Abstract
To evaluate the clinical outcomes and relationship between tumor size, lymph node status, and prognosis in a large cohort of patients with triple–negative breast cancer (TNBC).849 Patients were categorized by tumor size and nodal status. The Kaplan-Meier method and Cox proportional hazards models were used to determine the association of nodal status and tumor size with survival outcomes. A Sidak adjustment was used for pairwise group comparisons. We conducted six pairwise comparisons between different node status. In univariate and multivariate analyses, it was indicated that N0 patients had similar prognoses as N1 patients (P = 0.072), and the OS of both of these groups was significantly better than that of N2/N3 patients (N0 vs N2, P < 0.001; N0 vs N3, P < 0.001; N1 vs N2, P = 0.014; N1 vs N3, P = 0.005). In summary, we report that in Chinese patients with triple-negative breast cancer, a greater difference in survival was observed between N1 and N2 than between N0 and N1, warranting the possible need of more intensive chemotherapy for N2-3 patients. We also found that tumor size made an impact on survival when lymph nodes were extensively involved, a finding that needs longer follow-up and further validation.
Similar content being viewed by others
Introduction
The incidence rate of breast cancer is increasing almost every year throughout the world, although the mortality rate is declining in many countries, including China. Such advances are mainly due to the systematic use of adjuvant chemotherapy and the development of directed therapies for hormone receptor–positive and C-erbB-2 (HER2)–positive tumors. With a deeper insight into breast cancer over the past two decades, breast cancer is regarded as a heterogeneous disease and can be defined by five intrinsic subtypes: ‘luminal A’, ‘luminal B’, ‘HER2 overexpressing’, ‘basal-like’ and ‘normal breast-like’ tumors. Basal-like breast cancer is a subtype of breast tumor with unique characteristics in terms of clinical-pathological presentation, prognosis, and response to therapy, with poorer prognosis than luminal tumors. However, in clinical work, basal-like breast cancer is not convenient to identify. Three quarters of triple-negative breast cancers, which are defined as ER negative, PR negative and lacking overexpression of HER2, express basal markers; thus, the triple-negative type is frequently taken as a surrogate marker of basal-like breast cancer. This “triple-negative” category comprises 10 to 15% of all breast cancers. In retrospective studies, patients with TNBC have worse clinical outcomes when compared with those with non-TNBC. These tumors tend to present at a younger age, with higher histologic grade, larger size, higher rate of p53 mutations, and positive Ki-67 staining, and they have a tendency toward local and visceral metastases rather than bone metastases1.
Currently, the assessment of prognosis and the appropriate treatment is based on patient and standard tumor-related characteristics. Among these factors, tumor size and nodal status are known to be independent prognostic factors and play an important role in treatment decisions. It is well-known that in breast cancer, the size of the primary tumor and the number of positive lymph nodes has an inverse linear relationship with prognosis and survival. Previous studies also indicated that in cases of more than 7 nodes involved, the survival rate decreased, regardless of tumor size2. Additionally, lymph node metastasis was more important than tumor size for prognosis.
However, evidence have shown that in patients with TNBC, tumor size and lymph node status may not be linearly correlated with survival outcomes, and even the small tumors in the triple-negative group had a high rate of node positivity3. Moreover, once there is evidence of lymph node metastasis, the prognosis may not be affected by the number of positive lymph nodes1. However, clinical evidence are still conflicting and confusing. To better illustrate this point, we sought to analyze the clinical outcomes and relationship between lymph node status and prognosis in a large cohort of patients with confirmed TNBC.
Methods
Patients and follow-up
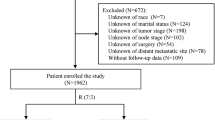
From the database of Shanghai Cancer Center/Hospital of Fudan University, we identified consecutive 849 women with TNBC with complete follow-up data who received treatment for early-stage breast cancer between January 2002 and December 2011. According to the inclusion criteria, all cases were confirmed as females with TNBC but no distant metastasis at the initial diagnosis. All patients received a complete physical examination, bilateral mammography, chest radioscopy, ECG and ultrasonography of the breast, axillary fossa, abdomen and pelvis. All patients were treated with a multidisciplinary approach. Most patients(93.6%) received adjuvant chemotherapy using different regimens (taxane-based, or a combined anthracycline/taxane-based and nonanthracycline/taxane-based regimen) according to the standards used at the time of surgery, followed by radiotherapy (if required). Exclusion criteria included ER or PR positivity, HER2 overexpression or amplification, unknown date of surgery, prior administration of neoadjuvant chemotherapy, absence of the date of last follow-up, additional malignancy and male sex. For all patients, information was available for patient age and menopausal status at diagnosis, tumor size, number of lymph nodes removed, number of positive lymph nodes, histological type, histological grade and treatment regimen. Information on date and cause of death came from linking to the Center for Disease Control records, using the unique personal identification number issued to every Chinese citizen. Causes of death were obtained from death certificates and were classified as either death as a result of breast cancer or death from other causes. In this study, we identified OS as the primary end point because the cause of death is two-year lagging behind survival information (simply dead or alive). OS was defined as time from surgery to death. The study was conducted according to the principles expressed in the Declaration of Helsinki and was approved by the institutional review board of Shanghai Cancer Center, Fudan University. All patients enrolled in this study signed the informed consent voluntarily.
Pathology methods
ER, PR and HER2 status were determined on representative paraffin sections of each tumor using immunohistochemical (IHC) staining that was performed after the patient underwent surgery. ER and PR antibodies were purchased from Dako (clones ER 1D5 1:35 and PR 636 1:50) and were evaluated by an avidin–biotin–peroxidase complex (ABC) assay as described by Shimada et al.4 ER and PR were considered positive in breast cancer cells if the positive nuclei number was ≥10%. After July 2010, tumors in which ≥1% of tumor cells staining for ER or PgR of any intensity were considered positive5. Cytoplasmic staining was ignored6. The overexpression of HER2 protein was evaluated using a monoclonal antibody (Dako, Clone PN2A 1:400), and a peroxidase-antiperoxidase (PAP) technique. Positive HER2 was defined as a complete membrane staining in >10% of tumor cells7, using a qualitative HercepTest scale of 0–3+, in which scores 0–1 were negative, and 3 was positive8. Fluorescence in situ hybridization tests were used when the IHC results were ambiguous (i.e., 2+), or for patients who could not be defined as HER2. The pathological and IHC outcomes were diagnosed under an Olympus light microscope with ×10 and ×40 magnifications by two independent senior pathologists in the Department of Pathology of the Cancer Hospital at Fudan University.
Statistical analysis
Based on the pathology review, the number of positive lymph nodes was categorized into one of four groups: N0 (negative lymph nodes), N1 (one to three positive lymph nodes), N2 (four to nine positive lymph nodes), and N3 (10 or more positive lymph nodes). For BCSS, we used the time from definitive surgery until death as a result of breast cancer or the date of last survival update. Patients who died before experiencing a disease recurrence were considered censored at their dates of death. The Kaplan-Meier method was used to estimate the survival outcomes of all patients by different categories; groups were compared using the log-rank statistic. The Sidak adjustment method was used for multiple group pairwise comparisons. Cox proportional hazards regression models were applied in multivariate analysis. The results are expressed in hazard ratios (HRs) and 95% CIs. P values less than 0.05 were considered statistically significant; all tests were two-sided. Statistical analyses were performed using SPSS Statistics 19.0 (IBM SPSS Statistics 19). P < 0.05 was considered significant.
Results
Patient demographics and tumor characteristics
A total of 849 women with TNBC were included in this study. Patient characteristics are listed in Table 1. The median age was 53 years (range, 23 to 87 years). Three hundred eight patients (44.8%) had a tumor size that was less than 2 cm (T1), 438 patients (51.6%) had a tumor size that was between 2 and 5 cm (T2), and 17 patients (2.0%) had a tumor size that was greater than 5 cm (T3/4). Of these patients, 398 (46.9%) were premenopausal, while 451 (53.1%) were not. As to nodal status, 551 patients (64.9%) were node-negative, and 298 (35.1%) were node-positive. A total of 795 patients (93.6%) received chemotherapy, which included taxanes, anthracyclines or a combination. Of the patients, 145 (17.1%) underwent breast-conserving surgery, while the other 704 (82.9%) underwent radical mastectomy or mastectomy.
Univariate and multivariate survival analyses of patients
The median follow-up of the entire cohort was 53 months (range, 0.7 month to 317 months). There were 106 deaths, and the 5-year OS rate was 83% (95% CI, 67 to 72%). For node-negative patients, the 5-year OS rate was 89%. As the number of lymph nodes increased, the 5-year OS rate decreased to 81%, 66%, and 58%, respectively, for N1, N2, and N3 patients (P < 0.001) (Table 2). Postmenopausal status (HR = 2.216, 95% CI = 1.445–3.400, P < 0.001), T3/T4 (HR = 5.629, 95% CI = 2.490–12.725, P < 0.001), lymph node involvement (HR = 2.563,95% CI = 1.725–3.809, P < 0.001), lymphatic vascular invasion (HR = 2.104, 95% CI = 1.358–3.258, P = 0.001) and local treatment, which includes mastectomy and radiotherapy (HR = 3.72, 95% CI = 1.085–12.871, P < 0.001), predicted worse overall survival in univariate analysis(Table 3). Multivariate analysis using the Cox model was also performed, indicating that postmenopausal status (HR = 2.537, 95% CI = 1.498–3.708, P < 0.001), T3/T4 (HR = 3.791, 95% CI = 1.533–9.376, P = 0.004) and lymph node involvement (HR = 2.135, 95% CI = 1.386–3.292, P = 0.001) were all independent prognostic factors for OS (Table 4).
Pairwise survival comparison by lymph node involvement
We conducted six pairwise comparisons (N0 vs N1, N2, N3; N1 vs N2, N3; and N2 vs N3) between different node statuses using univariate and multivariate methods. In univariate analysis, it was indicated the outcome of N0 and N1 patients was not significantly different (P = 0.072), while the prognosis of N0 patients was significantly better than the N2/N3 group (both P < 0.001). In a pairwise comparison using the multivariate method, this phenomenon was further validated. The prognosis of N0 patients was similar to that of N1 patients (P = 0.072), and the OS of both these groups was significantly better than that of N2/N3 patients (N0 vs N2, P < 0.001; N0 vs N3, P < 0.001; N1 vs N2, P = 0.014; N1 vs N3, P = 0.005). Moreover, no significant difference in survival was observed between N2 and N3 patients (P = 0.578) (Table 5).
Survival and comparisons by tumor size and lymph node status
We found that survival differed more between N1 and N2 than between N0 and N1, so we categorized N0 and N1 into one group and N2 and N3 into the other. Then, we conducted survival comparisons (T1N0-1 vs T1N2-3 vs T2-3N0-1 vs T2-3N2-3) between different node status and tumor sizes using univariate methods. The Kaplan-Meier curve showed there was no survival difference between T1N0-1 vs T2-3N0-1, which meant that tumor size did not affect survival when there was less lymph node involvement. The prognosis of the T1N2-3 group was worse than that of the T1N0-1 and T2-3N0-1 groups, but it was better than that of the T2-3N2-3 group (P < 0.001), which meant that tumor size did impact survival when more lymph nodes were involved (Fig. 1). This finding was somewhat different from our previous cognition.
Discussion
In this study, we evaluated the clinical outcomes and relationships between tumor size, lymph node status, and prognosis in a large cohort of Chinese patients with confirmed triple-negative breast cancer. In our study cohort, the OS rate decreased as the number of positive lymph nodes increased. This finding has been reported in previous studies9 many times and is in agreement with our previous cognition.
However, when we conducted pairwise comparisons using the Sidak adjustment method, our study makes the important observation that the outcome of N0 and N1 patients was not significantly different (P = 0.072), while the prognosis of N0 patients was significantly better than that of the N2/N3 group (P < 0.001, both). In other words, survival differed more between N1 and N2 than between N0 and N1 in patients with triple-negative breast cancer. This finding was consistent with the AJCC staging system10, which categorizes N2 and N3 together into Stage III while leaving N1 in Stage II. However, it was discordant with a previous study published in the Journal of Clinical Oncology by Leonel F. Hernandez-Aya, whose study concluded that once there is evidence of lymph node metastasis, the prognosis may not be affected by the number of positive lymph nodes1. The difference may due to different genetic backgrounds of patients and to the heterogeneity of triple-negative breast cancer itself. For example, black women were more likely to be diagnosed with triple-negative breast cancer than Chinese women and non-Hispanic white women11. Moreover, the actuarial probability of a woman dying due to small-sized breast cancer tumors was significantly higher for black women compared with non-Hispanic white women and Chinese women12. Therefore, the genetic background of triple-negative breast cancer patients was largely different between the study by Hernandez-Aya and ours. Moreover, the cases in that study were collected from 1980, when the chemotherapy regimen and lymph node dissection standard was quite different from the standards used today.
We found that survival differed more largely between N1 and N2 than between N0 and N1, so we categorized N0 and N1 into one group and N2 and N3 into the other. Then, we conducted survival comparisons (T1N0-1 vs T1N2-3 vs T2-3N0-1 vs T2-3N2-3) between different node statuses and tumor sizes using a univariate method. The prognosis of T1N2-3 group was worse than that of T1N0-1 and T2-3N0-1 but better than that of T2-3N2-3 (P < 0.001), which meant tumor size made an impact on survival when lymph nodes were more involved. This finding was not in concordance with the AJCC staging system. In the AJCC staging system, N2 and N3 were categorized as stage III no matter the tumor size (T4 excluded here). This phenomenon may be explained in two aspects. Although it is found that the “size-nodes” relationship in triple-negative breast cancer is distinct and that tumor size is not a strong indicator of prognosis in basal-like breast cancer3,13, the reason for this result may be due to the short follow-up time. In a follow-up to the triple-negative study in Toronto, women with basal-like breast cancer <2 cm in diameter had a survival advantage compared with women with larger basal-like breast cancers (hazard ratio = 0.5, 95% CI 0.4–0.7; P = 0.002) for the first 5 years, but after 8 years of follow-up, the proportion of cancer-related deaths was approximately 40% in both groups14. Thus, tumor size was predictive of short-term survival for women with triple-negative breast cancer, but it was not a predictor of long-term survival. The underlying mechanism might also be explained as follows: it has been proposed that a higher fixed proportion of cancer cells with stem-cell properties of self-renewing and pluripotency, which we call cancer stem cells, may be present15,16, and these cells may confer the capacity to small tumors to metastasize to distant sites. However, there are two dimensions to examine the degree of malignancy of cancer stem cells. One is migration ability, and the other is proliferation ability. Tumors with extensive lymph node involvement when they are small tend to have strong migration ability but not proliferation ability, while large tumors with extensive lymph node involvement tend to have strong migration ability and proliferation ability. Thus, for small tumors with extensive lymph node involvement, even if the cancer cells migrate to distant organs, it may take a longer time to form a clinically detectable tumor lesion, leading to comparatively later recurrence and death.
To the best of our knowledge, this is the largest study evaluating the prognostic significance of tumor size and lymph node status in Chinese patients with TNBC. Our survival data was from the Center of Disease Control records and death records—data with high accuracy. However, there are still limitations that should be mentioned. First, this is a retrospective study with inevitable bias. Additionally, we need to account for the significant exclusion of patients treated with neoadjuvant chemotherapy. This exclusion resulted in a highly select patient population that may not accurately reflect the entire cohort of patients with triple-negative breast cancers.
In summary, we report that in this large cohort of Chinese patients with triple-negative breast cancer, survival differed more between N1 and N2 than between N0 and N1, rendering the possible need of more intensive chemotherapy for N2-3 patients. We also found that tumor size had an impact on survival when lymph nodes were extensively involved, a finding which needs longer follow-up and further validation. This finding may enhance our understanding of the recurrence pattern in specific triple-negative breast cancer subpopulations.
References
Hernandez-Aya, L. F. et al. Nodal status and clinical outcomes in a large cohort of patients with triple-negative breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 29, 2628–2634, https://doi.org/10.1200/JCO.2010.32.1877 (2011).
Shen, Z. Z. [Relation of tumor size, lymph node status and prognosis in breast cancer]. Zhonghua wai ke za zhi [Chinese journal of surgery] 29(554–557), 589 (1991).
Dent, R. et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clinical cancer research: an official journal of the American Association for Cancer Research 13, 4429–4434, https://doi.org/10.1158/1078-0432.CCR-06-3045 (2007).
Shimada, A. et al. Immunocytochemical staining of estrogen receptor in paraffin sections of human breast cancer by use of monoclonal antibody: comparison with that in frozen sections. Proceedings of the National Academy of Sciences of the United States of America 82, 4803–4807 (1985).
Hammond, M. E. et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 28, 2784–2795, https://doi.org/10.1200/jco.2009.25.6529 (2010).
Rody, A. et al. Estrogen receptor alpha and beta, progesterone receptor, pS2 and HER-2/neu expression delineate different subgroups in ductal carcinoma in situ of the breast. Oncology reports 12, 695–699 (2004).
Gouvea, A. P. et al. Selecting antibodies to detect HER2 overexpression by immunohistochemistry in invasive mammary carcinomas. Applied immunohistochemistry & molecular morphology: AIMM 14, 103–108, https://doi.org/10.1097/01.pai.0000155794.64525.11 (2006).
Jacobs, T. W., Gown, A. M., Yaziji, H., Barnes, M. J. & Schnitt, S. J. Specificity of HercepTest in determining HER-2/neu status of breast cancers using the United States Food and Drug Administration-approved scoring system. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 17, 1983–1987, https://doi.org/10.1200/jco.1999.17.7.1983 (1999).
Rakha, E. A. et al. Prognostic markers in triple-negative breast cancer. Cancer 109, 25–32, https://doi.org/10.1002/cncr.22381 (2007).
Singletary, S. E. & Connolly, J. L. Breast cancer staging: working with thesixth edition of the AJCC Cancer Staging Manual. CA: a cancer journal for clinicians 56, 37–47; quiz50–31 (2006).
Carey, L. A. et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. Jama 295, 2492–2502, https://doi.org/10.1001/jama.295.21.2492 (2006).
Iqbal, J., Ginsburg, O., Rochon, P. A., Sun, P. & Narod, S. A. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. Jama 313, 165–173, https://doi.org/10.1001/jama.2014.17322 (2015).
Foulkes, W. D., Reis-Filho, J. S. & Narod, S. A. Tumor size and survival in breast cancer–a reappraisal. Nature reviews. Clinical oncology 7, 348–353, https://doi.org/10.1038/nrclinonc.2010.39 (2010).
Dent, R. et al. Time to disease recurrence in basal-type breast cancers: effects of tumor size and lymph node status. Cancer 115, 4917–4923, https://doi.org/10.1002/cncr.24573 (2009).
Visvader, J. E. & Lindeman, G. J. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nature reviews. Cancer 8, 755–768, https://doi.org/10.1038/nrc2499 (2008).
Wicha, M. S., Liu, S. & Dontu, G. Cancer stem cells: an old idea–a paradigm shift. Cancer research 66, 1883–1890, https://doi.org/10.1158/0008-5472.can-05-3153 (2006). discussion 1895–1886.
Author information
Authors and Affiliations
Contributions
Liu Yin, Hao Shuang and Shao Zhiming wrote the main manuscript text. Liu Yin and Hao Shuang made statistical analysis. Liu Yin, Hao Shuang, Chen Sheng and Huang Liang collected patients’ data. Sun Xiangjie,Yang Wentao performed the IHC procedures and review the IHC outcomes. Shao Zhiming made the study design. All authors reviewed the manuscript. All the authors of this manuscript have no conflicts to disclose.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yin, L., Shuang, H., Sheng, C. et al. The Prognostic Value of Nodal Staging in Triple-Negative Breast Cancer — A Cohort from China. Sci Rep 8, 9007 (2018). https://doi.org/10.1038/s41598-018-23999-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-23999-8
This article is cited by
-
Varying outcomes of triple-negative breast cancer in different age groups–prognostic value of clinical features and proliferation
Breast Cancer Research and Treatment (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.