Abstract
Assessment of macular thickness is important in the evaluation of various eye diseases. This study aimed to determine the influence of the optic disc-fovea distance (DFD) on macular thickness in myopic eyes. We determined the DFD and the macular thickness in 138 eyes from 138 healthy myopic subjects using the Cirrus HD-OCT. Correlation analysis and multiple linear regression were performed to determine the influence of DFD, axial length, disc area, and β-PPA on macular thickness. To further remove the confounding effect of ocular magnification on the DFD and OCT scan area, a subgroup analysis was performed in eyes with a limited axial length range (24–25 mm). DFD was significantly correlated with both regional (central, inner, and outer ETDRS subfields) and overall average macular thickness at a Bonferroni corrected P value of 0.004 (r ranging from −0.27 to −0.47), except for the temporal outer (r = −0.15, P = 0.089) and inferior outer (r = −0.22, P = 0.011) macular thickness. In the multivariable analysis, DFD was significantly associated with the average inner and outer macular thickness, the central subfield thickness, and the overall macular thickness (all P < 0.001), independent of ocular magnification and other covariates. Our findings indicate that eyes with a greater DFD have a lower macular thickness.
Similar content being viewed by others
Introduction
Myopia is a prevalent condition in Asia and a major risk factor for glaucoma and various forms of progressive maculopathy1,2,3,4,5,6,7,8. The introduction of optical coherence tomography (OCT) has facilitated the detection of glaucoma and myopic maculopathy9,10,11,12. While macular thickness measurements with OCT are useful to detect glaucoma and myopia associated maculopathy, significant variation of macular thickness in healthy individuals has confounded the detection of macular pathologies13,14,15,16,17,18,19,20,21,22,23,24,25.
In a population-based study, Gupta et al. reported that a longer axial length was significantly associated with a lower overall macular thickness as measured with spectral-domain OCT17. The distance between the optic disc center and the fovea (DFD) is another biometric variable that may influence the macular thickness. Myopic eyes are likely to have a large DFD; the elongation of the eyeball may stretch the fovea further away from the optic disc center26. It has been shown that the DFD is associated with the peripapillary retinal nerve fiber distribution in healthy eyes27. However, to our knowledge, the relationship between DFD and macular thickness has not been reported.
The objective of this study was to determine the influence of DFD on macular thickness. For this purpose, we performed spectral-domain OCT measurements in healthy myopic eyes.
Results
Nine subjects were excluded because of unreliable visual field tests (6 subjects) and poor OCT scan quality (3 subjects). As a result, we included 138 eyes from 138 subjects (60 females and 89 right eyes). Table 1 shows the demographics of the study population. The mean ± standard deviation refractive error and axial length were −5.12 ± 2.30 D and 25.57 ± 1.09 mm, respectively. The mean DFD was 4.58 ± 0.30 mm. Figure 1 displays the distribution of DFD across all subjects. The overall macular thickness and volume were 277.0 ± 12.1 μm and 9.98 ± 0.44 mm3, respectively. DFD was significantly associated with axial length (r = 0.45, P < 0.001) and disc area (r = −0.26, P = 0.002). Of all the included eyes, 49% had β-PPA. Of all scans, 90% had a signal strength of 8 or better (median value 9).
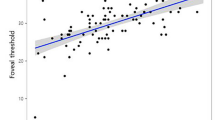
Table 2 demonstrates the associations between the macular thickness parameters and the ocular factors (including DFD, axial length, and refractive error). DFD was significantly correlated with all macular thickness parameters at a Bonferroni-corrected P value of 0.004 (0.05/12), with r ranging from −0.27 to −0.47, except for the temporal outer (r = −0.15, P = 0.089) and inferior outer(r = −0.22, P = 0.011) macular thickness. Axial length was mainly associated with the outer macular thickness parameters. This was less clear for the refractive error. Figure 2 shows the correlation between DFD and the summary macular thickness parameters. Age (r ranging from −0.08 to 0.06, all P > 0.3), signal strength (r ranging from −0.05 to 0.06, all P > 0.5), and visual field mean deviation (MD; r ranging from 0.01 to 0.11, all P > 0.1) did not correlate significantly with any of the macular parameters (within this group of subjects with a limited age range, signal strength range, and healthy eyes, that is, limited MD range). Eyes with β-PPA showed a thinner average macular thickness (273.7 versus 280.2 μm; P = 0.001) and a greater DFD (4.63 versus 4.53 mm; P = 0.002), compared to eyes without β-PPA. Table 3 presents the multivariable analysis. DFD, axial length, and gender were significantly associated with the average inner and outer macular thickness and the overall macular thickness; age and signal strength were not significant in the initial multivariable model and removed from the final model. Disc area and the presence of β-PPA were not significantly associated with any of the macular thickness measurements. Both DFD and gender were significantly associated with the central subfield thickness. There was no significant relationship between axial length and the central subfield thickness.
In order to remove the confounding effect of ocular magnification on the OCT scan area and DFD, a subgroup analysis was performed in eyes with axial length between 24 and 25 mm. Table 4 shows the results. Again, DFD was strongly associated with macular thickness; a larger DFD implies a thinner macula.
Discussion
Disc-fovea distance has a significant inter—individual variability in healthy myopic eyes and is significantly associated with macular thickness. Eyes with a greater DFD have a lower average macular thickness and a lower central subfield thickness, independent of axial length and gender.
Variability in macular thickness in healthy eyes has been described previously13,14,15,16,17,18,19,20,21,22,23,24,25. Several factors, including age, axial length, and gender, have been reported to be associated with macular thickness13,14,15,16,17,18,19,20,21,22,23,24,25. A lower macular thickness has been observed in myopic eyes13,16,18, an effect that could be explained by stretching of the globe. Related to this, it has also been reported that a greater DFD is associated with a longer axial length26. Together this could suggest that the observed relationship between macular thickness and DFD is just an epiphenomenon of myopia. However, we found that DFD was independently associated with macular thickness, that is, not (only) through the effect of axial length. Macular thickness decreased typically 20–30 μm per mm increase in DFD (Tables 3 and 4; these analyses were adjusted for axial length), which corresponds to approximately 10 um per standard deviation of DFD (Table 1)26. As a result, a clinical assessment of macular thickness can be improved significantly by taking DFD into account. Importantly, it is easy to take DFD into account: unlike axial length, you don’t have to measure it separately because it can be measured from the OCT scan itself. Currently this can be done by using the built-in manual measurement tool of the OCT device; in the future this could be added to the software and normative database.
Apart from the stretching as described in the previous paragraph, there is another possible explanation is the difference in scan area for the macular measurements for eyes with different DFD. In eyes with greater DFD, the fovea is farther away from the optic disc, which implies that the OCT scan area (centered at the fovea) is farther away from the optic disc (for a given axial length). According to previous histological and imaging studies28,29, the RNFL is thinner farther from the optic disc than it is closer to the optic disc margin. Thus, one would expect to find that macular thickness is lower in eyes with greater DFD. On the other hand, the retinal ganglion cell layer becomes thicker towards the fovea. As macular thickness data for individual layers are not available for the 6 × 6 mm region of the Cirrus OCT, further studies are warranted.
Regional variations of the association between axial length and macular thickness have been described in previous studies13,15,16,17,18,19. We also found region-specific correlations between axial length and macular thickness (Table 2). However, this could be an artifact arising from the axial length related ocular magnification30. For the OCT device used in the current study, the default axial length is set to 24.46 mm31. Thus, due to ocular magnification, the scan area would be different from 6 × 6 mm in an eye with a shorter or longer axial length30,32,33. With the OCT device used in this study, it is impossible to obtain macular thickness data for an ocular magnification adjusted scan area. To reduce the confounding effect of axial length, we evaluated the correlation between DFD and macular thickness in a subgroup of subjects with a very narrow axial length range. A similar effect of DFD on macular thickness was observed in the multivariable analysis in this subgroup, indicating that DFD is indeed associated with macular thickness, independent of ocular magnification and other covariates.
It has been shown that myopic eyes have a steeper posterior retinal curvature than emmetropic eyes34. Thus, the DFD, based on two-dimensional images, may be underestimated in myopic eyes, especially in eyes with a posterior staphyloma35. Of note, most of the eyes included in present study were not high myopic eyes (the mean refractive error was −5.12 D). Moreover, eyes with myopic macular degeneration including a posterior staphyloma were excluded from the study. Importantly, the results of our subgroup analysis (Table 4) indicate that the uncovered influence of DFD on macular thickness cannot be a spurious effect of the steeper posterior retinal curvature artifact.
The association between gender and macular thickness has been reported in previous studies. Males have been found to have a greater sectoral and overall macular thickness compared to females14,15,17,20,21. Consistent with the previous reports, we found that males have a greater macular thickness after adjusting for DFD and other covariates. It has been reported that eyes with β-PPA tend to have a thinner macular thickness36. In line with previous studies26,36, we found that eyes with β-PPA had a thinner macular thickness and a greater DFD. In the multivariable analysis, however, the presence of β-PPA was not associated with macular thickness. Previous studies have reported that age is significantly correlated with macular thickness14,15,17,20,21,22. In the present study, we did not detect an association between age and any of the macular thickness parameters. One possible explanation is that the age range in our study is relatively narrow. Most of the subjects included in our study were young myopic subjects (Table 1). It has been reported that the image quality of OCT scans affects the observed retinal layer thicknesses37,38,39, and image quality decreases with an increase in myopia40. Consistent with Lee et al.'s study40, we found a negative correlation between signal strength and axial length (r = −0.32, P < 0.001). However, we did not detect a significant relationship between image quality and macular thickness. This is presumably due to the fact that only OCT scans with a high image quality (minimum signal strength was set at 7; 90% of the scans had a signal strength of 8 or better) were included in the current study, which limits the variability of this variable.
There are limitations in the present study. One limitation is that only young myopic subjects of the same ethnicity (all were Chinese) were included. Thus, the current findings may not apply to other populations. Racial differences in macular thickness have been reported41. Further studies are needed to evaluate the association between DFD and macular thickness in other populations and for a wider age range.
In conclusion, a significant inter-subject variability exists in DFD and macular thickness in healthy myopic eyes. Eyes with a greater DFD have a lower macular thickness, independent of axial length. A clinical assessment of macular thickness should always be interpreted in the context of DFD.
Methods
Subjects
One hundred and forty seven Chinese healthy myopic subjects with a spherical equivalent less than −0.5 diopters (D) were consecutively recruited from the refractive surgery clinic of Joint Shantou International Eye Center. All the included subjects underwent a full ophthalmic examination including the measurement of visual acuity and intraocular pressure (IOP), refraction, perimetry (see below), and a dilated stereoscopic fundus examination including assessment of the presence of β-zone peripapillary atrophy (β-PPA). Axial length was determined using the IOL Master (Carl-Zeiss Meditec, Dublin, CA). Only measurements with a signal-to-noise ratio above 2.0 were included. We recorded five measurements and used the mean value for analysis. None of the included eyes had any concurrent ocular disease other than a refractive error. One eye from each subject was included for analysis; if both eyes were eligible, a random eye (based on a computer-generated randomization list) was selected. Subjects were excluded if the best corrected visual acuity was less than 20/40, the IOP over 21 mmHg, if they had a family history of glaucoma, or if they had a history of intraocular surgery, myopic macular degeneration, glaucoma, refractive surgery, neurological disease, or diabetes. The study was approved by the ethical committee of Joint Shantou International Eye Center with written informed consent obtained from each subject before enrolment. The present study followed the tenets of the declaration of Helsinki.
Visual field testing
Visual field testing was performed with standard automated perimetry using the 24–2 grid and the SITA standard strategy (Humphrey Field Analyzer II; Carl Zeiss Meditec, Inc.). Only reliable visual field tests (with fixation loss less than 20% and false positive and negative less than 10%) were included in the study. All visual field tests of the included subjects had a pattern standard deviation (PSD) P > 5% and were within normal limits in the glaucoma hemifield test (GHT).
Optical coherence tomography
All eyes received macular and optic disc imaging using the Cirrus High Definition OCT (software version 5.0.0.326; Carl Zeiss Meditec, Dublin, CA). The scan speed for this spectral-domain OCT is 27,000 A-scans per second and the axial resolution is 5 μm42. Both the Optic Disc Cube 200 × 200 protocol and the Macular Cube 200 × 200 protocol were performed. Disc area generated by the Optic Disc Cube 200 × 200 protocol was recorded for subsequent analysis. The Macular Cube 200 × 200 protocol was used for macular thickness measurements in an area of 6 × 6 mm. Eye movements were checked by reviewing the line-scanning ophthalmoscope (LSO) fundus images. Images with misaligned vessels within the scanning area were excluded and retaken. All the included images had minimum signal strength of 7. Images with segmentation errors were excluded from the analysis (3.5%).
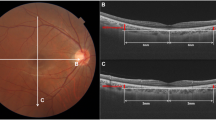
The Cirrus HD-OCT software was used to generate retinal thickness maps. The mean thickness was determined for nine retinal subfields in three concentric circles (with diameters of 1, 3, and 6 mm) centered at the fovea, as defined by the Early Treatment Diabetic Retinopathy Study (ETDRS). The retinal subfields in the retinal thickness map are central, inner superior, inner nasal, inner inferior, inner temporal, outer superior, outer nasal, outer inferior, and outer temporal. The central subfield thickness was defined by the innermost 1 mm diameter circle while the inner and outer subfields were bounded by the 1 and 3 and 3 and 6 mm diameter circles, respectively. Apart from the nine retinal subfields, the average of the four-quadrant macular thicknesses in the inner and outer rings was also calculated as average inner macular thickness and average outer macular thickness, respectively. Overall macular thickness in the entire grid area was also recorded from the retinal thickness maps.
Measurement of DFD
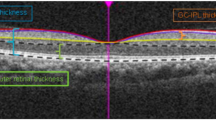
Measurement of DFD was performed with ImageJ software (available in the public domain at http://rsbweb.nih.gov/ij/; www.nih.gov, National Institutes of Health, Bethesda, MD, USA), based on the coordinates of the fovea and the center of the optic disc. For this purpose, we used carefully registered OCT projection images, as illustrated in Fig. 3. We used the LSO fundus image with the macular color thickness map overlay to localize the fovea. The fovea was automatically detected by the OCT software. Subsequently, the enface optic disc image (RNFL thickness deviation map) from the RNFL thickness report was exported and manually registered to the LSO fundus image with Illustrator CS4 software (Adobe Systems Inc., San Jose, California). For this, the transparency of the optic disc image was set to 50% to allow visualization of the underlying LSO fundus image; the retinal vessel trajectories were used as a reference. The optic disc center and the disc margin were determined by the built-in software and shown on the RNFL thickness deviation map, based on the margin of Bruch’s membrane. The DFD was then measured by using the ImageJ software on the overlaid images43,44.
Measurement of optic disc-fovea distance (DFD) on SLO fundus image. The fovea (point F) was automatically detected by the OCT software on the SLO fundus image with the macular color thickness map. The enface optic disc image (RNFL deviation map) with the optic disc center labelled (point D) by the OCT software was manually registered to the SLO fundus image with Illustrator CS4 software using the retinal vessels as reference. Measurement of DFD (distance from F to D) was performed with ImageJ software based on the coordinates of the fovea and the center of the optic disc.
Ocular magnification and subgroup analysis
The actual size of objects at the level of the retina may differ from the reported size, depending on the axial length related ocular magnification. We could adjust the DFD for this magnification, but the built-in software does not adjust the macular area over which the thicknesses and volume are reported. For that reason, we did not adjust DFD for ocular magnification in our main analysis. We also studied the effect of DFD on macular thickness in a subgroup of subjects with a very narrow axial length range, in order to circumvent the confounding effect of ocular magnification. For this analysis, we restricted the axial to a 1 mm range, 24 to 25 mm, centered around the default axial length of OCT of 24.46 mm31.
Statistical analysis
Univariable (Pearson’s or Spearman’s correlation coefficient depending on the distribution) and multivariable analysis (multiple linear regression) were performed to determine the effects of axial length/refractive error, age, gender, image quality, and DFD on the macular thickness. A P value of 0.05 was considered statistically significant; Bonferroni correction was applied if applicable. In the multivariable analysis, we used backward stepwise regression by including initially all variables and subsequently removing, one at a time, those variables with P > 0.05, starting with the variable with the highest P value. The statistical analyses were performed by using the SPSS software (ver. 17.0; SPSS Inc, Chicago, IL).
References
Katz, J., Tielsch, J. M. & Sommer, A. Prevalence and risk factors for refractive errors in an adult inner city population. Invest Ophthalmol Vis Sci. 38, 334–340 (1997).
Morgan, I. G., Ohno-Matsui, K. & Saw, S. M. Myopia. Lancet. 379, 1739–1748 (2012).
Pan, C. W., Ramamurthy, D. & Saw, S. M. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 32, 3–16 (2012).
Liang, Y. B., Wong, T. Y. & Sun, L. P. Refractive errors in a rural Chinese adult population: the Handan eye study. Ophthalmology. 116, 2119–2127 (2009).
Grossniklaus, H. E. & Green, W. R. Pathologic findings in pathologic myopia. Retina. 12, 127–133 (1992).
Koh, V. et al. Myopic Maculopathy and Optic Disc Changes in Highly Myopic Young Asian Eyes and Impact on Visual Acuity. Am J Ophthalmol. 164, 69–79 (2016).
Mitchell, P., Hourihan, F., Sandbach, J. & Wang, J. J. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 106, 2010–2015 (1999).
Marcus, M. W., de Vries, M. M., Junoy Montolio, F. G. & Jansonius, N. M. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 118, 1989–1994 (2011).
Huang, D. et al. Optical coherence tomography. Science. 254, 1178–1181 (1991).
Sun, C. B. et al. Myopic Macular Retinoschisis in Teenagers: Clinical Characteristics and Spectral Domain Optical Coherence Tomography Findings. Sci Rep. 13, 27952 (2016).
Ng, D. S. et al. Advances of optical coherence tomography in myopia and pathologic myopia. Eye (Lond). 30, 901–916 (2016).
Oddone, F. et al. Macular versus Retinal Nerve Fiber Layer Parameters for Diagnosing Manifest Glaucoma: A Systematic Review of Diagnostic Accuracy Studies. Ophthalmology. 123, 939–949 (2016).
Lam, D. S. et al. Regional variations in the relationship between macular thickness measurements and myopia. Invest Ophthalmol Vis Sci. 48, 376–382 (2007).
Duan, X. R. et al. Normal macular thickness measurements using optical coherence tomography in healthy eyes of adult Chinese persons: the Handan Eye Study. Ophthalmology. 117, 1585–1594 (2010).
Song, W. K., Lee, S. C., Lee, E. S., Kim, C. Y. & Kim, S. S. Macular thickness variations with sex, age, and axial length in healthy subjects: a spectral domain-optical coherence tomography study. Invest Ophthalmol Vis Sci. 51, 3913–3918 (2010).
Hwang, Y. H. & Kim, Y. Y. Macular thickness and volume of myopic eyes measured using spectral-domain optical coherence tomography. Clin Exp Optom. 95, 492–498 (2012).
Gupta, P. et al. Determinants of macular thickness using spectral domain optical coherence tomography in healthy eyes: the Singapore Chinese Eye study. Invest Ophthalmol Vis Sci. 54, 7968–7976 (2013).
Zhao, M. H., Wu, Q., Hu, P. & Jia, L. L. Macular Thickness in Myopia: An OCT Study of Young Chinese Patients. Curr Eye Res. 10, 1–6 (2016).
Patel, P. J. et al. Spectral-Domain Optical Coherence Tomography Imaging in 67 321 Adults: Associations with Macular Thickness in the UK Biobank Study. Ophthalmology. 123, 829–840 (2016).
Ooto, S. et al. Three-dimensional profile of macular retinal thickness in normal Japanese eyes. Invest Ophthalmol Vis Sci. 51, 465–473 (2010).
Ooto, S. et al. Effects of age, sex, and axial length on the three-dimensional profile of normal macular layer structures. Invest Ophthalmol Vis Sci. 52, 8769–8779 (2011).
Rao, H. L. et al. Predictors of normal optic nerve head, retinal nerve fiber layer, and macular parameters measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 52, 1103–1110 (2011).
Hashemi, H. et al. The Distribution of Macular Thickness and Its Determinants in a Healthy Population. Ophthalmic Epidemiol. Mar 23, 1–9, https://doi.org/10.1080/09286586.2017.1290808 (2017).
von Hanno, T. et al. Macular thickness in healthy eyes of adults (N = 4508) and relation to sex, age and refraction: the Tromsø Eye Study (2007-2008). Acta Ophthalmol. 95, 262–269 (2017).
Myers, C. E. et al. Retinal thickness measured by spectral-domain optical coherence tomography in eyes without retinal abnormalities: the Beaver Dam Eye Study. Am J Ophthalmol. 159, 445–456 (2015).
Jonas, R. A. et al. Optic Disc-Fovea Distance, Axial Length and Parapapillary Zones. The Beijing Eye Study 2011. PLoS One. 10, e0138701 (2015).
Hong, S. W., Ahn, M. D., Kang, S. H. & Im, S. K. Analysis of peripapillary retinal nerve fiber distribution in normal young adults. Invest Ophthalmol Vis Sci. 51, 3515–3523 (2010).
Gabriele, M. L. et al. Peripapillary nerve fiber layer thickness profile determined with high speed, ultrahigh resolution optical coherence tomography high-density scanning. Invest Ophthalmol Vis Sci. 48, 3154–3160 (2007).
Varma, R., Skaf, M. & Barron, E. Retinal nerve fiber layer thickness in normal human eyes. Ophthalmology. 103, 2114–2119 (1996).
Higashide, T. et al. Influence of Clinical Factors and Magnification Correction on Normal Thickness Profiles of Macular Retinal Layers Using Optical Coherence Tomography. PLoS One. 11, e0147782 (2016).
Kang, S. H., Hong, S. W., Im, S. K., Lee, S. H. & Ahn, M. D. Effect of myopia on the thickness of the retinal nerve fiber layer measured by Cirrus HD optical coherence tomography. Invest Ophthalmol Vis Sci. 51, 4075–4083 (2010).
Bennett, A. G., Rudnicka, A. R. & Edgar, D. F. Improvements on Littmann’s method of determining the size of retinal features by fundus photography. Graefes Arch Clin Exp Ophthalmol. 232, 361–367 (1994).
Leung, C. K. et al. Optic disc measurements in myopia with optical coherence tomography and confocal scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 48, 3178–3183 (2007).
Ohno-Matsui, K., Lai, T. Y., Lai, C. C. & Cheung, C. M. Updates of pathologic myopia. Prog Retin Eye Res. 52, 156–187 (2016).
Rahimy, E., Beardsley, R. M., Gomez, J., Hung, C. & Sarraf, D. Grading of posterior staphyloma with spectral-domain optical coherence tomography and correlation with macular disease. Can J Ophthalmol. 48, 539–545 (2013).
Kwon, J. W., Choi, J. A., Kim, J. S. & La, T. Y. Ganglion Cell-Inner Plexiform Layer, Peripapillary Retinal Nerve Fiber Layer, and Macular Thickness in Eyes with Myopic β-Zone Parapapillary Atrophy. J Ophthalmol. 2016, 3746791 (2016).
Jansonius, N. M., Cervantes, J., Reddikumar, M. & Cense, B. Influence of coherence length, signal-to-noise ratio, log transform, and low-pass filtering on layer thickness assessment with OCT in the retina. Biomed Opt Express. 7, 4490–4500 (2016).
Huang, J., Liu, X., Wu, Z. & Sadda, S. Image quality affects macular and retinal nerve fiber layer thickness measurements on fourier-domain optical coherence tomography. Ophthalmic Surg Lasers Imaging. 42, 216–221 (2011).
Darma, S. et al. Optical density filters modeling media opacities cause decreased SD-OCT retinal layer thickness measurements with inter- and intra-individual variation. Acta Ophthalmol. 93, 355–361 (2015).
Lee R. et al. Factors affecting signal strength in spectral-domain optical coherence tomography. Acta Ophthalmol. Apr 9, https://doi.org/10.1111/aos.13443 (2017).
Girkin, C. A. et al. Variation in optic nerve and macular structure with age and race with spectral-domain optical coherence tomography. Ophthalmology. 118, 2403–2408 (2011).
Carl Zeiss Meditec, Inc. Cirrus HD-OCT User Manual. Dublin, CA: Carl Zeiss Meditec, Inc. 4.18-9. Rev. A (2008).
Amini, N. et al. Influence of the disc-fovea angle on limits of RNFL variability and glaucoma discrimination. Invest Ophthalmol Vis Sci. 55, 7332–7342 (2014).
Pereira, I. et al. Multivariate Model of the Intersubject Variability of the Retinal Nerve Fiber Layer Thickness in Healthy Subjects. Invest Ophthalmol Vis Sci. 56, 5290–5298 (2015).
Acknowledgements
We would like to thank Prof. Christopher Kai-Shun Leung for his useful suggestions regarding this manuscript. The present study was supported by Grant No. 2013B022000091, from Science and Technology Planning Project of Guangdong Province, China; and by the University of Groningen Abel Tasman Talent Program (University Medical Center Groningen/Shantou University Medical College). The funding organizations had no role in the design, conduct, analysis, or publication of this research.
Author information
Authors and Affiliations
Contributions
Involved in study design and conduct (M.Z., K.Q.); data collection, management, analysis (K.Q., X.L., G.W., R.Z.), and interpretation (M.Z., K.Q., N.M. Jansonius); and manuscript preparation, review, or approval (M.Z., K.Q., N.M. Jansonius)
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qiu, K., Wang, G., Zhang, R. et al. Influence of optic disc-fovea distance on macular thickness measurements with OCT in healthy myopic eyes. Sci Rep 8, 5233 (2018). https://doi.org/10.1038/s41598-018-23479-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-23479-z
This article is cited by
-
Diagnostic ability of OCT parameters and retinal ganglion cells count in identification of glaucoma in myopic preperimetric eyes
BMC Ophthalmology (2020)
-
Age-related changes of individual macular retinal layers among Asians
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.