleading sounds. The positive response towards the ecologically irrelevant temperate habitat cues in fish treated with elevated CO2 was observed on the same day as when control fish were attracted to tropical estuarine soundscapes. These results are consistent with the only other study that investigated the effect of ocean acidification on the response to both relevant and irrelevant settlement cues6. Because that study6 focussed on olfactory habitat cues this suggests the existence of a common underlying mechanism that results in altered processing of sensory information regardless of the type of cue (i.e., olfactory or auditory). This is likely due to the fact that elevated CO2 concentration causes a reversal in the functioning of the GABA-A receptors from inhibitor to activator which has been shown to alter multiple sensory behaviours in various species of fish5,11,12. Hence, ocean acidification has the potential to reverse the natural deterrence of larvae towards ecologically irrelevant cues and attract them to cues and habitats that are not encountered or irrelevant in nature.
The behavioural alterations observed in this study may result in larvae settling in unsuitable habitats or delaying their settlement from the pelagic because of an inability to distinguish relevant from irrelevant environmental cues. Spending longer periods of time in the water column during the larval stage likely incurs elevated mortality, e.g. due to predation or starvation13. This risk is likely to be exacerbated where processing of multiple sensory cues, such as sounds and odours, are disrupted simultaneously. Such confounding of cues reduces scope for sensory compensation14 or compensation using alternative cues15. Because larval settlement into adult habitats is the major bottleneck in the life cycle of many coastal marine species, these results could highlight risk to the replenishment and connectivity of populations of some fish species. Moreover, because ocean acidification has the potential to attract larval fish to anthropogenic sounds such as white noise, this opens up the question whether larvae could also be attracted to other anthropogenic noises commonly found in the ocean such as those produced by boats and ships. As these artificial noises are becoming more and more widespread in our oceans16, ocean acidification may add to the already detrimental effects of anthropogenic sounds on species under contemporary climate conditions. We reveal that the effects of ocean acidification on animal behaviours go beyond that of altering responses to natural cues17. By reversing behaviour to relevant and irrelevant environmental cues during the critical settlement stage, larval navigation and their population persistence are at risk under future ocean acidification.
Methods
Study species
Barramundi is a tropical fish with a distribution ranging from the eastern Indian Ocean to the western Central Pacific, and is of high commercial and recreational importance15. Barramundi is a catadromous fish migrating from freshwater to the ocean to spawn. Saltwater (28–35 units) is required for optimal gonadal and larval development18. Barramundi eggs and larvae are often caught around river mouths and marine bays, while juveniles settle into mangroves and wetland habitats18,19. Our previous experiments on barramundi larvae obtained from the same hatchery (Robarra Broodstock Sanctuary and Hatchery, West Beach, South Australia) as the one for the current experiment, and raised using the same experimental setup, showed that the larvae undergo metamorphosis mostly between the 16 and 21 days post hatching (dph) and are receptive to settlement acoustic cues between the 16 and 18 dph8. The larvae settle around 3 weeks post-hatching. Therefore, in this study we tested larvae that were between 16 and 21 days old.
Larval rearing
Estuaries often have elevated and highly fluctuating pCO2 concentrations due to natural and human-driven processes like freshwater input, tidal exchange and eutrophication20,21. These processes can exacerbate seawater CO2 concentrations that are increasing due to greenhouse gas emissions. Projections for end of the century pCO2 levels in coastal and estuarine range from 1,700 to 3,200 µatm22. Because barramundi larvae settle in estuaries and coastal areas we therefore used experimental pCO2 levels of approx. 1,368 to 1,541 µatm (Table S1) to represent realistic future effects of ocean acidification in estuarine environments.
We used fertilized eggs as well as larvae of 9 dph, depending on the availability of developmental stage at the time of the experiment, both of which were sourced from a local hatchery (Robarra, 7th generation broodstock) and reared at The University of Adelaide. In both cases, two replicate closed-system larval rearing tanks were used for each CO2 treatment (i.e. 2 control vs 2 elevated CO2 tanks) and comprised individual recirculating tanks consisting of 60 l rearing tanks (containing larvae) connected to their respective 20 l sumps (containing a heater, pump, protein skimmer, UV steriliser, biological filter, and air bubbler) as described in Rossi et al.8. In summary, CO2-enriched air was constantly bubbled into the sumps of the elevated CO2-treatments with a Pegas 4000 MF gas mixer (Columbus). The sumps of the ambient treatments were bubbled with ambient air. pHNBS was measured daily within the rearing tanks using a SG2-ELK SevenGo pH probe (Mettler Toledo) calibrated with a three point calibration, whilst salinity was measured daily with a SR6 refractometer (Vital Sine). pCO2 values of the seawater in the rearing tanks was calculated using the software CO2SYS23 with the respective rearing tank values of seawater temperature, salinity, pHNBS and total alkalinity (TA), using constants K1 and K2 from Mehrbach24 and refit by Dickson & Millero25. Alkalinity was measured twice during each of the two experiments by Dynamic Endpoint Titration using an 888 Titrando (Metrohm) titrator, and values were within 1% accuracy of certified standards (reference materials from Dr. A. Dickson, Scripps Institution of Oceanography). Fish were fed ad libitum with rotifers for the first 12 days post hatching (dph), then with Artemia nauplii and a dry feed (Otohime) of increasing granule size as development progressed.
We tested the response of larvae to estuarine mangrove sounds and white noise using larvae raised from the egg stage at 1,540 µatm (26 July–14 August 2013), whereas for temperate reef sounds (representing an irrelevant cue from outside the species range) we used larvae raised from 9 days old at 1,360 µatm (5–16 December 2014). CO2 treatments (see Table S1) were started at 2 dph for the larvae raised from the egg stage and on 10 dph for the larvae obtained from the hatchery at 9 dph. Four days in elevated CO2 treatment were previously reported to be sufficient to trigger behavioural changes in fish26. Temperature was maintained at ~ 27 °C (Table S1).
Effect of elevated CO2 on larval fish audition
Three types of recordings were used for the behavioural trials: estuarine soundscapes, which acted as a natural settlement-habitat cue, and two irrelevant cues: temperate reefs and white noise. We used a hydrophone (HiTech HTI-96-MIN with inbuilt preamplifier, manufacturer-calibrated sensitivity −164.3 dB re 1 V/μPa; frequency range 0.02–30 kHz; calibrated by manufacturers; High Tech Inc., Gulfport MS) and digital recorder (2007 recordings: Edirol R-1, 44 kHz sampling rate, Roland Systems Group, Bellingham WA; 2013 recordings: PCM-M10, 48 kHz sampling rate, Sony Inc., Tokyo, Japan) to record the sounds. A recording of mixed estuarine habitats represented a biologically relevant acoustic cue (Figs S1, S2). We randomly mixed 30 sec fragments of mangrove habitat recordings from Tanzania (Kunduchi; obtained between 3:30 and 7 pm during 16–19 Feb. 2007 at 1 m depth during calm seas). The estuarine soundscape data have been published previously but in a different format: in the previous study8 larval responses were measured during 13–28 dph but pooled in blocks of 3 days, whilst in the present study only responses for 16–21 dph are used but these are shown for single days rather than pooled days. Soundscapes recorded on temperate rocky reefs in New Zealand (White Island) instead represented an irrelevant biological sound for tropical barramundi as temperate reefs occur outside the biogeographic range of adult habitats. The playback of temperate reef sounds consisted of a 10-minute recording of the dusk chorus, created by mixing various 30 sec fragments collected under full moon between 18–21 November 2013 with a hydrophone placed a few metres above the rocky reef. These recordings were dominated by snapping shrimps crackle, sea urchin raping sounds and the noise of breaking waves. White noise is an artificially generated signal with constant amplitude at every frequency (Figs S1, S2).
Little is known of the hearing sensitivity in barramundi larvae. However, fishes with an extension of their swim bladder to the otic region show increased acoustic sensory performance27. Also settlement-stage barramundi are known to have a forward extension of their swim bladder and a gas-filled chamber in the otic region8. This enables the transit of sound pressure oscillations from the swim bladder to the area housing the otoliths28.
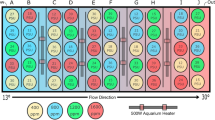
We used the same experimental set-up as designed by Rossi et al.8. In short, a choice chamber with 8 parallel lanes was used (see Rossi et al.8 Fig. S5). In each of the 8 lanes, one fish was released at the same time as all the others from the centre of their respective lane (4 control larvae and 4 elevated CO2 larvae per trial). Before release, larvae were allowed to acclimate to the experimental conditions for 2 min., during which the respective experimental sound was played for habituation. Upon release, the position of each larvae inside their lane was recorded from above using a camcorder (HF R406 Legria, Canon, Japan). The larvae could choose to swim towards the respective sound broadcast from an underwater speaker (UW-30; maximal output 156 dB re 1 μPa at 1 m, frequency response 0.1–10 kHz, Lubell Labs Inc., Columbus OH) that was located 8 cm from the edge of the chamber. As a control, a second, but silent speaker was located at the other far end of the speaker. The sides of the active vs silent speakers were switched on a daily basis to avoid any effects of tank orientation. Sound could travel inside the lanes as the lanes were separated from the remainder of the tank by mesh. The individual larvae in the 8 lanes are considered true replicates as the fish could not influence each others’ behaviours due to their separation by the PVC sides of the lanes (i.e. they could not hear or smell each other). All tests were done in seawater with ambient pCO2 levels as altered behavioural effects due to elevated CO2 are retained for > 24 hrs when fish arte transferred to ambient conditions26. After each trial with 8 fish, we cleaned the tank and chamber and replenished them with unused seawater to get rid of any chemical cues from the previously used fish.
We used EthoVision XT10 (Noldus Information Technology, Wageningen, The Netherlands) to automatically track the position and swimming speed of each larvae for 7 min. after they had been released inside their lane (i.e. after the 2-min habituation). We used this approach to avoid any biases due to observers presence and subjectivity in recording behaviours. Each lane was subdivided in two equal sections and Ethovision recorded the proportion of time each larvae spent in the two sections. The response to each of the three sound cues was investigated daily between 16 and 21 dph, using naïve larvae for each trial (i.e. newly sourced larvae from the rearing tanks). Replication per sound cue per day was: n = 20/day, half in each CO2 treatment for larvae raised from the egg stage, and n = 32/day, half in each treatment for larvae raised from 9 dph. No data were collected for the former on day 17 post hatching due to failure of playback equipment. The experiments were all performed from 2–6 pm. Each fish was used only once (i.e. different fish used for different sound experiments). Larvae that did not respond or showed startle behaviour upon release were not included in the statistical analysis (7% of all larvae). For the response to white noise we also tested the responses during 13–15 dph (we were unable to do this for temperate reef sounds). Whilst Rossi et al.8 showed that barramundi larvae have a very narrow window in which they respond to natural soundscapes (i.e. during 16–18 dph, and not during 13–15 dph or 19–28 dph), we did not have an a priori reason to assume this would be the same for responses to anthropogenic noise. Hence, we also investigated the response towards white noise at an earlier stage that the 16–18 dph window, viz. 13–15 dph.
Larvae can continuously sample sound pressure as they approach the sound source (conceptual model provided by Lillis et al.29) and hence we assumed that larvae inside the lanes could likewise be attracted to sound sources of interest. Besides sound pressure, also particle acceleration is important to consider. We calculated particle acceleration based on the simultaneous measurement of sound pressure by two hydrophones separated by 5.5 cm, and using the Euler equation30. Particle acceleration inside the lanes decreased linearly with distance from the active speaker (Fig. S2). Although it is difficult to replicate a far-field acoustic cue in small aquaria31, this did not limit our conclusions as we were not interested in determining the exact sensitivity levels of larvae, but were interested in the relative responses (i.e. between CO2 treatments) of larvae towards different types of relevant vs irrelevant acoustic cues. The sound pressure levels of the acoustic cues inside the choice chamber was similar to those obtained in situ near to the hydrophone, and decreased towards ambient sound levels with increasing distance away from the active speaker32 (Figs S1, S2).
Statistical analysis
Larvae that show no preference toward a sound cue are expected to be randomly distributed inside their lane and hence observed, on average, 50% of the total experimental time in each of the two sections. To test whether larvae spent significantly more than 50% of their time in a particular section (and hence showed a positive response to a particular cue), we used a non-parametric 1-sample Wilcoxon Signed Rank Test. A non-parametric test was used as a Shapiro-Wilk’s test showed that the data did not show a normal distribution. Furthermore, we tested the difference in response between larvae raised on contemporary vs. future CO2 conditions within each dph, irrespective of whether they were attracted or deterred towards a sound. This evaluates whether elevated CO2 alters the response of larvae towards soundscapes. A 2-way ANOVA was performed for each of the three sounds tested (main factors: CO2 treatment and dph), followed by post-hoc comparisons in case of a significant interaction. Response to temperate reef sound showed no significant main effects (treatment: p = 0.529, dph: p = 0.151) or interaction (p = 0.710).
Data accessibility
Data can be obtained by application to I. Nagelkerken.
Ethics statement
Research was carried out under approval of the University of Adelaide animal ethics committee (permit: S-2012–171) and according to the University’s animal ethics guidelines.
References
Leis, J. M. Are larvae of demersal fishes plankton or nekton? Adv. Mar. Biol. 51, 57–141 (2006).
Leis, J. M., Siebeck, U. & Dixson, D. L. How Nemo finds home: the neuroecology of dispersal and of population connectivity in larvae of marine fishes. Integr. Comp. Biol. 51, 826–843 (2011).
Huijbers, C. M. et al. A test of the senses: fish select novel habitats by integrating multiple cues. Ecology 93, 46–55 (2012).
Nagelkerken, I. & Munday, P. L. Animal behaviour shapes the ecological effects of ocean acidification and warming: moving from individual to community-level responses. Glob. Change Biol. 22, 974–989 (2016).
Nilsson, G. E. et al. Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat. Clim. Change 2, 201–204 (2012).
Munday, P. L. et al. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl. Acad. Sci. USA 106, 1848–1852 (2009).
Montgomery, J. C., Jeffs, A., Simpson, S. D., Meekan, M. & Tindle, C. Sound as an orientation cue for the pelagic larvae of reef fishes and decapod crustaceans. Adv. Mar. Biol. 51, 143–196 (2006).
Rossi, T. et al. Ocean acidification boosts larval fish development but reduces the window of opportunity for successful settlement. Proc. R. Soc. B 282, 20151954 (2015).
Castro, J. M. et al. Painted goby larvae under high-CO2 fail to recognize reef sounds. PLoS ONE 12, e0170838 (2017).
Simpson, S. D. et al. Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol. Lett. 7, 917–920 (2011).
Lai, F., Jutfelt, F. & Nilsson, G. E. Altered neurotransmitter function in CO2-exposed stickleback (Gasterosteus aculeatus): a temperate model species for ocean acidification research. Conserv. Physiol. 3, cov018 (2015).
Regan, M. D. et al. Ambient CO2, fish behaviour and altered GABAergic neurotransmission: exploring the mechanism of CO2-altered behaviour by taking a hypercapnia dweller down to low CO2 levels. J. Exp. Mar. Biol. Ecol. 219, 109–118 (2016).
Morgan, S.G. Life and death in the plankton: larval mortality and adaptation. In Ecology of marine invertebrate larvae (ed. McEdward, L.) 279–322 (CRC Press, Boca Raton, FL, 1995).
Goldenberg, S. U. et al. Ecological complexity buffers the impacts of future climate on marine animals. Nature Climate Change 8, 229–233 (2018).
Devine, B. M., Munday, P. L. & Jones, G. P. Rising CO2 concentrations affect settlement behaviour of larval damselfishes. Coral Reefs 31, 229–238 (2012).
Slabbekoorn, H. et al. A noisy spring: the impact of globally rising underwater sound levels on fish. Trends Ecol. Evol. 25, 419–427 (2010).
Rossi, T., Nagelkerken, I., Pistevos, J. C. A. & Connell, S. D. Lost at sea: ocean acidification undermines larval fish orientation via altered hearing and marine soundscape modification. Biol. Lett. 12, 20150937 (2016).
Russell, D., Rimmer, M., McDougall, A., Kistle, S. & Johnston, W. Stock enhancement of barramundi, Lates calcarifer (Bloch), in a coastal river system in northern Australia: stocking strategies, survival and benefit‐cost. Stock enhancement and sea ranching: developments, pitfalls and opportunities, second edition, pp. 490–500 (1999).
Moore, R. Spawning and early life history of burramundi, Lates calcarifer (Bloch), in Papua New Guinea. Mar. Freshw. Res. 33, 647–661 (1982).
Feely, R. A. et al. The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary. Estuar. Coast. Shelf Sci. 88, 442–449 (2010).
Hofmann, G. E. et al. High-frequency dynamics of ocean ph: a multi-ecosystem comparison. PLoS One 6, e28983 (2011).
Melzner, F. et al. Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar. Biol. 160, 1875–1888 (2012).
Pelletier, G., Lewis, E. & Wallace, D. A calculator for the CO2 system in seawater for Microsoft Excel/VBA. Washington State Department of Ecology, Olympia, WA, Brookhaven National Laboratory, Upton, NY (2005).
Mehrbach, C. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907 (1973).
Dickson, A. & Millero, F. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. 34, 1733–1743 (1987).
Munday, P. L. et al. Replenishment of fish populations is threatened by ocean acidification. Proc. Natl. Acad. Sci. USA 107, 12930–12934 (2010).
Popper, A. N. & Fay, R. R. Rethinking sound detection by fishes. Hear. Res. 273, 25–36 (2011).
Braun, C.B. & Grande, T. Evolution of peripheral mechanisms for the enhancement of sound reception. Fish bioacoustics. 99–144 (Springer, 2008).
Lillis, A., Eggleston, D. B. & Bohnenstiehl, D. Soundscape variation from a larval perspective: the case for habitat-associated sound as a settlement cue for weakly swimming estuarine larvae. Mar. Ecol. Prog. Ser. 509, 57–70 (2014).
Mann, D.A. Propagation of fish sounds. In Communication in fishes (eds. Ladich, F., Collin, S.P., Moller, P. & Kapoor, B.G.) 107–120 (Science Publishers, Enfield, 2006).
Rogers, P., Hawkins, A., Popper, A., Fay, R. & Gray, M. Parvulescu revisited: small tank acoustics for bioacousticians. In Effects of noise on aquatic life II (eds. Popper, A.N. & Hawkins, A.) 933–941 (Springer, New York, 2015).
Akamatsu, T., Okumura, T., Novarini, N. & Yan, H. Y. Empirical refinements applicable to the recording of fish sounds in small tanks. J. Acoust. Soc. Am. 112, 3073–3082 (2002).
Acknowledgements
We thank Jordan Jones and William Nichols for help in the laboratory, and M. Igulu for collecting the habitat sounds in Tanzania. This study was supported by an Australian Research Council Future Fellowship to I.N. (grant no. FT120100183).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design and writing of the article. T.R. collected and analysed the data.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rossi, T., Pistevos, J.C.A., Connell, S.D. et al. On the wrong track: ocean acidification attracts larval fish to irrelevant environmental cues. Sci Rep 8, 5840 (2018). https://doi.org/10.1038/s41598-018-24026-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24026-6
This article is cited by
-
Temporal dimensions of taxonomic and functional fish beta diversity: scaling environmental drivers in tropical transitional ecosystems
Hydrobiologia (2023)
-
The ocean in a box: water density gradients and discontinuities in water masses are important cues guiding fish larvae towards estuarine nursery grounds
Behavioral Ecology and Sociobiology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.