Abstract
Due to the substantial limitation of study population, Spindle cell sarcoma (SCS) was unexplored comprehensively. In this study, we investigated the clinical characteristics and disease specific prognostic factors of SCS. 3299 SCS cases were identified and extracted from Surveillance, Epidemiology, and End Results (SEER) database (1973–2017). White people account for 79.1% with median age of 57 years without predominance in any gender. Significant disease specific survival (DSS) and overall survival (OS) were found differentiated in age, T stage, N stage, M stage, AJCC stage, SEER historic stage, tumor locations, surgery, and pathologic grade. In the multivariate Cox analysis, the age >64 years (for DSS, P < 0.001 and for OS, P < 0.001; Reference age ≤64 years), AJCC stage III (for DSS, P = 0.006 and for OS, P = 0.04; Reference: AJCC stage I), and non-surgical treatment (for DSS, P < 0.001 and for OS, P < 0.001; Reference: surgery) were independently associated with worse DSS and OS. In brief, our study demonstrated that SCS mostly found in white people at fifth to seventh decades of life without gender predilection. The patient’s age, AJCC stage, tumor location and surgery were independent prognostic indicators for both DSS and OS of SCS.
Similar content being viewed by others
Introduction
Spindle cell neoplasm are diverse in nature by means of clinicopathologic and tumor biological heterogeneity1. Primary spindle cell sarcoma (SCS) is an extremely rare entity and one of the least reported tumor2. It is a type of connective tissue tumor and generally begins in layers of connective tissue such as that under the skin, between muscles, and surrounding organs. Only a handful of cases have been reported around the world from variety of body parts3,4,5,6,7,8,9. As such, SCS constitutes a diagnostic and therapeutic challenge10,11.
As morbidity, majority of the previous reports were single case reports and retrospective case series with more than five patients were even few. According to these case reports, the clinical presentations of SCS were similar to the benign lesion at early stage11,12,13,14,15,16,17,18. Like other sarcoma, SCS were treated aggressively with surgical therapy as a mainstay in the management and adjuvant (chemo) radiotherapy was implemented for patients with high risk behavior2,5,7,9,10,13,15,16,17,18.
Nowadays, the sophisticated molecular pathologic diagnostic techniques has made the diagnosis of SCS accurate and reliable2. However, owing to the rarity of SCS, there are lack of basic information regarding the tumor incidence, distinctive clinical characteristics, treatment outcome and disease specific prognostic factors. To address these, a retrospective investigation was carried out with study population from Surveillance, Epidemiology, and End Results (SEER) database.
Results
Summary statistics
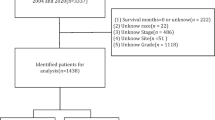
A total of 3299 cases were identified. The average follow-up time was 43 months (SD = 65), with the median follow-up time was 15 months (range, 1–481 months). Of these patients, the mean age at diagnosis of patients was 61 years (SD = 19, range from birth to 103 years) (Fig. 1). The incidence peaked during the seventh decade of life and the majority of cases were white people (80.9%, 2668/3299). There is no significant difference for gender distribution including 1605 females and 1694 males. According to this dataset, SCS could occur at almost any site of the body. The incidence was higher in superficial primary tumor site than internal primary tumor site (2041 vs. 1151 cases). More than 60% of overall case were treated surgery alone.
Among 3229 cases, 2115 cases were found with SCS specific mortality, in which included 1026 female and 1089 male with median age was 57 years (SD = 19). In this disease specific survival (DSS) group, white people account for nearly 80% of population (79.1%, 1674/2115). Regarding the pathological diagnosis, poorly differentiated cases were 27.5% (357/1300) and undifferentiated cases were 42.6% (554/1300). There were 302 early stage cases (AJCC stage I + II) and 367 advanced stage cases (AJCC stage III + IV). The basic clinic-pathological characteristics of overall study population and DSS subgroup summarized in Table 1.
Survival analysis
Survival analysis were performed as previously described19,20. There were significant differences depending on age (P < 0.001), marital status (P = 0.042), pathologic grade (P < 0.001), AJCC stage (P < 0.001), T stage (P < 0.001), N stage (P < 0.001), M stage (P < 0.001), SEER historic stage (P < 0.001), tumor site (P < 0.001) and treatment modality (P < 0.001) in overall survival (OS) (Fig. 2). While, the significant differences were also found DSS for age (P < 0.001), pathologic grade (P < 0.001), AJCC stage (P < 0.001), T stage (P < 0.001), N stage (P < 0.001), M stage (P < 0.001), SEER historic stage (P < 0.001), tumor site (P < 0.001) and treatment modality (P < 0.001) (Fig. 3).
In the univariate cox regression analysis, age, race, pathologic grade, AJCC stage, T stage, N stage, M stage, SEER historic and stage treatment were associated with DSS and OS (Table 2). More importantly, the age >64 years [HR 95% CI: 2.149 (1.619–2.851), P < 0.001, age ≤64 years – as Ref], AJCC stage III [HR 95% CI: 2.803 (1.352–5.813), P = 0.006, AJCC stage I – as Ref], and non-surgical treatment [HR 95% CI: 2.979 (2.154–4.120), P < 0.001, surgery – as Ref] were independently associated with worse DSS. Besides, the age, marital status, AJCC stage, T stage, N stage, SEER historic stage, tumor location and treatment were also independently correlated with OS (Table 3).
Discussion
According to current investigation, SCS affects people of almost all ages which was as same as soft tissue sarcomas21. SCS occur more commonly in middle and old age adult groups. In this series, SCS most frequently occurs during the seventh decades of life with the mean age at diagnosis of SCS is 61 years. In addition, there is no statistically significant difference on incidence rate in gender. However, there is predominance in male with a sex predilection of 1.11:1 male: female ratio in a previous report22. Besides, the overall race distribution includes 80.9% white, 11.4% black, 7.8% American Indian/Asian/Pacific Islander (Table 1). According to the survival analyses depending on demographic factors such as age, gender and race, it demonstrates that only age is an independent prognostic indicator for SCS in DSS and OS.
The treatment modalities were performed for SCS varied, including surgery, adjuvant radiotherapy and chemotherapy in previous available reports. In this study, we only concentrate on the obtainable treatment modality (surgery or not) and hopefully to confirm the role of surgery in SCS treatment. Despite of the difference in surgical style, the surgery group have absolute favorable survival in DSS and OS than non-surgery group. Thus, it indicates that surgical resection remains the mainstay of treatment for SCS. However, the value of extensive radical operation and lymphadenectomy is still ambiguous. Similarly, the descriptive results should not be misinterpreted as causal effects of surgery on survival because of the unavoidable severe treatment selection bias present in this retrospective data source. In addition, the use of adjuvant radiotherapy for SCS remains controversial, and the sensitivity of SCS to chemotherapy in the metastatic setting is highly variable23. Unfortunately, due to the lack of information on other therapies in this study, we are unable to determine the conclusion from this data that SCS patients cannot benefit from radiotherapy or chemotherapy.
The pathologic grade and TNM/AJCC stage are associated with outcome of sarcomas and it is important for treatment protocol planning24,25,26. In this study, although the pathologic grading data in this study was incomplete and half of them were missing in the SEER database, there are still 1986 cases available. According to the SEER Program user’s instruction, cases were listed with latest pathological grading system. Although two histological grading systems are mainly used for soft-tissue sarcoma: the National Cancer Institute (NCI) system and the French Federation of Cancer Centers Sarcoma Group (FNCLCC) system, but there is still no specific system can be used for spindle cell sarcoma. So we used the four-tiered grading system which was most commonly used, and recommended by the American Joint Commission on Cancer (National Cancer Institute, “Tumor Grade”, accessed 18 August, 2014)27. SCS were divided into four different pathologic grades basing on the degree of the cell differentiation28. In results, most of the cases are advanced grade at the first time when they are diagnosed, which includes 546 cases at grade III (pathologically poorly differentiated, 27.5%) and 873 cares at grade IV (pathologically undifferentiated, 42.6%). Previous reports demonstrated pathologic grade is a significant prognostic factor for outcome in soft tissue sarcomas29. Similarly the typical survival differences are found in pathologic grade for both DSS and OS (Figs 2C and 3C). Meanwhile, for TNM stage/AJCC stage survival analysis relatively complete data are available, including 1531cases for TNM staging data 1025 cases AJCC staging. By performing survival analysis, the significant survival difference in OS and DSS have been presented in T stage, N stage, M stage and AJCC stage (Figs 2E–H and 3E–H). Importantly, AJCC stage is one of the independent prognostic factors for SCS in DSS. Similarly, we confirmed SEER historic stage was another independent prognostic indicator for SCS patients. In this results, the SEER stage of distant metastasized tumor was unfavorably associated with DSS and OS for SCS (Localized tumor - as a ref).
The tumor origination is another important factor affecting the outcome of the tumor. SCS can occur in any anatomic location including soft tissue, bone, or viscera30. This study included all of the cases listed as spindle cell sarcoma which were pathologically confirmed (International Classification of Diseases for Oncology, Third Edition, Histologic Type ICD-O-3: 8801) including bone origination, meanwhile excluded undifferentiated high-grade pleomorphic sarcoma (8830/3) which is new category recognizes pleomorphic sarcomas that cannot be classified into any of the other categories. Above all, our study is the largest series of patients and intend to evaluate the primary tumor location as a prognostic factor for the first time. As previous studies, SCS occurred at any location of the body involving skin and subcutaneous connective tissue, tongue, sinus, trachea, atrium, vein, bone, etc.11,31,32. For better characterization and further evaluating, we categorized the tumor locations into two main groups according to the distribution of primary tumor site: superficial site (tumor involving skin and subcutaneous soft tissue in head & neck, upper limb & shoulder, lower limb & hip, thorax & breast, abdomen, pelvis, trunk and other) and interior site (included tumor involving bone or viscera of digestive system, respiratory system, reproductive system, locomotors system, urinary system, nervous system, endocrine system, circulatory system). In this categorization (Tables 4 and 5), we found that SCS was more likely to occur in the superficial site compare with the deep interior site (2014 superficial site cases versus 1151 interior site cases). And significant survival differences were found in both DSS and OS for SCS (Figs 2J and 3J). More importantly, tumor site is another independent prognostic indicator for SCS in both DSS and OS which means primary SCS locates in superficial site possibly have a better outcome.
The several important limitations that come with this study were acknowledged. Most importantly, the use of other treatment modalities is not recorded in the SEER database. Thus we could not identify the role of other treatment modalities, like radiotherapy or chemotherapy, in treatment for SCS. Besides, there are lack of information neither about surgery type nor resection margin status of the tumor. Similarly, it should be noted that some other important data specifically relevant to the tumor including TNM stage, AJCC stage, margin status, local or distant recurrence, lymphatic metastasis status, are either incomplete or absent. Additionally, we have to point out that the follow-up time in SEER is not even and long enough. However, this study is the first using such a large and comprehensive representative registry database to demonstrate the demographic features, clinic-pathologic characteristics, prognostic factors of spindle cell sarcoma.
In summary, it is definitely the largest data about SCS which came from SEER database. Despite its preliminary character, this study can clearly indicate the information on demographic features, distinctive clinicopathologic characteristics, tumor specific prognostic factors and treatment outcome by performing comprehensive analysis of the 3299 SCS cases from the database. The study does demonstrate that SCS mostly occurred during fifth to seventh decade of life in white people without gender specific. More importantly, we found that the ageå 64 years (≤64 years - as a ref), AJCC stage III (AJCC stage I - as a ref), SEER historic stage distant metastasized tumor (Localized tumor - as a ref) and primary tumor site in internal site (Tumor locate in superficial site - as a ref) were independent averse prognostic factors for SCS patients in DSS and OS. Despite the lack of the information about other treatment modalities (radiotherapy or chemotherapy), surgical resection shows the mainstay of treatment modality.
Materials and Methods
The data extraction and statistical analysis were performed as described previously19,20. In brief, the data were extracted with International Classification of Diseases for Oncology codes 8801/3 for SCS from 1973 to 2017 by using official software SEER*Stat, version 8.3.4. Overall statistical analysis was performed by utilizing the software of the Statistical Package for Social Sciences, version 23.0, for Windows (SPSS, Chicago, IL) and survival tabs were generated by GraphPad Prism, version 5.01. The survival curves were generated by using the Kaplan-Meier method, and the survival difference was evaluated by performing the log-rank test. Adjusted hazard ratios (HRs) along with 95% confidence intervals (CIs) were calculated by using the Cox proportional hazards regression model. Differences in the numerical variables were assessed using the Student’s test or non-parametric Wilcoxon test. Categorical variables comparisons were evaluated by the chi square test or Fisher exact test. When the P value was <0.05, the difference was regarded as statistically significant. All statistical tests were two tailed.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
Cerio, R. Spindle cell neoplasms. Journal of the European Academy of Dermatology and Venereology 11, S52 (1998).
Jo, V. Y. & Fletcher, C. D. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology-Journal of the RCPA 46, 95–104 (2014).
Teleb, M. et al. Spindle-cell sarcoma involving the major pulmonary arteries. Proceedings 30, 311–313 (2017).
Reddy, S. S., Sharma, S., Mysorekar, V., Sharma, P. & Kaur, A. Oral Spindle CellSarcoma: A Rare Case Report and Review of Literature. Journal of clinical and diagnostic research: JCDR 11, ZD23–ZD25 (2017).
Patel, K. N., Jha, R. K. & Pandya, S. J. Surgical Management of Primary Spindle Cell Sarcoma of Prostate. Urology 105, e7–e8 (2017).
Waran, E. Delayed diagnosis of spindle cell sarcoma presenting as a large mass on the back. BMJ case reports 2015 (2015).
Muturi, A., Kotecha, V., Ruturi, J., Muhinga, M. & Waweru, W. High-grade spindle cell sarcoma of the heart: a case report and review of literature. Journal of cardiothoracic surgery 10, 46 (2015).
Chen, J. et al. A spindle cell sarcoma of liver supplied by internal mammary artery. Diagnostic and interventional imaging 96, 103–105 (2015).
Siyad, A. K., Gopi, P., Jayan, N. P. & Augustine, J. Spindle cell sarcoma of thyroid: a case report. Indian journal of surgical oncology 5, 312–314 (2014).
Fletcher, C. D. The evolving classification of soft tissue tumours–an update based on the new 2013 WHO classification. Histopathology 64, 2–11 (2014).
Bills, V., Iqbal, R., Haqqani, M. & Clague, J. E. Spindle cell sarcoma: a rare cause of a large abdominal mass. Age and ageing 34, 88–89 (2005).
Cil, T., Altintas, A., Pasa, S., Buyukbayram, H. & Isikdogan, A. Primary Spindle Cell Sarcoma of the Breast. Breast care 3, 197–199 (2008).
Lokesh, V., Naveen, T. & Pawar, Y. S. Spindle cell sarcoma of esophagus: a rare case presentation. Journal of cancer research and therapeutics 6, 100–101 (2010).
Samdhani, S., Choudhary, A., Mahanta, V. R. & Srivastava, S. P. Spindle cell sarcoma of larynx. Indian journal of otolaryngology and head and neck surgery: official publication of the Association of Otolaryngologists of India 58, 305–306 (2006).
Bhaumik, G. & Chatterjee, K. Spindle cell sarcoma of the maxillary antrum. Journal of the Indian Medical Association 110, 657–658 (2012).
Martens, T., Vandekerckhove, K., Francois, K. & Bove, T. Spindle cell sarcoma of the mitral valve: an unusual cause of acute coronary syndrome in a child. The Annals of thoracic surgery 98, 1456–1459 (2014).
Adeleye, A. J., Palmeri, N., Wang, S. H., Liu-Jarin, X. & Wright, J. D. Spindle cell sarcoma of the vulva with myofibroblastic differentiation. Journal of lower genital tract disease 19, e38–39 (2015).
Mehra, S., Ibrahim, O., Moshiri, M., Cahill, J. & Bhargava, P. Spindle-cell sarcoma of the heart: A rare cause for a cardiac mass. Radiology case reports 7, 792 (2012).
Wushou, A., Jiang, Y. Z., Liu, Y. R. & Shao, Z. M. The demographic features, clinicopathologic characteristics, treatment outcome and disease-specific prognostic factors of solitary fibrous tumor: a population-based analysis. Oncotarget 6, 41875–41883 (2015).
Feng, L. et al. Spindle cell carcinoma: the general demographics, basic clinico-pathologic characteristics, treatment, outcome and prognostic factors. Oncotarget 8, 43228–43236 (2017).
Borden, E. C. et al. Soft tissue sarcomas of adults: state of the translational science. Clinical cancer research: an official journal of the American Association for Cancer Research 9, 1941–1956 (2003).
Corey, R. M., Swett, K. & Ward, W. G. Epidemiology and survivorship of soft tissue sarcomas in adults: a national cancer database report. Cancer medicine 3, 1404–1415 (2014).
Collini, P. et al. Sarcomas with spindle cell morphology. Seminars in oncology 36, 324–337 (2009).
Nystrom, L. M. et al. Multidisciplinary management of soft tissue sarcoma. The Scientific World Journal 2013, 852462 (2013).
Voelker, R. Progress for Soft Tissue Sarcoma. Jama 316, 2474 (2016).
Mathur, S., Kapila, K. & Verma, K. Accuracy of cytological grading of spindle-cell sarcomas. Diagnostic cytopathology 29, 79–83 (2003).
Edge, S. B. & Compton, C. C. The American Joint Committee on Cancer: the7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology 17, 1471–1474 (2010).
Coindre, J. M., Nguyen, B. B., Bonichon, F., de Mascarel, I. & Trojani, M. Histopathologic grading in spindle cell soft tissue sarcomas. Cancer 61, 2305–2309 (1988).
Callegaro, D., Miceli, R., Mariani, L., Raut, C. P. & Gronchi, A. Soft tissue sarcoma nomograms and their incorporation into practice. Cancer 123, 2802–2820 (2017).
Fisher, C. Spindle Cell Sarcomas. Surgical pathology clinics 4, 721–744 (2011).
Gurria, J. P. et al. Spindle cell sarcomatoid carcinoma of the trachea: first case report of surgical resection. Journal of cardiothoracic surgery 11, 128 (2016).
Carter, R. M., Uthappa, M. C. & Warakaulle, D. R. Case report: spindle cell sarcoma of the profunda femoris vein mimicking deep venous thrombosis. Clinical radiology 63, 231–235 (2008).
Acknowledgements
We are grateful to Dr. Kamila Abulimiti (MD) from Strayer University (USA) for checking the English of the manuscript. This study was partially supported by The Scientific Research Foundation of Shanghai Stomatological Hospital, Fudan University (SSDCZ-2016-01).
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of the study and writing of the manuscript. L.F. and M.W. wrote the main manuscript text. A.W. gathered the data. F.Y., H.Z. and Y.L.Y. performed the statistical analyses. F.R. prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feng, L., Wang, M., Yibulayin, F. et al. Spindle cell sarcoma: a SEER population-based analysis. Sci Rep 8, 5024 (2018). https://doi.org/10.1038/s41598-018-23145-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-23145-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.