Abstract
In riparian zones along the Tarim River in northeastern China, the co-dominance by Populus euphratica and Tamarix ramosissima at the early succession stage shifts to P. euphratica dominance in the late stages. However, little is known about how this shift is mediated by the highly variable water conditions in riparian zones. Here we conducted a mesocosm experiment in which we measured the physiological and morphological traits of these two co-occuring species grown in mixtures under simulated favorable groundwater condition and no groundwater availability. Results indicated that T. ramosissima, in comparison to P. euphratica, had much lower WUE, less proportion of root biomass under favorable groundwater condition. Under no groundwater condition, T. ramosissima also showed higher maximal quantum yield of PSII which allowed it to accumulate higher aboveground and total biomass. Therefore, regardless of groundwater conditions, T. ramosissima exhibited superior competitive advantages against P. euphratica under direct competition condition, which demonstrates that the dominance shift was not resulted from the direct competition at seedling stage. Our findings further imply that a strategy of “sit and wait” in P. euphratica might favor its growth and survival when suffered flooding disturbances, thus allowing P. euphratica not being excluded through competition at early successional stage.
Similar content being viewed by others
Introduction
Environmental variability is often considered as an important influence on community structure because of its effects on population growth and species interactions such as competition. In particular, temporal environmental variability is commonly believed to promote species diversity by preventing competitive exclusion that would otherwise occur1,2. In theory, given a constant environment, species with superior advantage would drive out the weaker ones. When the environment is variable, one species might not always have superior advantage over others, thus results in coexistence3,4,5. Study the competition effects under various environment, not only helps us to explain how species replacement and community succession, but also provides guidelines for forest management and reconstruction5,6.
Riparian plants are subject to high variability in water availability due to hydrological fluctuations7,8,9,10. The spatial-temporal variations of water availability, interacted with plant-plant competition, can further determine the riparian plant distribution11. Because competition takes place in the context of environmental conditions, competitive advantages need to be considered over a range of conditions12. In desert riparian environment, for example, species with the ability to grow rapidly in wet years and tolerate water deficit in drought years may be more competitive13.
Tarim River is a 1321-km-long inland river, located in the arid region of northwestern China. Desert riparian forest occurs along the river, dominated by a tree species Populus euphratica Oliv.14. However, at the seedling stage near the active river channel, there are seedlings of another species, T. ramosissima, co-establish and co-dominate the riparian community15. The shift from the dominance of both species at the seedling stage to the dominance of only P. euphratica at late successional stage suggests that P. euphratica out-competes T. ramosissima during the succession. Because P. euphratica is a tree species while T. ramosissima is a shrub species, earlier occupancy of the riparian space to avoid being suppressed by the other species would win a competitive advantage, so that early competition is critical for the riparian community succession.
Previous studies showed that competition during the succession is mediated by environment variability. Implications from the southwestern USA riparian ecosystems where Tamarix have replaced native popular and willow species16 indicate that the dominance of Tamarix was largely resulted from reduced competition intensity by native species due to groundwater decline and/or flood disappearance17. Another example was that Synedra was competitively advantage over Fragilaria in both constant and varying cultures, but the rate of competitive exclusion was slower in varying cultures18. As for the two species in the Tarim River riparian community, seedlings of T. ramosissima exhibits competitive advantages over seedlings of P. euphratica even under favorable water environment where P. euphratica was most likely expected to out compete T. ramosissima19. T. ramosissima was also more drought tolerant20 and able to accumulate more biomass under variable groundwater conditions in monocultures15. These studies all suggested that T. ramosissima was more competitive than P. euphratica, contrary to the dominant position of P. euphratica in the forests along the Tarim River. Thus further work on plant interactions between the two species under variable water environment is needed.
Since the growth of both species are highly depended on groundwater availability in Tarim River Basin21, here we examined the competition outcome between these two co-occurring species under simulated groundwater-available condition (the groundwater was shallow but no plants were inundated) and groundwater-unavailable environment (the groundwater is not available for seedlings). Under each condition, P. euphratica and T. ramosissima seedlings were grown in mixtures and ecophysiological traits relative to competitive ability were measured, including leaf photosynthesis, biomass allocation and root distribution. We asked: (1) whether competition between the two species was mediated by groundwater conditions, and then (2) whether the direct competition at the early stage is responsible for the shift of dominant species during the community succession in the fields of the Tarim desert riparian forests.
Results
Plant water status
ψ pd and ψ md in P. euphratica were significantly higher (P < 0.05) than those of T. ramosissima regardless of water environments (Fig. 1).
Predawn (ψ pd ) and (ψ md ) midday xylem water potential for P. euphratica and T. ramosissima under available groundwater and no groundwater environment. Values are mean ± SD (n = 3). Asterisk indicates significant difference with paired bars (* represents P < 0.05, NS represents no significance, the same below).
Leaf gas exchange
Maximum net photosynthesis rate (A max ) and water use efficiency (WUE) were significantly higher (P < 0.05) in P. euphratica than T. ramosissima under available groundwater condition, but there was no significant difference between the two species under no groundwater environment (Fig. 2a,b). Maximal quantum yield of PSII (F v /F m ) was significantly higher (P < 0.05) in T. ramosissima under no groundwater condition, while there was no significant difference under available groundwater environment (Fig. 2c).
Biomass allocation and root distribution
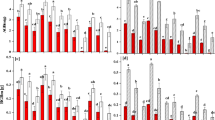
Total biomass and aboveground biomass were significantly higher (P < 0.05) in T. ramosissima than that of P. euphratica regardless of water environments (Fig. 3a,b). Root biomass was significantly higher (P < 0.05) in T. ramosissima than P. euphratica under available groundwater environment, while there was no significant difference between the two species under no groundwater condition (Fig. 3c).
The percentage of root biomass in P. euphratica was significantly higher (P < 0.05) than T. ramosissima, shoot biomass in P. euphratica was significantly lower (P < 0.05) than T. ramosissima correspondingly regardless of water condition (Fig. 4).
There was significant difference in total root length between the two species. Under available groundwater condition, with the increasing of soil depth, total root length for T. ramosissima increased at first, then decreased, it reached the maximum at 40 cm depth. While root length of P. euphratica increased firstly, then stayed constant from 40 cm to 80 cm, reached the peak at 100 cm depth. Under no groundwater condition, root length of T. ramosissima peaked at 100 cm depth, while P. euphratica peaked at 120 cm depth (Fig. 5).
Both species showed positive relationships between log (height) and log (aboveground biomass). Height of P. euphratica increased with aboveground biomass quicker (higher regression slope) than T. ramosissima (Fig. 6).
Relationship between log (biomass) and log (height) for P. euphratica and T. ramosissima under available groundwater and no groundwater environment. Data of P. euphratica under no (open circles) and high (filled circles) groundwater conditions were modeled with a dashed line, while T. ramosissima under no (open triangle) and high (filled triangle) groundwater conditions were modeled with a solid line.
Discussion
Our results indicated that T. ramosissima had competitive advantages over P. euphratica, evidenced by higher aboveground as well as total biomass regardless of groundwater conditions. The consistent results under two groundwater conditions suggested that the dominance shift from early to late successional stages in Tarim desert riparian forests was not resulted from early direct competition. Furthermore, we found that the competition outcome was associated with the growth strategies in the studied species at the early stage. P. euphratica allocated higher proportional biomass to roots under both groundwater conditions, a strategy favoring survival rather than growth in riparian environments. On contrary, the relationships between plant height and biomass suggested that T. ramosissima tends to occupy horizontal space, a strategy as weeds22,23 that suppress its competitors through rapid growth.
Ecophysiological traits of plants reflect adaption strategies to various environments, which provide guidelines for species replacement during forest community succession24,25. In agreement with Wu et al.19, in our study, T. ramosissima had superior advantages over P. euphratica under available groundwater condition (Figs 1 and 3). T. ramosissima accumulated more biomass in two ways. Firstly, the significantly lower WUE suggested that T. ramosissima accumulated carbon at high expense of water (Fig. 2), which was in agreement with Brotherson & Field26. Secondly, although T. ramosissima had lower net photosynthetic rate, and much lower percent of root biomass allocation in T. ramosissima (Fig. 4) indicated that it invested more to aboveground biomass with higher potential to increase leave areas, thus advantaged more CO2 accumulation. Li et al.15 demonstrated that T. ramosissima had significant higher percent leaf biomass than P. euphratica under various groundwater conditions. Benefited from these traits, T. ramosissima could suppress P. euphratica under available groundwater condition.
Under no groundwater environment, T. ramosissima showed more negative Ψ md than P. euphratica (−2.9 MPa for T. ramosissima versus −2.0 MPa for P. euphratica, Fig. 1). T. ramosissima also has a more negative water potential inducing 50% loss of hydraulic conductivity (P50) (−4.5 MPa for T. ramosissima and −0.70 MPa for P. euphratica)20,27, thus according to the estimation of hydraulic safety margin by Choat et al.28, it is clear that P. euphratica was in a higher risk of hydraulic failure. Furthermore, significantly lower maximal quantum yield of PSII F v /F m of P. euphratica (Fig. 2) also demonstrated the higher degree of water stress. Under no groundwater condition, both species had similar WUE, the higher drought tolerance ensured T. ramosissima to accumulate more biomass (Fig. 3). Overall, our results demonstrated that stronger competitiveness of T. ramosissima in our experiment benefited from its water waste strategy and higher aboveground biomass allocation under available groundwater condition, and more drought tolerance under no ground water condition.
Water availability in riparian zone in arid and semi-arid region was highly variable due to hydrological fluctuations10,29. Thus, root allocation strategy in such environment is critical for the survival and growth in species. Higher proportion of roots allocation and deeper roots distribution was advantage survival of species when water is unfavorable water environment, but would restrict plant growth under favorable water environments. Less proportion of root allocation and shallower roots distribution would advantage growth of species when water is favorable, but might have water stress risk when water environment is unfavorable7. In our study, in agreement with Busch and Smith30, Horton and Clark31, both species were phreatophytic, their roots nearly attached the bottom of the pools either in favorable or unfavorable water condition, but P. euphratica had higher roots biomass allocation and deeper root length distribution than that of T. ramosissima regardless of groundwater (Fig. 5), suggested advantage in growth for T. ramosissima under available groundwater condition and in survival for P. euphratica under no groundwater environment.
As a light-demanding species, P. euphratica should grow higher to avoid being shaded by T. ramosissima. Yokozawa and Hara32 indicated that allometric relationship between plant height and aboveground mass implied competitive strategies for light. Taller plants will are able to project their leaves in the highest positions of the canopy where they receive the more light intensities33,34. On the other hand, there are costs related to increase height: plants invest a disproportionate amount of biomass in support tissue. In our study, to access more light intensities, P. euphratica tended to increase height at the cost of lateral growth (Fig. 6). Such strategy was advantage to the survival of P. euphratica. But T. ramosissima tended to occupy horizontal space to suppress P. euphratica.
Based on the root allocation, relationship between height and biomass in P. euphratica, it is possible that it could “sit and wait” until conditions become favorable. For instance, T. ramosissima suffers from large percentage of biomass loss due to extreme stress or disturbance, thus releasing the intensity of competition between them. This is a reasonable hypothesis to explain the domination of P. euphratica. As desert riparian zone is highly disturbance by runoff of river, seedlings near channel were more vulnerable. Plants with higher aboveground biomass and horizontal growth have higher risk of being uprooted by extreme floods35. So plants invest more to aboveground biomass have higher risk to loss more in desert riparian zone, while the strategy in P. euphratica might make it suffer less loss from disturbance. Moreover, P. euphratica have lancet-like leaves at seedling stage, and such leaf shape is known to decrease flush resistance, thus favoring decreasing aboveground biomass loss during floods35. As suggested by the “storage effect” theory4, P. euphratica might “store” more underground biomass in various groundwater environments, which buffered the effects of disturbance. All the above competition strategies explained the co-existence of both species at the seedling stage. As long as P. euphratica can survive the early stage (as we observed that the mortality in P. euphratica seedlings was negligible although they were obviously suppressed by T. ramosissima), it will out compete T. ramosissima in the late stage of the plant community in the Tarim riparian zones.
Our study implied that the success of P. euphratica in Tarim riparian zone probably rely on disturbance that change or weaken competition direction in T. ramosissima. Shafroth et al.36 indicated that dam construction in the western USA altered disturbance thus accelerated the invasion of Tamarix. With more water consuming and manual control of runoff with the development of agriculture in Tarim region, the decrease of water volume and natural flow in Tarim River will be serious ecological problem37. The larger distribution area of T. ramosissima than that of P. euphratica38 suggested the changing of hydrological environment in Tarim region, which might lead to the expansion of T. ramosissima. Therefore, management of natural flow in Tarim River is critical for final community structure in Tarim region, and for the maintenance of P. euphratica forest.
Our study demonstrate that T. ramosissima has overwhelming competition advantages at the seedling stage over P. euphratica regardless of groundwater conditions, so that the early direct competition between the two species is not the reason for the dominance of P. euphratica in late successional stages in the Tarim River Basin. In details, seedlings of P. eupharitca allocated more proportional biomass to root, and tended to grow higher at the cost of lateral growth when suppressed by T. ramosissima. Such a strategy allows P. euphratica to survive at the early successional stage. A strategy of “sit and wait” in P. euphratica might favor its growth and survival when suffered flooding disturbances, which could release the intensity of direct competition at the early successional stage, leading to the dominance by P. euphratica in the riparian plant communities in fields. Therefore, it is critical to maintain natural flooding regimes for the management of the desert riparian forest in the Tarim Basin.
Materials and Methods
Study sites
Experiments were carried out at the Aksu Water Balance Station, Chinese Academy of Sciences (40°27′N, 80°45′E, hereafter Aksu Station), located in the Tarim Basin, northwestern China. The region is characterized by a hyperarid climate, with an annual mean precipitation of 45.7 mm but an annual mean potential evaporation greater than 2500 mm. In August 2011, seeds of both species were collected from natural populations and then sown in a common garden in the Aksu Station. Then in March 2013, seedlings with heights ranging from 35 to 42 cm were transplanted into outdoor concrete pools designed for simulating different groundwater conditions.
Experimental design
The experimental pools, 5 m2 of each in size, were filled with soil collected from riparian zones of the Tarim River, with drainage valves at different depths to control groundwater depth. Details of the experiment design were described by Wu et al.19. Briefly, seedlings of both species were grown alternatively at a space of 0.4 m * 0.4 m, total 28 seedlings in each pool that was similar in density to field communities at this stage. There were 6 pools in total, three for favorable groundwater treatment, and the other 3 for no groundwater available. For the available groundwater treatment, a drainage valve at 0.4 m depth in each pool was maintained open, and flooding irrigations were carried out weekly during the experiment, through which the groundwater was maintained within a range between 0.4 and 0.6 m below soil surface. For no groundwater treatment, irrigation was ceased after middle July when the seedlings were successfully established in pools. For the extremely low precipitation and great evaporative demand in Tarim region, rainfall was not excluded for the no groundwater treatment.
Data collections
We determined plant water status by measuring leaf water potential at predawn (Ψ pd ) and midday (Ψ md ) with a pressure chamber (PMS, Corvallis, OR, USA). Measurements were carried out between 06:30 and 07:30 for Ψ pd and between 15:30–16:30 for Ψ md . A minimum of nine fully expanded mature leaves from three individuals per species in each pool were sampled for leaf water potential measurements. Water status for P. euphratica and T. ramosissima were measured three times from July to September.
Leaf gas exchange was measured between 10:30 and 12:30 using a portable photosynthesis system equipped with a CO2 injector (Li6400, Li-Cor, Lincoln, USA). Based on preliminary trials, the photosynthetic photon flux density was set at 1500 μmol m−2s−1 to ensure that light-saturated photosynthesis rates were reached for the two study species. Ambient CO2 was maintained at 400 μmol mol−1. Similar to the sampling way for leaf water potential measurements, a minimum of nine fully expanded mature leaves from three individuals per species in each pool were selected, for the quantification of maximum net CO2 assimilation rate (A max ), stomatal conductance (g s ) and transpiration rate (T r ), leaf water use efficiency (WUE) was calculated as A max /T r . Chlorophyll fluorescence parameters (F v /F m ) were measured in a standard fluorescence leaf chamber with a Li-6400 portable photosynthesis system. Prior to the measurement in the early morning, a clip was placed on each leaf for 30–40 min for dark adaptation39. Gas exchange for P. euphratica and T. ramosissima were also measured three times from July to September.
At the end of the experiment, nine seedlings of each species for each treatment were harvested to measure aboveground biomass. We dug a ditch around the pool thus made an earth cube in the center of the pool. Then the cube was soaked with water for a few hours to facilitate removal of roots from the loamy soils with a spray nozzle. We used tape to measure root length at each layer for the selected individuals. All biomass was dried in an oven at 65 °C for 72 h and then weighed.
Data analysis
We use t-test to analysis xylem water potential, above-, below-biomass, A max , WUE, F v /F m between species in each water treatment. We fitted the relationship between log-transformed height and aboveground biomass with linear models. All statistical tests were performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA).
References
Chesson, P. et al. Resource pulses, species interactions, and diversity maintenance in arid and semi-arid environments. Oecologia 141, 236–253 (2004).
Descamps-Julien, B. & Gonzalez, A. Stable coexistence in a fluctuating environment: an experimental demonstration. Ecology 86, 2815–2824 (2005).
Chesson, P. L. & Warner, R. R. Environmental variability promotes coexistence in lottery competitive systems. Am. Nat. 117, 923–943 (1981).
Chesson, P. Multispecies competition in variable environments. Theor. Popul. Biol. 45, 227–276 (1994).
Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–66 (2000).
Martorell, C. & Freckleton, R. P. Testing the roles of competition, facilitation and stochasticity on community structure in a species-rich assemblage. J. Ecol. 102, 74–85 (2014).
Naiman, R. J. & Décamps, H. The ecology of interfaces: riparian zones. Annu. Rev. Ecol. Syst. 28, 621–658 (1997).
Lytel, D. A. & Poff, N. L. Adaptation to natural flow regimes. Trends Ecol. Evol. 19, 94–100 (2004).
Stromberg, J. C., Tluczek, M. G. F., Hazelton, A. F. & Ajami, H. A century of riparian forest expansion following extreme disturbance: spatio-temporal change in Populus/ Salix/Tamarix forests along the Upper San Pedro River, Arizona, USA. For. Ecol. Manage. 239, 1181–1189 (2010).
Stella, J. C., Rodríguez-González, P. M., Dufour, S. & Bendix, J. Riparian vegetation research in Mediterranean-climate regions: common ecological processes, and considerations for management. Hydrobiologia 719, 291–315 (2013).
Kotowski, W. et al. Waterlogging and canopy interact to control species recruitment in floodplains. Funct. Ecol. 24, 918–926 (2010).
Sher, A. A. & Marshall, D. L. Seedling competition between native Populus deltoides (Salicaceae) and exotic Tamarix ramosissima (Tamaricaceae) across water regimes and substrate types. Am. J. Bot. 90, 413–422 (2003).
Cleverly, J. R., Smith, S. D., Sala, A. & Devitt, D. A. Invasive capacity of Tamarix ramosissima in a Mojave Desert floodplain: the role of drought. Oecologia 111, 12–18 (1997).
Zhang, Y. M., Chen, Y. N. & Pan, B. R. Distribution and floristics of desert plant communities in the lower reaches of Tarim River, southern Xinjiang, People’s Republic of China. J. Arid Environ. 63, 772–784 (2005).
Li, J. et al. Physiological and morphological responses of Tamarix ramosissima and Populus euphratica to altered groundwater availability. Tree Physiol. 33, 57–68 (2013).
Glenn, E. P. & Nagler, P. L. Comparative ecophysiology of Tamarix ramosissima and native trees in western U.S. riparian zones. J. Arid Environ. 61, 419–446 (2005).
Merritt, D. M. & Poff, N. L. Shifting dominance of riparian Populus and Tamarix along gradients of flow alteration in western North American rivers. Ecol. Appl. 20, 135–152 (2010).
Grover, J. P. Dynamics of competition in a variable environment: experiments with two diatom species. Ecology 69, 408–417 (1988).
Wu, G. et al. Competition between Populus euphratica and Tamarix ramosissima seedlings under simulated available groundwater availability. J. Arid Land 8, 293–303 (2016).
Gries, D. et al. Growth and water relations of Tamarix ramosissima and Populus euphratica on Taklamakan desert dunes in relation to depth to a permanent water table. Plant Cell Environ. 26, 725–736 (2003).
Zhang, X. W., Cheng, T. F., Chen, H. W. & Tian, X. M. Underground water monitoring and analysis on Tarim River Basin. Journal of Shihezi University: Natural Science 25, 364–368 (2007). (In Chinese).
Tomaso, D. J. M. Impact, biology, and ecology of saltcedar (Tamarix spp.) in the southwestern United States. Weed Technol. 12, 326–336 (1998).
Dudley, T. L. & Deloach, C. J. Saltcedar (Tamarix spp.), Endangered Species, and Biological Weed Control—Can They Mix? Weed Technol. 18, 1542–1551 (2004).
McGill, B. J., Enquist, B. J., Weiher, E. & Westoby, M. Rebuilding community ecology form functional traits. Trends Ecol. Evol. 21, 178–185 (2006).
Zhu, S. D., Song, J. J., Li, R. H. & Ye, Q. Plant hydraulics and photosynthesis of 34 woody species from different successional stages of subtropical forests. Plant Cell Environ. 36, 879–891 (2013).
Brotherson, J. D. & Field, D. Tamarix: Impacts of a successful weed. Rangelands 9, 110–112 (1987).
Hukin, D. et al. Cavitation vulnerability in roots and shoots: does Populus euphratica Oliv., a poplar from arid areas of Central Asia, differ from other poplar species? J. Exp. Bot. 56, 2003–2010 (2005).
Choat, B. et al. Global convergence in the vulnerability of forests to drought. Nature 491, 752–755 (2012).
Stromberg, J. C. et al. Altered stream-flow regimes and invasive plant species: the Tamarix case. Global Ecol. Biogeogr. 16, 381–393 (2007).
Busch, D. E. & Smith, S. D. Mechanisms associated with decline of woody species in riparian ecosystems of the Southwestern US. Ecol. Monogr. 65, 347–370 (1995).
Horton, J. L. & Clark, J. L. Water table decline alters growth and survival of Salix gooddingii and Tamarix chinensis seedlings. Forest Ecol. Manag. 140, 239–247 (2001).
Yokozawa, M. & Hara, T. A canopy photosynthesis model for the dynamics of size-structure and self-thinning in plant populations. Ann. Bot. 70, 305–316 (1992).
Weiner, J. & Thomas, S. C. Size variability and competition in plant monocultures. Okios 47, 211–222 (1986).
Anten, N. P. R. & Werger, M. J. A. Canopy structure and nitrogen distribution in dominant and subordinate plants in a dense stand of Amaranthus dubius L. with a size hierarchy of individuals. Oecologia 105, 30–37 (1996).
Usherwood, J. R., Ennos, A. E. & Ball, D. J. Mechanical and anatomical adaptations in terrestrial and aquatic buttercups to their respective environments. J. Exp. Bot. 48, 1469–1475 (1997).
Shafroth, P. B., Stromberg, J. C. & Patten, D. T. Riparian vegetation response to altered disturbance and stress regimes. Ecol. Appl. 12, 107–123 (2002).
Chen, Y. et al. Progress, challenges and prospects of eco-hydrological studies in the Tarim River basin of Xinjiang, China. Environ. Manag. 51, 138–153 (2013).
Zhu, X. C. et al. Spatial pattern of riparian vegetation in desert of the lower Tarim River basin. Chinese Journal of Plant Ecology 39, 1053–1061 (2015). (in Chinese).
Yi, Y. H., Fan, D. Y., Xie, Z. Q. & Chen, F. Q. Effects of waterlogging on the gas exchange, Chlorophyll Fluorescence and water potential of Quercus Variabilis and Pterocarya Stenoptera. J. Plant Ecol-UK 30, 960–968 (2006).
Acknowledgements
We would like to thank Tian Xiaohua and Wang Wenliang for their assistance during the experiment. This work was supported by grants from the National Natural Science of China (41171037 and U1403281).
Author information
Authors and Affiliations
Contributions
L.J. and W.G.L. designed and performed the experiment, analyzed and interpreted data, and wrote the manuscript. J.S.W. performed the experiment. L.H., Z.S.D., Z.Y., Z.D.D. and L.Q. wrote the manuscript. All authors have read, reviewed the intellectual content, corrected and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, G., Jiang, S., Liu, H. et al. Early direct competition does not determine the community structure in a desert riparian forest. Sci Rep 8, 4531 (2018). https://doi.org/10.1038/s41598-018-22864-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-22864-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.