Abstract
This work explored the biotechnological potential of the medicinal halophyte Artemisia campestris subsp. maritima (dune wormwood) as a source of health promoting commodities. For that purpose, infusions, decoctions and tinctures were prepared from roots and aerial-organs and evaluated for in vitro antioxidant, anti-diabetic and tyrosinase-inhibitory potential, and also for polyphenolic and mineral contents and toxicity. The dune wormwood extracts had high polyphenolic content and several phenolics were identified by ultra-high performance liquid chromatography–photodiode array–mass-spectrometry (UHPLC-PDA-MS). The main compounds were quinic, chlorogenic and caffeic acids, coumarin sulfates and dicaffeoylquinic acids; several of the identified phytoconstituents are here firstly reported in this A. campestris subspecies. Results obtained with this plant’s extracts point to nutritional applications as mineral supplementary source, safe for human consumption, as suggested by the moderate to low toxicity of the extracts towards mammalian cell lines. The dune wormwood extracts had in general high antioxidant activity and also the capacity to inhibit α-glucosidase and tyrosinase. In summary, dune wormwood extracts are a significant source of polyphenolic and mineral constituents, antioxidants and α-glucosidase and tyrosinase inhibitors, and thus, relevant for different commercial segments like the pharmaceutical, cosmetic and/or food industries.
Similar content being viewed by others
Introduction
Medicinal plants are increasingly explored by the food industry for their health-promoting benefits either as readily available for herbal teas (e.g. Matricaria chamomilla [chamomile], Cymbopogon citratus [lemongrass]) or as sources of additives for functional foods and drinks (e.g. Aloe vera [aloe], Aspalathus linearis [rooibos])1,2. Yet, medicinal halophytes remain largely unexplored and underutilized despite their outstanding potential as a reservoir of bioactive compounds and innovative health promoting products3. Recently, different scientific efforts have unveiled some of these halophytes’ prospective commercial uses namely as food (e.g. Arthrocnemum macrostachyum4), herbal functional beverages (e.g. Helichrysum italicum subsp. picardii5, Crithmum maritimum6, Limonium algarvense7), or as raw material for pharmaceutical and other related industries (e.g. Lithrum salicaria8, Polygonum maritimum9).
Halophytes live and thrive in saline biotopes characterized by highly fluctuating abiotic constraints. To deal with such unfavourable conditions these salt-tolerant plants developed adaptive responses including the synthesis of highly bioactive molecules with potent antioxidant capacity, such as phenolic compounds, terpenoids and vitamins, to counteract reactive oxygen species (ROS) production and accumulation, inhibit oxidative chain-reactions and protect cellular structures3. These natural antioxidants usually display strong biological activities, like radical-scavenging, metal-chelating and enzyme-inhibiting abilities, leading to beneficial therapeutic properties, which can help explain the use of some halophytes in traditional medicine and as dietary plants3,10. For example, the aromatic Crithmum maritimum is used in folk medicine as diuretic, antiscorbutic, digestive or anti-inflammatory, and is traditionally consumed as condiment, pickle, and in salads11. Another aromatic halophyte, Helichrysum italicum subsp. picardii, is often used as a spice and has folk therapeutic uses such as anti-inflammatory, analgesic or anti-microbial12. Besides their traditional use as food and folk remedies, halophytes can be produced in otherwise uncultivable saline soils and marine-influenced environments and serve as alternative cash crops in saline agriculture. In fact, these plants could be explored for diverse commercial segments, from human and animal nutrition to pharmaceutical and cosmetic industries13,14.
Artemisia campestris L. subsp. maritima Arcangeli (Asteraceae), commonly named dune wormwood (“madorneira” or “erva-lombrigueira” in Portugal), is an aromatic and medicinal halophytic shrub common in coastal sand dunes throughout the temperate European Atlantic coast15,16. Usually consumed as herbal tea made from stems and leaves, it is described as a remedy to treat gastric disorders, hypertension and rheumatics, being also used for its anthelmintic and abortifacient properties15. The species, A. campestris, has additional ethnomedicinal uses described such as anti-diabetic, anti-inflammatory and antipyretic16. Although several studies have already profiled the phytochemical content and bioactivities of A. campestris (revised in Dib et al.16), only few reports focused on the subspecies A. campestris subsp. maritima. Research on this particular plant reports compounds like phenolic acids, flavonoids, coumarins, sesquiterpenes and acetophenone derivatives, determined on organic extracts17,18,19,20, and describes the antioxidant and anti-microbial activities of methanolic extracts20.
In folk medicine, water (infusions and decoctions) and hydro-alcoholic (tinctures) extracts are commonly used to convey the plants’ healing properties21. Considering the potential health benefits of such botanical extracts, medicinal plants can offer a wide range of bioactive components (e.g. polyphenols) and can be explored as raw material for herbal beverages, foods products or constituents in health promoting commodities1. In fact, natural products are currently in high demand and substances with anti-ageing or beauty-enhancement properties (e.g. skin whitening) are on top of consumers list of interest1. Other sought beneficial outcomes include management of diabetes mellitus and improvement of cognitive functions, associated with the intake of antioxidants22. Biochemical studies on medicinal plants can therefore be extremely useful to identify new sources of relevant products for pharmaceutical, cosmetic and/or food industries, and many Artemisia species already find extensive uses as food additives and in perfumery23. In this sense, Artemisia campestris subsp. maritima could be a potential reservoir of bioactive compounds, representing a commercial underexplored opportunity. Therefore, this work’s goal was to explore the dune wormwood’s biotechnological potential as source of bioactive phytochemicals. For that purpose, infusions, decoctions and tinctures were prepared from above and below-ground organs of A. campestris subsp. maritima and assessed for polyphenolic and mineral contents, and in vitro antioxidant, anti-diabetic and tyrosinase-inhibition potential. A preliminary in vitro toxicological assessment was also carried out using mammalian cells. To the best of our knowledge, this is the first time that such an attempt is made with this plant.
Results and Discussion
Phytochemical profile
The polyphenolic content of the extracts was firstly assessed in terms of their total contents of phenolics (TPC), flavonoids (TFC), condensed tannins (CTC), hydroxycinnamic acid derivatives (HAD), flavonols and anthocyanins (Table 1). Phenolic compounds are some of plants most widely occurring secondary metabolites24. Although there is no instituted classification in terms of high/low values of total phenolics, some authors state that natural extracts can be considered rich in phenolic compounds when their TPC is higher than 20 mg GAE/g DW8,25,26. In this sense, all of A. campestris subsp. maritima extracts have high phenolics content considering that TPC was between 114 and 134 mg GAE/g DW, with the highest value determined in aerial-organs’ tincture. This extract also had the highest flavonoid content (40.8 mg RE/g DW), higher HAD together with aerial-organs’ infusion and decoction (89.4–88.4 mg CAE/g DW), and higher anthocyanins along with roots’ tincture (3.46 and 3.36 mg CCE/g DW). Flavonols, on the other hand, were highest in roots’ tincture (66.2 mg QE/g DW). As for tannins content, it was not found in the dune wormwood samples (below the limit of quantification, which was 0.78 mg/g DW). Working with the same sub-species, Megdiche-Ksouri et al.20 reported similar total phenolics (159 mg GAE/g DW) but higher flavonoid (175 mg CE/g DW) and tannin (8.7 mg CE/g DW) contents in methanolic extracts from shoots. These differences could be ascribed not only to the different solvent and extraction procedure, which several studies have showed to greatly influence results, but also to the different analytical methods used13. In similar aqueous and hydro-alcoholic extracts from the aerial parts of the species A. campestris, other authors determined different level of TPC and TFC, either higher, similar or lower than those presently found27,28,29,30,31,32,33. These discrepant phytochemical contents may be explained by species-specific factors, harvesting time and/or environmental characteristics, since these variables affect the biosynthesis of secondary metabolites in plants3,13. Nevertheless, authors generally consider A. campestris rich in phenolic compounds16,30.
To further explore the phytochemical profile of infusions, decoctions and tinctures from A. campestris subsp. maritima a generic LC-PDA-MS (liquid chromatography – photodiode array – mass spectrometry) method for moderately polar phytochemicals was employed. The analytical methodology was adapted from De Paepe et al.34, previously validated by those authors for quantitation of phenolic constituents in apple cultivars, and is fully detailed in Pereira et al.5 including performance characteristics, quantification procedures and compound tentative identification specifics. The aim was to (tentatively) identify phytochemical constituents in the dune wormwood extracts, getting an estimate of their concentrations and/or relative abundances when no reference standards were available. The phenolics and respective concentrations are presented in Table 2. As some standards can be expensive or not available, tentative identification of other compounds was accomplished based on available chromatographic and spectral information (Table 3). To get clean product ion spectra of the detected analytes, data dependent fragmentation was used. Product ions are substructures of precursor ions (ions of a particular mass over charge-range [m/z-range]), formed during fragmentation: structures were assigned to unknown peaks when both the m/z-values and molecular formulae/structures of the precursor and product ions were in agreement. Further information for de-replication was obtained from PDA spectra, in-house and commercial compound databases (PubChem35, Dictionary of Natural Products36, ChemSpider37) and peer reviewed publications (a more detailed explanation is given in Pereira et al.5). MS and diagnostic chromatographic data used for compound identification plus literature used for confirmation of compound identity can be found in Table S2 (supplementary material). It is important to mention that during LC-MS analysis different compounds can have different ionization efficiencies and so no absolute quantitative comparison can be made, although relative abundances per compound in-between samples can be calculated (based on the area of their most abundant ion). In this sense, the “maximum area detected” provides semi-quantitative information of compound abundance. Table 3 shows the relative abundances of these tentatively identified constituents. To visualize the extracts’ main detected compounds, the UV-chromatograms at combined wavelengths (280–330 nm, the absorption maxima of phenolics) are represented in Fig. 1, despite not showing all the constituents identified (compounds with no assigned peaks had low abundances or possibly their peaks overlapped).
According to Table 2, the dune wormwood aerial-organs’ extracts had greater diversity and higher levels of practically all phenolics found. Aerial-organs’ tincture in particular had higher concentrations of most of the determined compounds adding up to a total of 45 µg/mg DW. From this total, quinic acid amounts to half (24 µg/mg DW), followed by chlorogenic (16 µg/mg DW) and caffeic (1.6 µg/mg DW) acids. In fact, these phenolic acids were the main constituents determined in all extracts particularly quinic (roots: 13–15 µg/mg DW, aerial-organs: 24 µg/mg DW) and chlorogenic (roots: 8.4–10 µg/mg DW, aerial-organs: 10–16 µg/mg DW) acids, both higher in tinctures. Rutin was also preferentially detected in aerial-organs aqueous and hydro-alcoholic samples (0.7–1.3 µg/mg DW), followed by protocatechuic acid (0.27–0.43 µg/mg DW), luteolin (0.19–0.47 µg/mg DW) and coumaric acid (0.17–0.33 µg/mg DW), along with isoquercitrin, isorhamnetin and salicylic acid (~0.1–0.2 µg/mg DW). In roots’ extracts, protocatechuic acid in all extracts (0.09–0.11 µg/mg dw), and coumaric and salicylic acids in tincture (0.1 and 0.09 µg/mg dw, respectively) were also found in higher levels, although in comparatively lower concentrations than in the aerial-organ’s extracts. In Table 3 and Fig. 1 it is also possible to observe the higher compound diversity in extracts from aerial-organs, especially tinctures. However, relative abundance of some major constituents such as coumarin sulfates (peaks 12, 14 and 17) and dicaffeoylquinic acids (peaks 25 and 26) was higher in roots’ extracts, particularly tincture. Aerial-organs’ extracts had higher amounts of another coumarin sulfate (peak 13) and dicaffeoylquinic acid (peak 28), along with a chlorogenic acid isomer (peak 5) and an ethoxy/dimetoxycinnamic acid (peak 36). Again, it should be stated that Table 3 provides relative quantitative measures of abundance, not to be interpreted as absolute quantitative comparison. Overall, tinctures of both organs showed higher abundance and diversity of constituents comparatively to aqueous extracts and, between organs, extracts from aerial-organs had greater variety of phenolics, generally in higher levels. To the best of our knowledge, this is the first report comparing anatomical organs in this Artemisia species. Megdiche-Ksouri et al.20 also report a wide assortment of phytochemicals in dune wormwood’s shoots, several of them also presently determined, but studies detailing compound abundance in A. campestris extracts other than essential oils are extremely scarce. In fact, only Jahid et al.33 reports levels of phenolics in leaves’ hydro-alcoholic extracts with the main components catechin and vanillic acid (>20 mg/g DW), not being found in the current study, syringic (6 mg/g DW) and coumaric (0.9 mg/g DW) acids, presently determined at lower concentrations (0.05–0.08 mg/g DW and 0.06–0.33 mg/g DW, respectively), and caffeic acid (0.2 mg/g DW), being one of the current main constituents particularly in aerial-organs’ tincture (1.6 mg/g dw). These authors33 also consider that compound nature and abundance are related to environmental conditions, a well-established notion when comparing intra-species phytochemical content3,13,38,39.
Nevertheless, and although differing considerably between subspecies38, the phenolic profiles of A. campestris compiled in literature are generally in agreement with that reported here and include compounds like phenolic acids such as caffeic, chlorogenic, isochlorogenic and other dicaffeoylquinic acids, flavonoids such as apigenin, rutin, luteolin, kaempferol and quercetin, or hydroxycoumarins like aesculetin and scopoletin16,19,20,40,41,42. In fact, from the wide variety of phenolic constituents (tentatively) identified in A. campestris subsp. maritima extracts (Tables 2 and 3), most if not all were already described in the Artemisia genus. However, for the species A. campestris no reports were found detailing quinic, protocatechuic, p-hydroxybenzoic and salicylic acids, 4-hydroxybenzaldehyde, cynaroside, isoquercitrin and taxifolin (although its derivatives are described), which are, to the best of our knowledge, here described for the first time in the species. Moreover, chlorogenic, syringic, caffeic, coumaric and ferulic acids, luteolin, apigenin and kaempferol were not found reported in the literature for the subspecies under study (although derivatives for the three later are reported) and are therefore here firstly described in A. campestris subsp. maritima.
Mineral composition
Aqueous extracts like herbal teas can be considered an added source of minerals for the human diet2,6. In this context, the presence of these essential nutrients in the dune wormwood’s extracts could be of added value for their potential use as food products or in herbal beverages. Hence, A. campestris subsp. maritima extracts were analysed for mineral content and Table 4 summarizes the results. The most abundant element was Na (9.10–32.6 mg/g DW), followed by K (3.32–15.6 mg/g DW) and Ca (0.09–4.53 mg/g DW), all in higher levels in aerial-organs aqueous extracts. Magnesium (Mg: 0.39–1.67 mg/g DW) and Fe (22–1059 µg/g DW) were also relatively abundant but with higher levels in roots aqueous extract. Mn and Zn were determined in lower concentrations (Mn: 3.31–87.9 µg/g DW; and Zn: 2.30–18.3 µg/g DW). Mn was more abundant in aerial-organs aqueous samples and Zn had similar levels on aqueous extracts of both above and below-ground organs. Moreover, tinctures had consistently lower mineral content showing that water extracts are better at extracting these nutrients from the plant. In fact, herbal teas are usually considered good sources of many elements such as Na, Ca, K, Mg, Fe, Mn or Zn2. Considering the adult daily dietary reference mineral intakes (Na: 1200–1500, Ca: 1000–1300, K: 4700–5100, Mg: 255–350, Fe: 5.0–23, Mn: 1.8–2.6 and Zn: 6.8–10.9 mg/day43), one gram of the dune wormwood’s aqueous extracts could supply up 5% of Mn and 21% of Fe (with regard to the minimum reference values), without reaching the maximum recommended daily intake of Na, and therefore may contribute to the adult daily intakes of some major and minor elements. Moreover, values of Cu and Cr can be considered low and safe for consumption as they are below the recommended dietary allowance values (Cu: 700–1000, Cr: 20–45 µg/day)43 and potentially toxic minerals like Ni, Cd and Pb were not detected (below the LOQs). Even if these were present in the extracts at undetected levels, they would not constitute a threat since the LOQs, when adjusted to the equivalent units based on the extraction yields (Table 1), are below legislated values for plants (Pb 0.3 μg/g and Cd 0.2 μg/g of plant material; EC Regulation 1881/2006). Overall, results highlight a possible nutritional role of the dune wormwood’s extracts, particularly aerial-organs and aqueous extracts, as an additional mineral source.
Toxicological evaluation
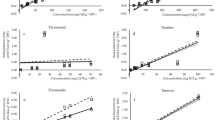
The potential toxicity of new herbal products for human use, such as plant extracts, must be determined to establish its safe consumption. Preliminary toxicological evaluations can be made by in vitro models that address the sensitivity of mammalian cell lines to possible toxic effects of the extracts, delivering reliable and quick results and reducing in vivo testing5,6,7,44,45. Aiming at such a predictive toxicity screening, the dune wormwood extracts were tested for cytotoxicity towards three mammalian cell lines and the resulting cellular viabilities are presented in Fig. 2. The aqueous extracts showed overall low toxicity with cell viability values higher than those obtained for tinctures. Infusions and decoctions exerted no toxic effects in the hepatocarcinoma (HepG2) cells while tinctures had moderate to low toxicity with cellular viabilities between 62% (aerial-organs) and 72% (roots). For the microglia (N9) cell line, toxicity of the aerial-organs aqueous extracts was very low (>90% viabilities) while that of aerial-organs tincture (73% viability) and roots infusion and decoction (63–68% viabilities) can be considered moderate to low; roots’ tincture exerted a more toxic effect with 50% of cellular viability. For the stromal (S17) cells, roots’ aqueous extracts had low toxicity (71–73% viabilities) whereas roots’ tincture (61% viability) and aerial-organs water extracts (66–68% viabilities) were only moderately toxic; aerial-organs’ tincture resulted in 55% of cellular viability. As a preliminary safety evaluation of A. campestris subsp. maritima extracts, results suggest that they may be regarded as safe for consumption, although some caution is advised regarding the use of hydro-alcoholic extracts. Nevertheless, for comparison purposes, the widely consumed green tea had cellular viabilities as low as 30% in S17 cells7. Moreover, acute toxicity tests of A. campestris leaves aqueous extracts on mice showed that up to 3200 mg/kg body weight administered orally neither killed nor impaired behaviour42 and intraperitoneal injections rendered a LD50 equivalent to 2500 mg/kg b.w.28.
Toxicity of infusions, decoctions and tinctures (100 μg/mL extract dw) from Artemisia campestris subsp. maritima organs on mammalian cell lines: (A) N9, (B) S17 and (C) HepG2. Cells treated only with cell culture medium were used as controls; H2O2 was used as positive control for cell toxicity. Values represent the mean ± SD of at least three experiments performed in triplicate (n = 9). In each graph, different letters mean significant differences (p < 0.05).
Biological activities
Antioxidants can be considered a group of medicinally preventive molecules also used as food additives to inhibit food oxidation. Hence, natural antioxidant sources are increasingly sought after as an alternative to synthetic antioxidants in the food, cosmetic and therapeutic industries3,22. Antioxidants are scavengers of free radicals or ROS and deactivators of metal catalysts by chelation, among other activities, reducing oxidative stress and consequent cell damage. It is increasingly documented that dietary antioxidant phytochemicals effectively prevent oxidative damage, reducing the risk of oxidative-stress related conditions like neurodegenerative and vascular diseases, carcinogenesis or inflammation10,22,46. Their intake is also associated with the management of diabetes mellitus22 and amelioration of skin ageing conditions47.
In this work, the antioxidant potential of the dune wormwood’s extracts was assessed by eight different methods targeting radical scavenging activity (RSA) and metal-related potential (Table 5). The extracts were overall effective as scavengers of DPPH, ABTS, NO and O2•— radicals and at reducing iron, but their chelating properties were moderate for copper and low for iron. In the DPPH assay the aerial-organs’ tincture had the lowest IC50 value (240 µg/mL), lower than that obtained for the positive control (BHT; IC50 = 320 µg/mL), followed by aerial-organs’ infusion (330 µg/mL), decoction (340 µg/mL) and roots decoction (370 µg/mL), all similar to BHT (p < 0.05). High RSA against DPPH was also reported by Megdiche-Ksouri et al.20 in methanolic extracts from shoots of the same A. campestris subspecies. Aerial-organs’ tincture also had the strongest NO scavenging activity allowing an IC50 of 290 µg/mL, comparable to that of this organs’ decoction (490 µg/mL, p < 0.05); most interestingly all extracts were better NO scavengers than the positive control (ascorbic acid, IC50 = 2.31 mg/mL). This was also the case with O2•— scavenging as catechin had the highest IC50 (620 µg/mL). For this radical’s assay, however, the lowest IC50 value was obtained after the application of roots’ decoction (180 µg/mL), followed by infusions from both organs (roots: 210 µg/mL, aerial-organs: 230 µg/mL). Roots decoction was also the best ABTS scavenger (IC50 = 370 µg/mL), statistically similar to the result obtained with the aerial-organs’ tincture (IC50 = 400 µg/mL; p < 0.05). As for the iron reducing capacity, the best result was obtained with the aerial-organs’ infusion with an IC50 of 170 µg/mL, followed by aerial-organs’ tincture (230 µg/mL), roots tincture (240 µg/mL) and decoction (250 µg/mL). This is in accordance with Megdiche-Ksouri et al.20 findings of a high FRAP in this subspecies. Conversely, the extracts iron-chelating activity was comparatively low, with IC50 values higher that 5 mg/mL, while the capacity to chelate copper was moderate (best IC50 = 1.3 mg/mL in aerial-organs’ water extracts). Tannins were not detected in any of the extracts, which may partially explain its low chelating potential since tannins are known metal chelating agents48. The aerial-organ’s water extracts had the highest capacity to chelate both metals (CCA, IC50 = 1.30–1.31 mg/mL; ICA, IC50 = 6.33–6.47 mg/mL). Several studies previously highlighted the high antioxidant capacity of similar aqueous and hydro-alcoholic extracts from A. campestris27,29,30,32,33,42, which confirms our results of strong in vitro antioxidant potential for this subspecies. Most of these authors also credited the pronounced antioxidant activity of the extracts to the polyphenolic content which is, in fact, an association widely reported by several studies that confirm the phenolics’ role as antioxidants, especially in halophyte plants3. Accordingly, aerial-organs’ tincture had the highest levels of almost all phenolics groups (Table 1) and was also of the best-scoring extracts in terms of antioxidant activity. Actually, that extract also had overall higher abundance and variety of individual phenolic constituents (Tables 2 and 3), altogether corroborating the hypothesis that phenolics play a major role in the sample’s strong antioxidant potential. For example, the main components quinic, chlorogenic and caffeic acids, determined in higher amounts in aerial-organs’ tincture (Table 2), are known antioxidant compounds49,50,51. Nevertheless, roots’ extracts showed greater relative abundances of some major constituents (Table 3), such as the dicaffeoylquinic acid (peak 25, Fig. 1) in roots’ decoction, and quinic, chlorogenic and caffeic acids, although in lower levels than in aerial-organs’ samples, were the predominant constituents. Synergistic and/or additive effects between these phytoconstituents may also account for the equally high antioxidant activity of roots’ decoction.
Besides antioxidant activity, other bioactivities have been ascribed to extracts from A. campestris as for example hypoglycaemic effects28. Type 2 diabetes mellitus (T2DM) is a common health disorder characterized by high blood glucose levels that can lead to major metabolic complications if left untreated52. One effective strategy to manage T2DM is to inhibit carbohydrate-hydrolysing enzymes, such as α-glucosidase, delaying carbohydrate digestion and uptake and resulting in reduced postprandial blood glucose levels, therefore lowering hyperglycaemia linked to T2DM52,53. In this sense, the dune wormwood’s extracts were tested for their capacity to inhibit microbial and mammalian α-glucosidases as an assessment of their anti-diabetic potential.
All extracts had the ability to inhibit the microbial α-glucosidase but the most active samples were roots’ aqueous extracts and aerial-organs’ decoction (IC50 = 0.89–1.13 mg/mL). Interestingly, all of the extracts were more efficient at inhibiting the microbial α-glucosidase than the positive control used acarbose (IC50 = 3.14 mg/mL), a clinically used inhibitor of this enzyme. However, only the roots’ extracts were able to inhibit mammalian α-glucosidase, particularly roots’ tincture (IC50 = 2.90 mg/mL), still more active than acarbose (IC50 = 4.64 mg/mL). Roots’ extracts were less active towards the mammalian enzyme than for the microbial counterpart, an outcome already described for some compounds showing that enzyme origin can influence the extracts’ inhibition of α-glucosidase54. Nevertheless, and despite the notion that the mammalian enzyme is a more reliable proxy for in vivo activity54, the in vivo anti-diabetic potential of A. campestris aqueous extracts from leaves was demonstrated by Sefi et al.28, having significantly reduced blood glucose levels in diabetic rats. Those authors considered that the in vivo hypoglycaemic activity of A. campestris extracts could be related to its strong antioxidant properties, and stated the role that this plant’s water extracts can have on the treatment of diabetic patients28. It is recognized that polyphenolic compounds, besides potent antioxidants3,10, can also have glucosidase-modulating activities therefore contributing to the management of T2DM52. The dune wormwood’s extracts had a high phenolic content and contained some compounds with described hypoglycaemic activity, namely chlorogenic, caffeic and ferulic acids50,51, and with reported α-glucosidase inhibitory activity, like isoquercitrin, luteolin, quercetin and apigenin52. Overall, our results suggest that all dune wormwood’s extracts could be beneficial in managing T2DM by its capacity to inhibit dietary carbohydrate digestive enzymes, which was higher than acarbose, and consequently controlling glucose levels. Furthermore, as oxidative stress has been considered a mediator in diabetic complications55, the extracts’ strong antioxidant potential can also be an adjuvant in preventing or attenuating the disease’s symptoms when used in combined anti-diabetic strategies.
Skin hyperpigmentation (e.g. melasma, freckles, age spots) is a result of melanin over-production but, as tyrosinase is essential in melanin biosynthesis, inhibition of this enzyme can help prevent and/or manage undesired skin darkening47,56. Tyrosinase is also responsible for unwanted browning of fruits and vegetables, which decreases their market value56,57. Hence, tyrosinase inhibitors from natural sources are increasingly sought not only for cosmetic and medicinal purposes but also for their potential in improving food quality47,56,57. In this context, the tyrosinase inhibitory potential of the dune wormwood’s extracts was evaluated and results are depicted on Table 6. All extracts were active, particularly aerial-organs’ infusion (IC50 = 4.13 mg/mL), although less effective than the used positive control (arbutin, IC50 = 0.48 mg/mL). Tyrosinase is a copper-containing enzyme56 and thus the extracts’ moderate copper chelating activity could be related to their tyrosinase inhibitory capacity. In fact, metal chelating and ROS-scavenging properties are mechanisms often thought to be related with the reducing activity of flavonoids47. Some flavonoids were already identified as tyrosinase inhibitors, as for example quercetin, kaempferol and taxifolin, the last being as effective as arbutin57. All these compounds were detected in the dune wormwood’s extracts, possibly contributing to their tyrosinase inhibitory activity. To the best of our knowledge, this is the first report on the tyrosinase inhibitory potential of A. campestris subsp. maritima.
This study reports for the first time a comprehensive assessment of the biotechnological potential of A. campestris subsp. maritima as a source of innovative products with health promoting properties. Overall, our results point to the potential role of infusions, decoctions and tinctures of the dune wormwood in the prevention of oxidative-stress related diseases and in the management of diabetes and skin-hyperpigmentation conditions. More specifically, those formulations can be considered an unexplored source of polyphenolic and mineral constituents, antioxidants and α-glucosidase and tyrosinase inhibitors that could deliver raw material to different commercial segments including the pharmaceutical, cosmetic and/or food industries. Further studies are being pursued aiming to fully explore the health-promoting benefits of this plant’s extracts, namely their in vivo effects.
Methods
Plant collection
Artemisia campestris L. subsp. maritima Arcang. (Compositae) plants were collected in South Portugal, within the area of the Ria Formosa coastal lagoon, near Faro (Ludo, 37°2′6.526′′N 7°58′58.465′′W), in June of 2013. The taxonomical classification was carried out by Dr. Manuel J. Pinto, botanist in the National Museum of Natural History, University of Lisbon, Botanical Garden, Portugal, and a voucher specimen (voucher code MBH34) is kept in the herbarium of Marbiotech’s laboratory. Plants were divided in roots and aerial-organs (stems and leaves), oven dried at 50 °C until complete dryness (3 days), milled and stored at −20 °C until use.
Extracts preparation: infusions, decoctions and tinctures
Water extracts were prepared similarly to a regular cup-of-tea: 1 g of dried plant material was homogenized in 200 mL of ultrapure water. For infusions, the biomass was immersed in boiling water for 5 min; for decoctions, the biomass was boiled in water for 5 min. Hydro-ethanolic extracts were prepared similarly to a home-made tincture: 20 g of dried plant material was left homogenising in 200 mL of 80% aqueous ethanol for a week. Independent extractions (n ≥ 3) for each combination of method + plant-part were made. All extracts were filtered (Whatman n° 4), vacuum and/or freeze-dried and stored in a dark, cool and moist-free environment. Extracts were re-suspended in water or aqueous ethanol to a concentration of 10 mg/mL to determine (spectrophotometric) phenolic content and test for bioactivities. For these assays, no significant differences were found among corresponding extracts from the different extractions and therefore freeze-dried extracts were pooled accordingly for the remaining analyses.
Phytochemical composition of the extracts
Total polyphenols (TPC), flavonoids (TFC) and condensed tannin (CTC) content
The TPC, TFC and CTC were estimated by spectrophotometric methods, respectively: Folin-Ciocalteau, aluminium chloride colorimetric and 4-dimethylaminocinnamaldehyde (DMACA), as described in Rodrigues et al.26. Gallic acid, quercetin and catechin were used as standards and results are presented as milligrams of standard equivalents per gram of extract dry weight (GAE, QE and CE, respectively; mg/g dw). Further information pertained to these methods is presented in Table S1 (supplementary material).
Hydroxycinnamic acid derivatives (HAD), flavonols and anthocyanins content
Total contents in HAD, flavonols and anthocyanins were assessed spectrophotometrically as described previously26 using caffeic acid, quercetin and cyanidin chloride as standards, respectively. Results are presented as milligrams of standard equivalents per gram of extract dry weight (CAE, QE and CCE, respectively; mg/g dw). Further information pertained to these methods is presented in Table S1 (sup. material).
Profile of moderately polar compounds by UHPLC
Standard stock solutions were prepared at 1 mg/mL in UHPLC-grade methanol and stored at 4 °C in the dark. Standard dilutions were prepared in 60:40 (v:v) methanol:40 mM ammonium formate buffer (reference standards: apigenin, apigenin-7-O-glucoside (apigetrin), catechin, cyanidin-3-O-arabinoside, cyanidin-3-O-galactoside chloride (ideain chloride), cyanidin-3-O-glucoside chloride (kuromanin chloride), cyanidin-3-O-rutinoside chloride (keracyanin chloride), (+)-dihydrokaempferol ((+)-aromadendrin), epicatechin, epigallocatechin, epigallocatechin gallate, flavone, galangin, hesperidin, hesperidin methyl chalcone, 4-hydroxybenzaldehyde, kaempferol, kaempferol-3-O-glucoside (astragalin), limonin, luteolin, naringenin, naringin, neohesperidin dihydrochalcone, phloretin, phloretin-O-20-glucoside (phloridzin), procyanidin B2, protocatechuic acid, propyl gallate, quercetin, quercetin-3-O-arabinoside (avicularin), quercetin-3-O-galactoside (hyperin), quercetin-3-O-glucoside (isoquercitrin), quercetin-3-O-rhamnoside (quercitrin), rutin, uvaol, and caffeic, chlorogenic, coumaric, dihydrocaffeic, ellagic, ferulic, gallic, gentisic, m-hydroxybenzoic, hydroferulic, p-hydroxybenzoic, oleanolic, quinic, rosmarinic, salicylic, sinapinic and syringic acids). Freeze-dried pooled extracts (approx. 15 mg) were dissolved in 20 mL of 60:40 methanol:water +40 mM ammonium formate, followed by 1 min vortex mixing, 30 min sonication (40 kHz, 100 W, room temperature) and 10 min centrifugation (3000 rpm). Supernatants were diluted 100-fold and stored along with undiluted extracts at 4 °C, until analysis. Both undiluted and diluted extracts were analysed with a generic ultra-high performance liquid chromatography – photodiode array – accurate mass mass spectrometry (UHPLC-PDA-amMS) method for moderately polar phytochemicals adapted from De Paepe et al.34 and fully detailed in Pereira et al.5. Briefly, for analysis 5 µL of extract was injected on an UPLC BEH SHIELD RP18 column (3.0 mm × 150 mm, 1.7 µm; Waters, MA) and thermostatically eluted (40 °C) with a quaternary solvent manager and a ‘Hot Pocket’ column oven. The mobile phase consisted of water +0.1% formic acid (A) and acetonitrile +0.1% formic acid (B), following a gradient of (min/%A): 0.0/100, 9.91/74, 18.51/35, 18.76/0, 23.76/0, 23.88/100, 26.00/100. For detection, a Q Exactive MS (Thermo Fisher Scientific, Bremen, Germany) was used with heated electrospray ionization (HESI). For quantitative analysis, full scan data were acquired using polarity switching with a mass/charge (m/z) range of 120–1800 and resolving power set at 70 000 at full width at half maximum (FWHM). Data were also recorded using data dependent fragmentation (ddMS2) in positive and negative ionization mode to obtain additional structural information. The PDA detector was set to scan from 190 to 800 nm during all analyses. The lowest calibration point included in the calibration curve was used to calculate the limits of quantitation (LOQs). The concentration ranges described by De Paepe et al.34 were also used during the present work. Results regarding concentrations of identified compounds were calculated as µg/mg of extract dry weight.
Mineral composition
Freeze-dried pooled extracts were digested in a combination of nitric acid (HNO3) and hydrogen peroxide on a hot plate and evaporated until dryness (up to 24 h). Digested samples were diluted in 20 mL of 5% HNO3 and analysed for mineral content by Microwave Plasma-Atomic Emission Spectrometer (MP-AES; Agilent 4200 MP-AES, Agilent Victoria, Australia), as described in Pereira et al.6. Instrumental detection limits were as follows: Ca: 0.04 μg/L, Cd: 1.4 μg/L, Cr: 0.3 μg/L, Cu: 0.5 μg/L, Fe: 1.7 μg/L, K: 0.6 μg/L, Mg: 0.031 mg/L, Mn: 0.1 μg/L, Na: 0.1 μg/L, Ni: 1.1 μg/L, Pb: 2.5 μg/L and Zn: 3.1 μg/L. Results were expressed as mg or μg/g of extract dry weight (DW). Appropriate blanks were also produced and analysed.
Toxicological evaluation of the samples
Samples’ toxicity was assessed using murine microglia (N9), murine bone marrow stromal (S17) and human hepatocellular carcinoma (HepG2) cell lines. The N9 cell line was provided by the Faculty of Pharmacy and Centre for Neurosciences and Cell Biology (University of Coimbra, Portugal), S17 and HepG2 cells were delivered by the Centre for Biomedical Research (CBMR, University of Algarve, Portugal). Cell culture was maintained as described in Pereira et al.6. Toxicity was evaluated according to Rodrigues et al.7. Briefly, N9 cells where plated at an initial density of 1 × 04 cells/well while S17 and HepG2 cells were seeded at 5 × 103 cells/well, all in 96-well plates. Freeze-dried pooled extracts were dissolved in culture medium (100 μg/mL) and incubated with cells for 72 h; culture medium was used as negative control and hydrogen peroxide (H2O2) as positive control. Cell viability was determined by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and results were expressed in terms of cell viability (%).
Biological activities
Antioxidant activity assessed by four radical-based assays
The extracts’ radical scavenging capacity against the DPPH (1,1-diphenyl-2picrylhydrazyl), ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), NO (nitric oxide) and O2•— (superoxide) radicals was assessed as described in Rodrigues et al.7,26. BHT (butylated hydroxytoluene), ascorbic acid and catechin were used as positive controls. Results were calculated as percentage of antioxidant activity in relation to a control containing ultrapure water or aqueous ethanol, and expressed as IC50 values (mg/mL; half maximal inhibitory concentration, ascertained for extracts with activities higher than 50% at 10 mg/mL).
Antioxidant activity assessed by three metal-related assays
The extracts’ chelating ability towards copper (CCA) and iron (ICA) and their Fe3+ reducing capacity (ferric reducing antioxidant power, FRAP) were assessed as described previously26. EDTA (ethylenediamine tetraacetic acid) and BHT were used as positive controls. Results were calculated as percentage of antioxidant activity relative to a positive control for FRAP, and in relation to a negative control (ultrapure water/aqueous ethanol) for CCA and ICA, and were expressed as IC50 values (mg/mL).
In vitro anti-diabetic activity: inhibition of microbial and mammalian α-glucosidases
The microbial α-glucosidase enzyme was obtained from the yeast Saccharomyces cerevisiae; rat’s intestine acetone powder was used to obtain a crude enzyme extract as an example of a mammalian-origin α-glucosidase. The extracts’ capacity to inhibit both enzymes was assessed following Kwon et al.53 and using acarbose as positive control. Results are expressed as IC50 values (mg/mL), calculated as percentage of inhibitory activity in relation to a control (ultrapure water/aqueous ethanol).
In vitro tyrosinase inhibition
The extracts’ ability to inhibit tyrosinase was assessed following Custódio et al.58, using arbutin as positive control. Results, calculated as percentage of inhibitory activity in relation to a control (ultrapure water/aqueous ethanol), are expressed as IC50 values (mg/mL).
Statistical analysis
Experiments were conducted at least in triplicate and results were expressed as mean ± standard deviation (SD). Significant differences (p < 0.05) were assessed by one-way analysis of variance (ANOVA) followed by Tukey pairwise multiple comparison test or, when parametricity of data did not prevail, Kruskal Wallis one-way analysis of variance on ranks followed by Dunn’s test. Statistical analyses were executed using XLStat® version 19.4. IC50 values were computed by curve fitting in GraphPad Prism® version 6.0c.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Gruenwald, J. G. Novel botanical ingredients for beverages. Clin. Dermatol. 27, 210–216 (2009).
Pohl, P. et al. The determination of elements in herbal teas and medicinal plant formulations and their tisanes. J. Pharm. Biomed. Anal. 130, 326–335 (2016).
Ksouri, R. et al. Medicinal halophytes: potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 32, 289–326 (2012).
Barreira, L. et al. Halophytes: Gourmet food with nutritional properties? Food Comp. Anal. 59, 35–42 (2017).
Pereira, C. G. et al. Chemical profiling of infusions and decoctions of Helichrysum italicum subsp. picardii by UHPLC-PDA-MS and in vitro biological activities comparatively with green tea (Camellia sinensis) and rooibos tisane (Aspalathus linearis). J. Pharm. Biomed. Anal. 145, 593–603 (2017).
Pereira, C. G. et al. Searching for new sources of innovative products for the food industry within halophyte aromatic plants: In vitro antioxidant activity and phenolic and mineral contents of infusions and decoctions of Crithmum maritimum L. Food Chem. Toxicol. 107, 581–589 (2017).
Rodrigues, M. J. et al. In vitro antioxidant and anti-inflammatory properties of Limonium algarvense flowers’ infusions and decoctions: A comparison with green tea (Camellia sinensis). Food Chem. 200, 322–329 (2016).
Lopes, A. et al. Natural products from extreme marine environments: Searching for potential industrial uses within extremophile plants. Ind. Crops Prod. 94, 299–307 (2016).
Rodrigues, M. J. et al. In vitro and in silico approaches to appraise Polygonum maritimum L. as a source of innovative products with anti-ageing potential. Ind. Crops Prod. 111, 391–399 (2018).
Shahidi, F. & Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects: A review. J. Funct. Foods 18, 820–897 (2015).
Atia, A., Barhoumi, Z., Mokded, R., Abdelly, C. & Smaoui, A. Environmental eco-physiology and economical potential of the halophyte Crithmum maritimum L. (Apiaceae). J. Med. Plants Res. 5(16), 3564–3571 (2011).
Viegas, D. A., Palmeira-de-Oliveira, A., Salgueiro, L., Martinez-de-Oliveira, J. & Palmeira-de-Oliveira, R. Helichrysum italicum: From traditional use to scientific data. J. Ethnopharmacol. 151, 54–65 (2014).
Buhmann, A. & Papenbrock, J. An economic point of view of secondary compounds in halophytes. Funct. Plant Biol. 40(9), 952–967 (2013).
Hasanuzzaman, M. et al. Potential use of halophytes to remediate saline soils. BioMed Res. Int. Article ID 589341, 12 pages (2014).
Almargem – Projeto “Biodiversidade a seus pés, n° 0400212”. http://almargem.org/biodiv/, (accessed 08/2017) (2017).
Dib, I., Angenot, L., Mihamou, A., Ziyyat (Pr), A. & Tits, M. Artemisia campestris L.: Ethnomedicinal, phytochemical and pharmacological review. J. Herb. Med. 7, 1–10 (2016).
Rauter, A. P. et al. Flavonoids from Artemisia campestris subsp. maritima. Phytochemistry 28(8), 2173–2175 (1989).
Sanz, J. F., Garcia-Lliso, V., Marco, J. A. & Vallés-Xirau, J. A cadinane derivative from Artemisia crithmifolia. Phytochemistry 30(12), 4167–4168 (1991).
Vasconcelos, J. M. J., Silva, A. M. S. & Cavaleiro, J. A. S. Chromones and flavanones from Artemisia campestris subsp. marítima. Phytochemistry 49(5), 1421–1424 (1998).
Megdiche-Ksouri, W. et al. Artemisia campestris phenolic compounds have antioxidant and antimicrobial activity. Ind. Crops Prod. 63, 104–113 (2015).
Handa, S. S. Chapter 1, An Overview of Extraction Techniques for Medicinal and Aromatic Plants in Extraction Technologies for Medicinal and Aromatic Plants (eds Handa, S. S., Khanuja, S. P. S., Longo, G. & Rakesh, D. D.) 21–54 (ICS-UNIDO, 2008).
Sindhi, V. et al. Potential applications of antioxidants – A review. J. Pharm. Res. 7, 828–835 (2013).
Silva, A. M. S. et al. Chemical composition of Artemisia campestris and Hibiscus cannabinus. In Natural Products in the New Millennium: Prospects and Industrial Application (eds Rauter, A. M., Palma, F. B., Justino, J., Araújo, M. E. & Santos, S. P.) 47–57 (Springer Science+Business Media, B.V., 2002).
Balasundram, N., Sundram, K. & Samman, S. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem. 99, 191–203 (2006).
Kähkönen, M. P. et al. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 47(10), 3954–3962 (1999).
Rodrigues, M. J. et al. Unravelling the antioxidant potential and the phenolic composition of different anatomical organs of the marine halophyte Limonium algarvense. Ind. Crop. Prod. 77, 315–322 (2015).
Djeridane, A. et al. Screening of some Algerian medicinal plants for the phenolic compounds and their antioxidant activity. Eur. Food Res. Technol. 224, 801–809 (2007).
Sefi, M., Fetoui, H., Makni, M. & Zeghal, N. Mitigating effects of antioxidant properties of Artemisia campestris leaf extract on hyperlipidemia, advanced glycation end products and oxidative stress in alloxan-induced diabetic rats. Food Chem. Toxicol. 48, 1986–1993 (2010).
Saoudi, M., Allagui, M. S., Abdelmouleh, A., Jamoussi, K. & El Feki, A. Protective effects of aqueous extract of Artemisia campestris against puffer fish Lagocephalus lagocephalus extract-induced oxidative damage in rats. Exp. Toxicol. Pathol. 62, 601–605 (2010).
Akrout, A., Gonzalez, L. A., Jani, H. E. & Madrid, P. C. Antioxidant and antitumor activities of Artemisia campestris and Thymelaea hirsuta from southern Tunisia. Food Chem. Toxicol. 49, 342–347 (2011).
Boulanouar, B., Abdelaziz, G., Aazza, S., Gago, C. & Miguel, M. G. Antioxidant activities of eight Algerian plant extracts and two essential oils. Ind. Crops Prod. 46, 85–96 (2013).
Ghlissi, Z., Sayari, N., Kallel, R., Bougatef, A. & Sahnoun, Z. Antioxidant, antibacterial, anti-inflammatory and wound healing effects of Artemisia campestris aqueous extract in rat. Biomed. Pharmacother. 84, 115–122 (2016).
Jahid, A. A., Essabaq, S., Elamrani, A., Blaghen, M. & Eddine, J. J. Chemical composition, antimicrobial and antioxidant activities of the essential oil and the hydro-alcoholic extract of Artemisia campestris L. leaves from southeastern Morocco. J. Biol. Act. Prod. Nat. 6(5–6), 393–405 (2016).
De Paepe, D. et al. An improved mass spectrometric method for identification and quantification of phenolic compounds in apple fruits. Food Chem. 136, 368–375 (2013).
PubChem online chemistry database. https://pubchem.ncbi.nlm.nih.gov, (accessed 05/2017) (2017).
Dictionary of Natural Products, version, Chapman and Hall/CRC, DVD, ISBN 9780412491504 (2016).
ChemSpider chemical structure database - Royal Society of Chemistry. http://www.chemspider.com/, (accessed 05/2017) (2017).
Valant-Vetschera, K. M., Fischer, R. & Wollenweber, E. Exudate flavonoids in species of Artemisia (Asteraceae – Anthemideae): new results and chemosystematic interpretation. Biochem. Syst. Ecol 31, 487–498 (2003).
Jallali, I. et al. Variability of antioxidant and antibacterial effects of essential oils and acetonic extracts of two edible halophytes: Crithmum maritimum L. and Inula crithmoïdes L. Food Chem. 145, 1031–1038 (2014).
Akkari, H. et al. In vitro evidence that the pastoral Artemisia campestris species exerts an anthelmintic effect on Haemonchus contortus from sheep. Vet. Res. Commun. 38, 249–255 (2014).
Riedel, H., Cai, Z. & Smetanska, I. Obtaining phenolic acids from cell cultures of various Artemisia species. Afr. J. Biotechnol. 9, 8805–8809 (2010).
Sebai, H. et al. Protective effect of Artemisia campestris extract against aspirin-induced gastric lesions and oxidative stress in rat. RSC Adv. 4, 49831–49841 (2014).
Otten, J. J., Hellwig, J. P. & Meyers, L. D. Dietary reference intakes: the essential guide to nutrient requirements (eds Otten, J. J., Hellwig, J. P. & Meyers, L. D.). 1329pp. (The National Academies Press, 2006).
Saad, B., Azaizeh, H., Abu-Hijleh, G. & Said, O. Safety of traditional Arab herbal medicine. Evid. Based Complement. Alternat. Med. 3, 433–439 (2006).
Nogueira, D. R., Mitjans, M., Infante, M. R. & Vinardell, M. P. Comparative sensitivity of tumor and non-tumor cell lines as a reliable approach for in vitro cytotoxicity screening of lysine-based surfactants with potential pharmaceutical applications. Int. J. Pharm. 420, 5–58 (2011).
Saeidnia, S. & Abdollahi, M. Toxicological and pharmacological concerns on oxidative stress and related diseases. Toxicol. Appl. Pharmacol. 273, 442–455 (2013).
Ribeiro, A. S., Estanqueiro, M., Oliveira, M. B. & Lobo, J. M. S. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2, 48–65 (2015).
Karamać, M. Chelation of Cu(II), Zn(II), and Fe(II) by tannin constituents of selected edible nuts. Int. J. Mol. Sci. 10, 5485–5497 (2009).
Pero, R. W., Lund, H. & Leanderson, T. Antioxidant metabolism induced by quinic acid. Increased urinary excretion of tryptophan and nicotinamide. Phytother. Res. 23, 335–346 (2008).
Meng, S., Cao, J., Feng, Q., Peng, J. & Hu, Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: a review. Evidence-Based Complementary Altern. Med. Article ID 801457, 11 pages (2013).
Alam, A. et al. Hydroxycinnamic acid derivatives: a potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 27, 13 pages (2016).
Kumar, S., Narwal, S., Kumar, V. & Prakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 5(9), 19–29 (2011).
Kwon, Y. I., Apostolidis, E. & Shetty, K. In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresour. Technol. 99, 2981–2988 (2008).
Oki, T., Matsui, T. & Osajima, Y. Inhibitory effect of α-Glucosidase inhibitors varies according to its origin. J. Agric. Food Chem. 47, 550–553 (1999).
Panigrahy, S. K., Bhatt, R. & Kumar, A. Reactive oxygen species: Sources, consequences and targeted therapy in Type-IIDiabetes. J. Drug Target. 25, 93–101 (2016).
Khan, M. T. H. Molecular design of tyrosinase inhibitors: A critical review of promising novel inhibitors from synthetic origins. Pure Appl. Chem. 79(12), 2277–2295 (2007).
Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 10, 2440–2475 (2009).
Custódio, L. et al. Methanol extracts from Cystoseira tamariscifolia and Cystoseira nodicaulis are able to inhibit cholinesterases and protect a human dopaminergic cell line from hydrogen peroxide-induced cytotoxicity. Pharm. Biol. 54(9), 1687–1696 (2016).
Acknowledgements
This work was supported by FCT – Foundation for Science and Technology funding (CCMAR/Multi/04326/2013) and Portuguese National Budget. Luísa Custódio was supported by FCT Investigator Programme (IF/00049/2012). Catarina Guerreiro Pereira acknowledges FCT for the PhD grant (SFRH/BD/94407/2013), Sebastiaan Bijttebier thanks the Research Foundation - Flanders (FWO) for a post-doc grant (12M8315N).
Author information
Authors and Affiliations
Contributions
C.G.P., L.C. and L.B. designed the study. S.B. analysed the phytochemical profile of the extracts by LC-PDA-amMS; C.M. performed the toxicological evaluation of the samples; T.F.S. assessed the extracts’ mineral contents; C.G.P. undertook the remaining work. C.G.P. wrote the main manuscript text with the contribution of L.C., S.B., L.P., J.V. and L.B. L.C. and L.B. jointly supervised the work. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pereira, C.G., Barreira, L., Bijttebier, S. et al. Health promoting potential of herbal teas and tinctures from Artemisia campestris subsp. maritima: from traditional remedies to prospective products. Sci Rep 8, 4689 (2018). https://doi.org/10.1038/s41598-018-23038-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-23038-6
This article is cited by
-
A metabolomics approach to evaluate the effect of lyophilization versus oven drying on the chemical composition of plant extracts
Scientific Reports (2021)
-
Phytochemistry and pharmacological activity of the genus artemisia
Archives of Pharmacal Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.