Abstract
YNbO4 phosphors with various Er3+ and Yb3+ concentrations were synthesized via a traditional high-temperature solid-state reaction method. Their crystal structure was investigated by means of X-ray diffraction (XRD) and Rietveld refinements, and it was confirmed that the obtained samples exist in monoclinic phase. The Er3+ and Yb3+ concentration-dependent up-conversion (UC) luminescence was studied under 1550 nm excitation. By inspecting the dependence of UC intensity on the laser working current, it was found that four-photon and three-photon population processes were co-existent for generating the green UC emissions in the samples with higher Yb3+ concentrations. In addition, it was observed that the temperature sensing properties of YNbO4: Er3+/Yb3+ phosphors were sensitive to both Er3+ and Yb3+ doping concentrations. Furthermore, based on the obtained temperature response of the UC luminescence phosphors, 1550 nm laser-irradiation-induced thermal effect was studied, and it was discovered that the sample temperature was very sensitive to the doping concentrations of Er3+ and Yb3+ and the excitation power.
Similar content being viewed by others
Introduction
Rare-earth (RE) ion-doped up-conversion (UC) luminescence materials have received considerable attention due to their widespread applications in many fields, such as UC lasers, sensors, solar cells, three dimension display and so on1,2. In practical applications, the UC phosphors with high luminescence efficiency and reliable temperature sensing ability may be preferable. Nevertheless, the UC materials possessing both highly efficient UC efficiency and high temperature sensitivity are very fewer3. Therefore, it is necessary to explore a new type material to meet this requirement.
On the one hand, choosing proper trivalent RE ions used as absorption and emission centers in the design of UC luminescence materials is very important in order to obtain highly efficient UC emissions4,5,6. Among trivalent RE ions, Er3+ is one of the most preferable RE ions for UC luminescence due to its uniform energy level distribution and proper energy distance between some metastable energy levels, which allows Er3+ to be effectively excited by different laser wavelengths, such as 800, 980 and 1550 nm7. Moreover, Er3+ is expected as an effective temperature sensing unit with high temperature sensing sensitivity based on its two thermally coupled emitting energy levels 2H11/2 and 4S3/2, whose energy distance is about 770 cm−1 8,9. In order to further enhance the UC luminescence efficiency of Er3+, Yb3+ is often employed as a sensitizer owing to its large absorption cross-section at around 980 nm and efficient energy transfer to Er3+ 10. Thus, Er3+/Yb3+ co-doped UC luminescence phosphors have been extensively studied4,5,6,8,9. On the other hand, the characteristics of the host materials, such as their phonon energy, chemical and thermal stabilities, have significant effects on the UC luminescence properties of the UC phosphors11,12,13,14. In general, oxide compounds have good chemical and physical stabilities, which are beneficial to the applications in high temperature environments, have received increasing attention in recent years15,16,17,18,19,20,21,22. Among various oxide matrixes, ANbO4 (A = Gd, Y, La) compounds, with a fergusonite structure and low phonon energy, are good host candidates for UC luminescence phosphors17,18,19,20,21,22. Recently, the UC luminescence properties of Er3+/Yb3+ co-doped ANbO4 materials under 980 nm excitation have been reported17,18. It was found that the ANbO4 host presented high chemical and physical stabilities, low lattice vibration energy and high accommodating concentration for RE luminescent centers; additionally they can be easily prepared via the traditional solid-state reaction method. However, the UC luminescence properties reported in these niobate systems are mainly focused on the 980 nm excitation. In addition to 980 nm excitation, the Er3+ can also be excited by 1550 nm. 1550 nm laser has obtained considerable attention due to its eye-safe nature and wide applications in optical communication and solar cells; meanwhile, it is also an effective excitation source to investigate the UC luminescence properties of RE3+-doped materials23,24,25. It is reported that efficient UC emission from Er3+ was obtained under monochromatic excitation around 1500 nm24. Previously, we have studied the UC luminescence properties of Er3+ in Er3+ mono-doped YNbO4 phosphors under 1550 nm excitation22. The results showed that the relative intensity of the green and the red UC emissions were significantly different in down-conversion from that in UC luminescence processes because of the different population routes and pumping conditions. Furthermore, it was confirmed that energy transfer process was the main mechanism for UC emissions of Er3+ in Er3+ mono-doped YNbO4 phosphors excited at 1550 nm. In addition, we have also studied the effect of Mo6+ on the UC luminescence and temperature sensing sensitivity of Er3+ in ANbO4 phosphors excited at 1550 nm20. However, there is little work on the study of UC luminescence, temperature sensing property and laser-induced thermal effect of Er3+/Yb3+ co-doped ANbO4 phosphors under 1550 nm excitation.
Hereby, in this study, Er3+/Yb3+ co-doped YNbO4 phosphors were prepared by a traditional high-temperature solid-state reaction method. Er3+ and Yb3+ concentration-dependent UC luminescence processes under 1550 nm excitation were discussed. Different concentration dependent UC luminescence processes for the green and red UC emissions were discovered. In addition, the effects of Er3+ and Yb3+ concentrations on the optical temperature sensing properties were also investigated according to fluorescence intensity ratio (FIR) technique. Furthermore, the laser-irradiation-induced thermal effect was evaluated by persistent irradiation of the 1550 nm laser. It was observed that both the temperature sensing property and laser-induced thermal effect were dependent on the Er3+ and Yb3+ concentrations.
Experimental Details
Sample preparation
Usually, doping concentration has significant effect on the luminescent performance of the UC luminescence materials. Previously, we have investigated the spectroscopic properties of Er3+ mono-doped YNbO4 phosphor and obtained the optimum Er3+ concentration (5 mol%)22. According to our previous results16,25, two sets of YNbO4: Er3+/Yb3+ phosphors were prepared by a traditional high-temperature solid-state reaction method. In one set, the concentration of Er3+ was fixed to be 5 mol%, and the doping concentrations of Yb3+ were designed to be 0, 2, 6, 10, 14, 16 and 20 mol%. In the other set, the concentrations of Er3+ were respectively set as 0.5, 1, 3, 5, 7, 9, 12 and 15 mol%, and the concentration of Yb3+ was fixed to be 6 mol%, which was the optimum doping concentration derived from the experimental results of the first set of the samples.
The starting materials Y2O3 (99.99%), Yb2O3 (99.99%) and Er2O3 (99.99%) used in this work were purchased from Shanghai Second Chemical Reagent Factory (China), Nb2O5 (99.99%) were purchased from Tianjin Reagent Chemicals Co., Ltd (China). They were first weighed according to the designed stoichiometric ratio and fully ground in an agate mortar for 30 min until the mixtures were uniformly mixed. Furthermore, the mixture was transferred into a crucible, and then it was put into a muffle furnace (SX-GO 3163, Tianjin Zhonghuan Experimental Electric Furnace Co., Ltd., China) and calcined at 1300 °C for 4 h. Last, the final products were obtained after the furnace was naturally cooled to room temperature.
Sample characterization
A SHIMADZU X-ray diffractometer (XRD-6000) with CuKα1 radiation (λ = 0.15406 nm) was used to analyze the crystal structure of those as-prepared samples. Temperature dependent UC luminescence spectra in the visible light region were measured by a Hitachi F-4600 fluorescent spectrometer equipped with a power-controllable 1550 nm fiber laser as an excitation source. The output power of the 1550 nm fiber laser shows a linear dependence on its working current. The fluorescence spectra in the wavelength range of 800–1100 nm were obtained by a near-infrared spectrometer (NIR QUEST-256) with an external 1550 nm fiber laser. The temperatures of the samples were controlled by a self-made sample temperature controlling system, DMU-TC 450, with a controlling accuracy of about ±0.5 °C.
Results and Discussion
Identification of the crystal structure
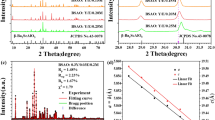
In order to identify the crystal structure of the as-prepared phosphors, XRD measurements were performed on all the samples. The results showed that all the samples almost had the same diffraction patterns. To better study the effect of Er3+, Yb3+ doping concentrations on the crystal structure of YNbO4, a Rietveld refinement procedure was carried out with non-commercial software, named General Structure Analysis System (GSAS)26,27. Figure 1 shows the XRD Rietveld refinement results of YNbO4: x mol% Er3+/y mol% Yb3+ (x = 5, y = 0 and 20; y = 6, x = 9 and 15) phosphors as representatives. In the refinement processes, the crystallographic data of monoclinic YNbO4 (space group of C2/c (15), see JPCDS card No. 83–1319) were used as the initial crystal structure model. The measured, calculated results, Bragg position, and the difference between experimental and calculated diffraction patterns are also shown in Fig. 1. It can be seen that all the diffraction peaks in the experimental data can be well fitted by the Rietveld theoretical model, and the deviation between the experimental and the calculated data are acceptable. The cell parameters and the Rietveld refinement results are shown in Table 1. It can be found that the lattice constants decrease with the increase of both Er3+ and Yb3+ concentrations, which confirms the disordered lattice induced by the substitution of larger Y3+ (0.90 Å) by smaller Er3+ (0.88 Å) and Yb3+ (0.86 Å). However, all the observed diffraction peaks for those samples satisfy the reflection conditions, and our as-prepared samples are of monoclinic phase.
XRD patterns of YNbO4: x mol% Er3+/y mol% Yb3+ (x = 5, y = 0 and 20; y = 6, x = 9 and 15) phosphors. Asterisks represent the experimental data, the red curves accompanying the asterisks show the Rietveld refinement results, the green sticks mark the Bragg reflection positions, and the blue solid lines represent the differences between experimental and calculated data.
Up-conversion luminescence properties
It is well known that the UC luminescence intensity greatly depends on the doping concentrations of RE ions, thus we have studied the effect of Er3+ and Yb3+ concentrations on the UC luminescence properties excited at 1550 nm wavelength. Er3+ and Yb3+ concentrations dependent UC luminescence spectra measured at room temperature are displayed in Fig. 2. Figure 2(a) shows the UC luminescence spectra of the as-prepared YNbO4 phosphors with fixed Er3+ concentration of 5 mol% and various Yb3+ concentrations of 0, 2, 6, 10, 14, 16 and 20 mol%. Several characteristic emissions of Er3+ can be found in the UC luminescence spectra. Among them, two strong green emissions centered at around 545 and 565 nm can be assigned to the 2H11/2, 4S3/2 → 4I15/2 transitions, and the red emission ranging from 650 to 740 nm originates from 4F9/2 → 4I15/2 transition28,29. In addition, an extremely weak near infrared emission located at 820 nm corresponding to 4I9/2 → 4I15/2 transition can also be observed30. The inset of Fig. 2(a) shows the dependences of the green and the red UC emission intensities on Yb3+ concentration. It can be seen that the concentration of Yb3+ has significant influence on both green and red UC emission intensities. With an increase in Yb3+ concentration, both green and red UC emission intensities gradually increase and reach their own maximum values when the concentration of Yb3+ is 6 mol%, and then the intensities decline as Yb3+ content further increases. Moreover, with increasing Yb3+ concentration the integrated intensity ratio (I R /I G ) of the red to the green UC emissions exhibits an interesting variation. As can be seen from the inset of Fig. 2(a), the intensity ratio I R /I G first decreases with an increase in Yb3+ concentration, and then significantly increases when the concentration of Yb3+ is higher than 6 mol%. The effect of Yb3+ concentration on the intensity ratio I R /I G will be discussed later.
UC luminescence spectra for YNbO4 samples doped with various Yb3+ concentrations (a) and Er3+ concentrations (b) excited at 1550 nm. The insets show the dependences of the green and the red UC luminescence intensities and the intensity ratio (I R /I G ) of red to green UC emissions on Yb3+ and Er3+ concentrations.
To examine the effect of the concentration of Er3+ on the UC luminescence of YNbO4: Er3+/Yb3+ under 1550 nm excitation, the UC luminescence spectra of those samples doped with 6 mol% Yb3+ and x mol% Er3+ (x = 0.5, 1, 3, 5, 7, 9, 12 and 15) were measured under an identical experimental conditions and are shown in Fig. 2(b). The intrinsic emissions of Er3+ as appearing in Fig. 2(a) are also observed. And when the concentration of Er3+ reaches 9 mol%, both green and red UC emission intensities are the strongest, and then decrease with further increase in Er3+ concentration, which can be seen in the inset of Fig. 2(b). In addition, from the inset of Fig. 2(b), it can also be found that the intensity ratio I R /I G decreases with an increase in Er3+ concentration in the whole of our studied concentration range, which will be discussed later, too.
In order to further analyze the UC luminescence processes of Er3+ in YNbO4: Er3+/Yb3+ phosphors under 1550 nm excitation, the laser working current dependence of UC luminescence of YNbO4: 5 mol% Er3+/y mol% Yb3+ (y = 0, 6, 14 and 20) and YNbO4: x mol% Er3+/6 mol% Yb3+ (x = 0.5, 3, 9 and 15) phosphors were studied. As examples, the UC luminescence spectra for both YNbO4: 5 mol% Er3+/6 mol% Yb3+ and YNbO4: 6 mol% Yb3+/9 mol% Er3+ samples under 1550 nm excitation at different laser working current are shown in Fig. 3(a,b). It should be pointed out that these two samples display the strongest UC luminescence in each set of phosphors in this study. From Fig. 3, it can be seen that the UC luminescence intensities of both samples monotonically increase with an increase of the laser working current. Strong green and red UC emissions were both observed. To further clarify the UC luminescence mechanism of Er3+, the integrated intensities of the green and the red UC emissions are calculated separately, and the results for various concentrations of Yb3+ and Er3+ doped YNbO4 phosphors are shown in Fig. 4(a,b). For any unsaturated UC luminescence process, the UC emission intensity (I up ) is depended on the laser working current (iLD), and fulfills the following relation31:
where n is the number of required photon, a and b are constants. Equation (1) is used to fit the experimental data depicted in Fig. 4(a,b), and the fitting results are also shown in Fig. 4(a,b). As can be seen, all these n values of the red UC emission (nR) are around 3, indicating that the red UC emissions are dominated by three-photon process for different concentrations of Yb3+ and Er3+ doped samples. The little differences for those nR values may be caused by the calculation error of the integrated intensity and the error in the fitting process. As can be seen in Fig. 4(a), for the green UC emissions the fitted n values (nG) are also around 3. But the value of nG continues to grow as Yb3+-concentration increases, and it is greater than 3 when Yb3+-concentration reaches 14 mol%, indicating much more 1550 nm photons are needed to achieve the green UC emissions in the case of high Yb3+-concentration.
To better describe the concrete UC luminescence processes of Er3+ in YNbO4: Er3+/Yb3+ phosphors excited at 1550 nm wavelength, Fig. 5 depicts the simplified energy level diagrams of Er3+, Yb3+ and the possible energy transfer processes. In principle, there are three possible basic population mechanisms involved in the UC luminescence processes, including energy transfer up-conversion (ETU), ground state absorption (GSA) and excited state absorption (ESA)10. As can be seen in Fig. 5, the UC luminescence of Er3+ under 1550 nm excitation can be obtained by both direct light absorption of Er3+ and ET from Yb3+. In this case, for Er3+ single-doped sample the population of 2H11/2 and 4S3/2 levels can be achieved by successive absorbing three 1550 nm photons via GSA and two ESA or ET (from Er3+ to Er3+) processes, and then yielding the green UC emissions24,32. The possible UC luminescence process of the red UC emission is that Er3+ in 4I9/2 level non-radiatively relaxes to 4I11/2 level and absorbs one 1550 nm photon or receives an energy transferred from a neighboring Er3+, yielding the population of 4F9/2 level, and then red UC emissions can be achieved.
Compared with Er3+ mono-doped YNbO4 phosphors, introducing Yb3+ as a sensitizer can influence the UC luminescence process of Er3+ though Yb3+ cannot directly absorb the energy of 1550 nm photons. From the inset of Fig. 2(a), it can be found that Yb3+ concentration has significant effect on the UC luminescence intensity of Er3+. With an increase of Yb3+ concentration, the UC luminescence intensity of Er3+ first increases and then decreases, which may be caused by the back energy transfer (BET, 4I11/2 (Er3+) + 2F7/2 (Yb3+) → 4I15/2 (Er3+) + 2F5/2 (Yb3+)) process from Er3+ to Yb3+ 15. Er3+ ions first absorb the energy of two 1550 photons to achieve the population of 4I9/2 level, and then complete the population of 4I11/2 level by non-radiative relaxation process. Because the energy distance between 2F5/2 level and 2F7/2 level of Yb3+ matches well with that of 4I11/2 level and 4I15/2 level of Er3+, energy transfer from Er3+ to Yb3+ can occur. The existence of the BET process can be confirmed by the UC luminescence spectra shown in Fig. 6(b), which were measured under 1550 nm excitation in the near infrared region. As can be seen, the UC emission intensity from Yb3+ increases first and then decreases with an increase in Yb3+ concentration. Considering the efficient ET process from Yb3+ to Er3+, Er3+ → Yb3+ → Er3+ ETU process should be an important mechanism for the UC luminescence of Er3+ when excited at 1550 nm11,33,34. Therefore, the UC luminescence mechanism of Er3+ can be described by the following processes. For Er3+ and Yb3+ co-doped case, Er3+ first absorbs two 1550 nm photons populating the 4I9/2 level and achieves the population of 4I11/2 level via a following non-radiative relaxation process. Next, Er3+ transfers the energy to an adjacent Yb3+ via the BET process, and then Yb3+ is excited to its excited state 2F5/2 level. Then, the excited Yb3+ transmits part energy to a neighboring Er3+, achieving the populations of 2H11/2/4S3/2 and 4F9/2 levels of Er3+ via ET2 and ET1 processes, respectively. Finally, the green and the red UC emissions can be achieved. For the green UC emissions, the above-mentioned BET process needs to absorb two 1550 nm photons, and another two 1550 nm photons are needed to be absorbed to accomplish the population of 4I11/2 level via ET2 process. Thus, four 1550 nm photons are needed for the green UC emission when Yb3+ acts as the sensitizer of Er3+. Because those values nG derived from the fitting process are larger than 3 especially in the case of high concentration of Yb3+ doping, it can be concluded that three-photon and four-photon absorption processes co-existent in the green UC luminescence process. That is to say, ETU processes between Er3+ and Yb3+ and between Er3+ ions are co-existent in the green UC luminescence of Er3+ under 1550 nm excitation.
Furthermore, the influences of Er3+ and Yb3+ concentrations on the UC luminescence intensity and the intensity ratio I R /I G can also be better explained by the energy level diagrams of Er3+ and Yb3+. For the case of varying Yb3+ concentration, when the concentration of Yb3+ is not higher than 6 mol%, much more energy can be transferred from Er3+ to Yb3+ with an increase of Yb3+ concentration, which means BET process occurs. Then, higher energy is re-transferred from Yb3+ to Er3+ via ET1 and ET2 processes, achieving the populations of the green and the red excited state levels 2H11/2/4S3/2 and 4F9/2. However, the increase of the population of 4I13/2 level will be suppressed compared with that of 4I11/2 level due to the increased BET process. Finally, the UC luminescence intensity enhances, but the intensity ratio I R /I G of Er3+ decreases. In the case of higher concentration of Yb3+ (greater than 6 mol%) doping, the interionic distance between Er3+ and Yb3+ decreases and those aggregated Yb3+ may become quenching center, resulting in the quenching of UC luminescence of Er3+ 35. In addition, at higher doping concentration of Yb3+, the population of 4I11/2 level may be reduced via BET process much easier than that of 4I13/2 (as can be seen in Fig. 6(b), the UC luminescence intensity of the emission from 4I11/2 → 4I15/2 transition decreases when the concentration of Yb3+ reaches 6 mol%), which further affects the populations of 2H11/2/4S3/2 and 4F9/2 levels. Thus, the intensity of the green and the red UC emissions decrease and the ratio I R /I G increases with an increase of Yb3+ concentration.
For variable concentrations of Er3+-doped case, the UC luminescence intensity also increases first and then decreases with an increase in Er3+ concentration. At higher concentration of Er3+, the reduction of UC luminescence intensity of Er3+ can be ascribed to the concentration quenching, which may be caused by the non-radiative ET and the cross-relaxation (CR) between Er3+ due to the intensified interaction between Er3+ for the closer interionic distance. The possible CR processes are shown in Fig. 525. As can be seen, CR1-CR4 processes are responsible for the depopulation of the green emitting levels 2H11/2 and 4S3/2, but CR5 and CR6 processes take the responsibility for the depopulation of the red emission level 4F9/2, which will cause the quenching of the corresponding green and red UC emissions, respectively. With an increase in Er3+ concentration, the BET process from Er3+ to Yb3+ can also be enhanced, and those aggregated Yb3+ may be one type of quenching center, which may further induce the quenching of the UC luminescence of Er3+ 34. In addition, as mentioned above, the intensity ratio I R /I G decreases with an increase of Er3+ concentration in the whole of the studied concentration range, which indicates that the depopulation of the red emitting level 4F9/2 is much faster than that of the green emitting levels 2H11/2 and 4S3/2. As can be seen from Fig. 5, CR1 process could increase the population of 4F9/2 level, so this CR process can be ignored in the present case. The other CR processes may simultaneously influence the population of the green and the red emitting levels assisted by other ET, energy absorption or non-radiative transition processes.
Temperature sensing properties
Temperature is an important physical quantity in both science and industrial fields, and it has a great influence on the spectroscopic properties of the luminescent materials. However, it is difficult to monitor the actual temperature of the materials via traditional contact measurement due to the interference from the introduced thermometers. Thus, it is necessary to find a non-contact method to measure the internal temperature of one material.
The energy distance between the two levels 2H11/2 and 4S3/2 of Er3+ is small and these two levels exist in a state of thermal equilibrium, thus their populations fulfill the Boltzmann’s distribution36. And then the FIR of these two green emissions is temperature dependent. Therefore, Er3+ can be used as temperature sensing unit to achieve temperature detection via probing the FIR of the two green emissions. According to the Boltzmann’s distribution theory, the dependence of the FIR value (R, I H /I S ) on temperature for Er3+-doped samples can be expressed as follows37:
where I H and I S are the integrated fluorescence intensities of the emissions from 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions, respectively. ΔE is the energy distance between 2H11/2 and 4S3/2 levels, k is Boltzmann’s constant, and T is absolute temperature. To investigate the optical temperature sensing behavior of Er3+ in YNbO4: Er3+/Yb3+ phosphors, the UC luminescence spectra of the samples doped with 5 mol% Er3+/y mol% Yb3+ (y = 0, 2, 6 and 20) and x mol% Er3+/6 mol% Yb3+ (x = 0.5, 1, 9 and 15) were measured at various temperatures ranging from 293 K to 723 K. Figure 7 shows the temperature dependent UC luminescence spectra for the samples with the maximum UC luminescence intensity of the two groups in our study. As can be seen, the two green emission bands originated from 2H11/2, 4S3/2 → 4I15/2 transitions can be observed, and the overall luminescence intensity decreases with an increase of temperature, indicating temperature dependent luminescence quenching occurs. Generally, non-radiative relaxation, energy transfer and crossover process are the main three reasons which may cause the temperature quenching of luminescence16,25. With an increase of sample temperature, the non-radiative relaxation rate of the emitting levels, the energy transfer probability originating from the emitting levels, and the crossover process between the emitting levels of the luminescent centers and the charge transfer band of them or the absorption band of the matrix in high energy region will be enhanced, which will result in the quenching of the luminescence intensity. Similar to our previous research results, the non-radiative relaxation process would be the main mechanism for the temperature dependent UC luminescence quenching of Er3+ in the present case16,25. In addition, it can also be found that the peak positions of them almost unchanged but the relative intensity and the changing trend of each green UC emission are different with an increase of temperature. It can be seen that the intensity of the green UC emission originated from 2H11/2 → 4I15/2 transition first decreases slightly and then remains approximately unvaried. However, the intensity of the emission from 4S3/2 → 4I15/2 transition monotonically decreases with an increase of temperature. However, the intensity ratios R monotonically increases with increasing the sample temperature, which can be seen from Fig. 8(a,b). As can be seen in Fig. 8(a,b), all the experimental data can be fitted well by equation (2). The confirmed equations for different samples are also shown in Fig. 8(a,b). As can be seen, all of the values of ΔE/k are around 1000. The corresponding ΔE value was 694.5 cm−1, which is in good agreement with the value (713.8 cm−1) derived from the measured UC luminescence spectra in Fig. 2. This fact means that equation (2) can explain well the temperature dependence of the FIR of the two green UC emissions.
With regard to the application in optical temperature sensing, it is extremely important to be aware of the temperature sensing sensitivity. Usually, the sensor sensitivity can be defined as the change rate of R per unit temperature and can be expressed as follows38:
By taking the values of A and ΔE/k derived from the fitting processes in Fig. 8(a,b) into equation (3), the sensitivities of the samples were calculated and are shown in Fig. 9(a,b). It can be seen that the sensitivities of all the samples have the same changing trend, and all of them are dependent on the sample temperature. With an increase of sample temperature, the sensitivity first increases and reaches the maximum value at around 536 K, and then dramatically decreases. In addition, it can also be found that the concentrations of Er3+ and Yb3+ have a great effect on the temperature sensing sensitivity. The sensitivity decreases with an increase of the doping concentrations of Er3+ and Yb3+. Similar phenomenon were observed in YNbO4: Er3+ and NaGdTiO4: Er3+, Yb3+ phosphors in our previous studies, which was attributed to the different optical transition rate of Er3+ in different samples doped with various concentrations of Er3+ 22,39. Therefore, if we want to obtain high temperature sensitivity, the doping concentrations of Er3+ and Yb3+ should be further reduced. However, when the concentration is too low, the luminescence of the sample is too weak, which is likely to cause large spectral measurement errors. Therefore, we still need to select the doping concentration of RE ions properly in practical applications. In our present study, the maximum sensitivity of Er3+ is 83.80 × 10−4 K−1 for 0.5 mol% Er3+/6 mol% Yb3+ doped sample.
Laser-induced thermal effect
The UC luminescence process is often accompanied by heat generation, especially when the luminescence materials are excited at long wavelength laser source with high power density. The laser irradiation can result in an elevation of the sample temperature, and further bring temperature dependent effect on the spectroscopic properties of the luminescence materials, thus it is necessary to study the laser-induced thermal effect of the UC luminescence materials.
To understand the thermal effect induced by 1550 nm laser irradiation on the samples, the UC emission spectra of YNbO4: Er3+/Yb3+ phosphors with various doping concentrations of Er3+ and Yb3+ were measured when these samples were continuously irradiated by 1550 nm laser with working current of 2.00A for 10 minutes. The integrated intensity ratios of the two green emissions were calculated according to the UC luminescence spectra, and the sample temperatures were derived by taking the integrated intensity ratios into equation (1). Figure 10(a,c) show the dependence of the sample temperature on the irradiation time of 1550 nm laser for the Er3+ and Yb3+ concentration-varied samples, the working current of 1550 nm laser is fixed at 2.00 A. It can be seen that the temperatures of all the samples change very slightly with irradiation time, indicating that all the samples can quickly reach thermal equilibrium. In addition, it can also be found that the sample temperatures are higher than the room temperature, suggesting the existence of laser-induced thermal effect. Figure 10(b,d) show the dependence of the sample temperatures on the working current of 1550 nm laser for the Er3+ and Yb3+ concentration-varied samples. It can be seen that the sample temperature increases with an increase of the laser working current, indicating significant laser-induced thermal effect in high power density (corresponding to large working current of 1550 nm laser) pumped case. Moreover, it is also observed that the higher the Er3+ (Yb3+) concentration, the higher the sample temperature.
Conclusion
YNbO4 phosphors co-doped with various concentrations of Er3+ and Yb3+ were synthesized via a high temperature solid-state reaction method. XRD results indicated that monoclinic YNbO4 phosphors were obtained. The concentration dependent UC luminescence properties were analyzed and it was found that the UC luminescence intensities greatly depended on the doping concentrations of Er3+ and Yb3+. By the analysis of the laser working current dependent UC luminescence spectra, it was confirmed that three-photon processes were dominated in the red UC emissions, and three-photon and four-photon processes co-existed in the green UC emissions under 1550 nm excitation. In addition, the study of the temperature sensing properties showed that the temperature sensing sensitivity is concentration dependent, and the lower concentrations of Er3+ and Yb3+, the higher temperature sensitivity. Furthermore, the YNbO4: Er3+/Yb3+ phosphors can quickly reach thermal equilibrium excited by 1550 nm laser with high laser power density, and the sample temperature induced by 1550 nm laser irradiation was significantly dependent on both the RE doping concentration and the laser working current. The above results demonstrate that the concentrations of Er3+ and Yb3+ have significant influences on the UC luminescence processes, the temperature sensitivity of Er3+ and the laser-induced thermal effect of YNbO4: Er3+/Yb3+ phosphors. Our results can provide a good reference for choosing proper doping concentrations of RE ions in the study of Er3+ related UC luminescence and temperature sensing.
References
Chen, G. Y., Liang, H. J. & Liu, H. C. Anomalous power dependence of upconversion emissions in Gd2O3: Er3+ nanocrystals under diode laser excitation of 970 nm. J. Appl. Phys. 105, 1–5 (2009).
Li, T. G., Liu, S. W., Wang, E. H. & Song, L. J. Visable and ultraviolet upconversion luminescence of Y2O3: Yb3+, Tm3+ nanomaterials. Acta Phys. Sin. 60, 073201–0732149 (2011).
Chen, G. Y., Kumar, R., Ohulchanskyy, T. Y., Prasad, P. & Ågren, H. Ultrasmall monodisperse NaYF4: Yb3+/Tm3+ nanocrystals with enhanced near-infrared to near-infrared upconversion photoluminescence. ACS Nano. 4, 3163–3168 (2010).
Sokolnicki, J. Upconversion luminescence from Er3+ in nanocrystalline Y2Si2O7: Er3+ and Y2Si2O7: Yb3+, Er3+ phosphors. Mater. Chem. Phys. 131, 306–312 (2011).
Singh, V., Rai, V. K., Al-Shamery, K., Nordmann, J. & Haase, M. NIR to visible upconversion in Er3+/Yb3+co-doped CaYAl3O7 phosphor obtained by solution combustion process. J. Lumin. 131, 2679–2682 (2011).
Yan, K. et al. Investigation of the mechanism of upconversion luminescence in Er3+/Yb3+co-doped Bi2Ti2O7 inverse opal. Opt. Lett. 11, 041602–041605 (2013).
Huang, F. F., Hu, L. L. & Chen, D. P. NIR to visible upconversion in Er3+-doped fluoride glass under 1550 and 980 nm excitations. Ceram. Int. 41, 189–193 (2015).
Tian, Y. et al. Size-dependent upconversion luminescence and temperature sensing behavior of spherical Gd2O3: Yb3+/Er3+ phosphor. RSC Adv. 5, 14123–14128 (2015).
Dwivedi, Y., Mishra, K. & Rai, S. B. Synthesis of bright multicolor down and upconversion emitting Y2Te4O11: Er3+/Yb3+ nanocrystals. J. Alloys Compd. 572, 90–96 (2013).
Auzel, F. Upconversion and anti-stokes processes with f and d ions in solids. Chem. Rev. 104, 139–173 (2004).
Haase, M. & Schäfer, H. Upconverting nanoparticles Angew. Chem. Int. Ed. 50, 5808–5829 (2011).
Ivanova, S. & Pellé, F. Strong 153 μm to NIR-VIS-UV upconversion in Er-doped fluoride glass for high-efficiency solar cells. J. Opt. Soc. Am. B. 29, 1930–1938 (2009).
Maciel, G. S., Rakov, N., Fokine, M., Carvalho, I. C. S. & Pinheiro, C. B. Strong upconversion from Er3Al5O12 ceramic powders prepared by low temperature direct combustion synthesis. Appl. Phys. Lett. 89, 109–110 (2006).
Ivanova, S. et al. Upconversion luminescence dynamics of Er-doped fluoride crystals for optical converters. J. Lumin. 128, 914–917 (2006).
Calderón-Villajos, R., Zaldo, C. & Cascales, C. Enhanced upconversion multicolor and white light luminescence in SiO2-coated lanthanide-doped GdVO4 hydrothermal nanocrystals. Nanotechnology 23, 505205–505215 (2012).
Zhou, T. M., Zhang, Y. Q., Wu, Z. L. & Chen, B. J. Concentration effect and temperature quenching of up-conversion luminescence in BaGd2ZnO5: Er3+/Yb3+ phosphor. J. Rare Earth. 33, 686–692 (2015).
Tian, Y. Y. et al. Effect of Yb3+ concentration on upconversion luminescence and temperature sensing behavior in Yb3+/Er3+ co-doped YNbO4 nanoparticles prepared via molten salt route. Chem. Eng. J. 297, 26–34 (2016).
Zhou, Y. Y., Ma, Q., Lǚ, M. K., Qiu, Z. F. & Zhang, A. Y. Combustion synthesis and photoluminescence properties of YNbO4-based nanophosphors. J. Phys. Chem. C 112, 19901–19907 (2008).
Dwivedi, A., Sinqh, A. K. & Rai, S. B. Down-shifting and upconversion photoluminescence in Ho3+/Yb3+ codoped GdNbO4: effect of the Bi3+ ion and the magnetic field. Dalton Trans. 43, 15906–15914 (2014).
Tian, B. N. et al. Improved upconversion luminescence and temperature sensing in Mo6+-doped LuNbO4: Er3+ phosphor under 1550 nm excitation. Mater. Res. Express 3, 116201–116206 (2016).
Singh, A. K., Singh, S. K., Gupta, B. K., Prakash, R. & Rai, S. B. Probing a highly efficient dual mode: down-upconversion luminescence and temperature sensing performance of rare-earth oxide phosphors. Dalton Trans. 42, 1065–1070 (2013).
Wang, X. et al. Concentration-dependent spectroscopic properties and temperature sensing of YNbO4: Er3+ phosphors. RSC Adv. 7, 23751–23758 (2017).
Huang, F. F., Tian, Y., Li, H. H., Xu, S. Q. & Zhang, J. J. Ho3+/Er3+ co-doped fluoride glass sensitized by Tm3+ pumped by a 1550 nm laser diode for efficient 2.0 μm laser applications. Opt. Lett. 40, 4297–4300 (2015).
Martin-Rodriguez, R. et al. Highly efficient IR to NIR upconversion in Gd2O2S:Er3+ for photovoltaic applications. Chem. Mater. 25, 1912–1921 (2013).
Tong, L. L. et al. Microwave-assisted hydrothermal synthesis, temperature quenching and laser-induced heating effect of hexagonal microplate β-NaYF4: Er3+/Yb3+ microcrystals under 1550 nm laser irradiation. Sensor. Actuat. B-Chem. 246, 175–180 (2017).
Von Dreele, R. B. & Larson, A. C. Los Alamos National Laboratory Report. LAUR 86–748 (1994).
Toby, B. H. EXPGUI, a graphical user interface for GSAS. J. Appl. Cryst. 34, 210–213 (2001).
Ming, C. G. et al. Research on up- and down-conversion emissions of Er3+/Yb3+ co-doped phosphate glass ceramic. Opt. Mater. 35, 244–247 (2012).
Zhang, Y. Q. et al. Dually functioned core-shell NaYF4: Er3+/Yb3+@NaYF4:Tm3+/Yb3+ nanoparticles as nano-calorifiers and nano-thermometers for advanced photothermal therapy. Sci. Rep. 7, 11849–11861 (2017).
Tong, L. L. et al. NaYF4: Sm3+/Yb3+@NaYF4: Er3+/Yb3+core-shell structured nanocalorifier with optical temperature probe. Opt. Express 14, 16047–16058 (2017).
Lu, W. L. et al. The concentration effect of upconversion luminescence properties in Er3+/Yb3+-codoped Y2(MoO4)3 phosphors. Physica B 16, 3284–3288 (2010).
Fu, Y. et al. High-purity red up-conversion emission of Ba5Zn4Y8O21: Er3+, Yb3+ phosphor excited by 1550 nm laser diode. Mater. Res. Bull. 84, 346–349 (2016).
Martín-Rodríguez, R., Rabouw, F. T., Trevisani, M., Bettinelli, M. & Meijerink, A. Upconversion dynamics in Er3+ -doped Gd2O2S: influence of excitation power, Er3+ concentration, and defects. Adv. Opt. Mater. 3, 1–10 (2015).
Martín-Rodríguez, R. et al. Highly efficient IR to NIR upconversion in Gd2O2S: Er3+ for photovoltaic applications. Chem. Mater. 25, 1912–1921 (2013).
Xu, D. K., Liu, C. F., Yan, J. W., Yang, S. H. & Zhang, Y. L. Understanding energy transfer mechanisms for tunable emission of Yb3+- Er3+ codoped GdF3 nanoparticles: concentration-dependent luminescence by near-infrared and violet excitation. J. Phys. Chem. C 119, 6852–6860 (2015).
Zheng, H. et al. Temperature sensing and optial heating in Er3+ single-doped and Er3+/Yb3+ codoped NaY(WO4)2 particles. RSC Adv. 4, 47556–47564 (2014).
Shen, Y., Wang, X., He, H. C., Lin, Y. H. & Nan, C. W. Temperature sensing with fluorescence intensity ratio technique in epoxy-based nanocomposite filled with Er3+-doped 7YSZ. Compos. Sci. Technol. 5, 1008–1011 (2012).
Vetrone, F. et al. Temperature sensing using fluorescent nanothermometers. ACS Nano. 4, 3254–3258 (2010).
Li, X. P. et al. Effects of Er3+ concentration on down-/up-conversion luminescence and temperature sensing properties in NaGdTiO4: Er3+/Yb3+ phosphors. Ceram. Int. 42, 14710–14715 (2016).
Acknowledgements
This work was partially supported in part by the NSFC (National Natural Science Foundation of China (Grant nos. 11774042 and 11104023), in part by the Natural Science Foundation of Liaoning Province (No. 2015020190), in part by the Scientific Research Foundation for Doctoral Program of Liaoning Province (No. 201601072), in part by the High-level personnel in Dalian innovation support program (No. 2016RQ037), and in part by the Fundamental Research Funds for the Central Universities (Grant Nos 3132017056 and 3132016333).
Author information
Authors and Affiliations
Contributions
Xin Wang prepared the samples and wrote the first edition of the manuscript. Xiangping Li made the research proposal and provided the financial support for this work, and also took the responsibility of all correspondence. Hua Zhong made the XRD measurements and help to finish the Rietveld refinement procedure with a non-commercial software, GSAS. Sai Xu and Baojiu Chen revised the manuscript, made the final check. Lihong Cheng, Jinsu Zhang, Jiashi Sun and Lei Li established the sample temperature controlling setup.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Li, X., Zhong, H. et al. Up-conversion luminescence, temperature sensing properties and laser-induced heating effect of Er3+/Yb3+ co-doped YNbO4 phosphors under 1550 nm excitation. Sci Rep 8, 5736 (2018). https://doi.org/10.1038/s41598-018-23981-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-23981-4
This article is cited by
-
Near-infrared optical nanothermometry via upconversion of Ho3+-sensitized nanoparticles
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.