Abstract
While resistance to anticoagulant rodenticides is known to occur in many European populations of Norway rat and house mouse, to-date no data is available on the occurrence in Ireland of such resistance. No genetic evidence for the occurrence of resistance was found in 65 Norway rat samples analysed, indicative of an absence, or low prevalence, of resistance in rats in at least the Eastern region of the island of Ireland. The presence of two of the most commonly found amino acid substitutions Leu128Ser and Tyr139Cys associated with house mouse resistance to anticoagulant rodenticides was confirmed. The occurrence of two such mutations is indicative of the occurrence of resistance to anticoagulant rodenticides in house mice in the Eastern region of the island of Ireland.
Similar content being viewed by others
Introduction
Following their introduction in the 1950s, warfarin and the structurally similar anticoagulant rodenticides (e.g. chlorophacinone and coumatetralyl), resulted in radical changes in rodent pest management practice. The newly introduced rodenticides had delayed action, with the result that mortality followed days or weeks after initiation of treatment, an effect that rendered them especially suitable for use to control Norway rats, also known as brown rats (Rattus norvegicus), a species that displays neophobic reactions. Reflecting the higher tolerance of house mice (Mus musculus) to such compounds, the use of warfarin and other first generation anticoagulants for their control was frequently unsatisfactory1.
Following the identification in 1958 of warfarin resistant rat populations in Scotland2, such resistance was subsequently confirmed at locations in Wales, in England3, in Denmark4, in Germany5, in Belgium6 and is now considered widespread. The first reports of warfarin-resistant mice populations were published in the early 1960s7.
As a consequence of increasing concerns generated by the identification of warfarin resistant rodent populations, industry developed the more potent anticoagulant rodenticides bromadiolone, brodifacoum, difenacoum, difethialone and flocoumafen, known as second generation anticoagulant rodenticides. As in the case of the first-generation compounds, these reflect the 4-hydroxycoumarin structure, but display increased lipophilicity that results in their having longer half-lives8.
Anticoagulant rodenticides being vitamin K antagonists, block Vitamin K metabolism in the liver by preventing the enzyme vitamin K epoxide reductase (VKOR) from reducing vitamin K epoxide to vitamin K. Vitamin K, an essential co-factor for the activation of a number of vitamin K-dependant coagulation factors, plays an important role in blood coagulation9. As a consequence of anticoagulant binding with VKOR, a deficiency of vitamin K and coagulation factors occur, resulting in spontaneous bleeding and eventual death.
In populations of resistant rats, VKOR is slightly changed thus preventing correct binding with the rodenticide which then fails to work10, a mechanism based on single nucleotide polymorphisms (SNPs) in the VKORC1 gene that codes for the VKOR enzyme11. The VKOR1 gene which has 6126 base pairs and three exons, codes for the VKOR1 protein that contains 163 amino acids12. Specific amino acid substitutions in VKORC1 have been shown to confer resistance to anticoagulant rodenticides and are considered to be a prerequisite for the development of rodenticide-resistant populations of rats and mice. Independent mutation events appear to have caused the emergence of resistant populations in different areas, affecting certain amino acid positions of the VKORC1 protein13,14. It has been suggested that at least seven independent polymorphisms occur in the VKORC1 gene in Norway rats that provide the genetic basis for anticoagulant resistance in that species, while at least two occur in house mice, although the mutations identified do not explain all aspects of resistance that have been reported13.
Scientists in Denmark, Germany and the UK that monitored the evolution and distribution of resistance3,4,15, observed that resistance expanded geographically and progressed from warfarin to the more potent active substances bromadiolone and difenacoum. Resistance to brodifacoum has also been reported16. Resistance to different anticoagulant rodenticides is known as cross-resistance and evolves from first- to second-generation anticoagulants5. As a consequence, resistance to second-generation anticoagulant rodenticides will always be accompanied by resistance to first-generation compounds.
While there have been anecdotal reports of the occurrence of resistance in rodent pest populations in Ireland, to-date there has been no scientific investigation of the matter. The current study was intended to establish if genetic evidence of resistance to anticoagulant rodenticides is present in rodent pest populations on the island of Ireland and if possible to establish the geographical distribution of such populations.
Materials and Methods
Whole carcases or tail segments of 65 Norway rats and tails of 50 house mice taken between October 2015 and February 2017, were provided by professional pest control officers working in the eastern region of the island of Ireland. The sites from which the samples were provided were distributed throughout the region (Figs 1 and 2). The number of samples provided was considerably less than had been planned and resulted in the project being terminated following the sampling of the eastern region of Ireland. Nevertheless as rodent pests are commensal and as the population of the counties in which sampling took place contain approximately 51% of the population of Ireland in the case of Norway rats and 43% in the case of house mice17, the areas sampled provide a satisfactory representation of a large part of the island of Ireland.
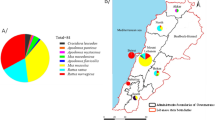
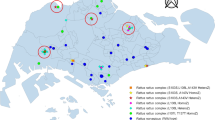
Distribution of House Mouse Sample Sites. More than one sample was taken at some locations: Yellow indicators show the locations of sampling 7 mice with a mutation on Exon 3, Codon 128, homozygous Leu128ser L128s, a mutation associated with resistance to warfarin and in some cases to bromadiolone and difenacoum. Red indicators show the locations of sampling 7 mice with a mutation on Exon 3, Codon 139, homozygous Tyr139Cys Y139c, a mutation indicative of a high degree of resistance to warfarin and bromadiolone. Green indicators show the locations of sampling 28 mice that had heterozygous mutations associated with resistance to anticoagulant rodenticides and 8 that did not have any mutations.
On receipt in the laboratory, samples were logged in and stored at −80 °C pending analysis.
Rat tail tissue was excised from tail bone using a scalpel and a fragment of tissue was shaved from the internal membrane of the tail. Mice tails were finely chopped with a scalpel. The tissue fragments were added to 800 µl MagNa Pure Total Nucleic Acid Isolation Kit lysis buffer (Roche Diagnostics Cat No. 03038505001) and 20 µL Proteinase K @ 20 mg/mL (Sigma Cat No. P2308) and digested at 55 °C for 8–24 hours. Total Nucleic acid was extracted from the supernatant on a MagNA Pure LC automated extraction instrument using MagNa Pure Total Nucleic Acid Isolation Kit (Roche Diagnostics Cat No. 03038505001) in accordance with the manufacturer’s instructions. The quality of the extracted nucleic acid was evaluated by testing all extracts for the reference gene ß-actin.
The three exons of the rat VKORC1 gene were amplified by PCR using primers listed in Table 1. The primer sequences for the mouse VKORC1 exons were received from S. Rost, Institut für Humangenetik, Würzburg and were referenced in14. PCR reactions on the extracted rat samples were performed with Accustart II PCR ToughMix (Quanta Biosciences Cat. No. 95142-800) at 94 °C for 3 min followed by 40 cycles of 30 sec at 94 °C, 30 sec at 60 °C and 1 min at 72 °C and a final 10 minutes at 72 °C. For the mouse samples the annealing temperature was reduced to 57 °C. The PCR products were cleaned with Illustra ExProStar 1-step Enzymatic PCR and Sequence Reaction Clean-up Kit (GE Healthcare Life Sciences Cat No. US77720), according to the manufacturer’s instructions. The treated extracts were quantified by Qubit prior to sequencing.
Sanger sequencing of the extracts, provided by the Molecular Virology Laboratory of the Department of Agriculture, Food and the Marine was conducted by the contract laboratory Source BioScience18.
Results and Discussion
Sixty five Norway rat samples (Fig. 1) and fifty house mouse samples (Fig. 2) were successfully extracted and sequenced for all three exons (Tables 2 and 3). The rat sequences were aligned with the NCBI Rattus norvegicus VKORC1 reference sequence (NM_203335.2). The mice sequences were aligned with the NCBI Mus musculus VKORC1 reference sequence (NM_178600.2). The sequence trace files were visually analysed for the presence of mutations in the three exons when compared with the reference sequences.
Rattus novegivcus
There were no polymorphisms (95% CI: 0.00% to 5.58%) in exons 1 and 3 of the rat samples when compared with the Rattus norvegicus VKORC1 reference strain (NM_203335.2). A single nucleotide polymorphism (SNP) was observed in codon 82 of exon 2. This point mutation, Ile82Ile, did not result in an amino acid substitution. Fourteen (21.5%, 95% CI: 12.3% to 33.5%) were heterozygous for the point mutation and 7 (11%, 95% CI: 4.4% to 20.9%) were homozygous. The silent mutation Ile82Ile has been detected in Rattus norvegicus in England, Europe, Indonesia, Korea, the Azores and North and South America at a high frequency14. There is no evidence to date that the silent mutation Il82Il is implicated in anticoagulant resistance, however it has been suggested that the mutation may alter the efficiency of translation of the VKORC1 gene and therefore alter the biological response to anticoagulant exposure19.
Rats with the Tyr139Cys, Tyr139Ser and Leu128Gln substitutions in the VKORC1 gene have been found throughout Germany and Switzerland. These mutations are known to confer resistance to anticoagulants or to reduce VKOR activity13. Warfarin resistant rats have been shown to be both heterozygous and homozygous for the mutation Tyr139Cys11. In Kent the substitution Tyr139Phe was also found in resistant Norway rats at a site treated with bromadiolone bait. All rats tested from the Kent site were found to be homozygous for the mutation19.
Mutations associated with resistance to anticoagulant rodenticides have not been detected in Norway rats in this study, indicative of an absence, or low prevalence of genetic evidence of resistance to anticoagulant rodenticides in Norway rats in at least the Eastern region of the island of Ireland.
Mus musculus domesticus
Leu128Ser and Tyr139Cys are the most frequent amino acid substitutions that have been found to occur in the VKORC1 gene in resistant house mice in the UK and other European countries20. A group of linked substitutions Arg12Trp/Ala26Ser/Ala48Thr/Arg61Leu have also been found in mice in Germany and Switzerland and are linked to reduced house mouse susceptibility to anticoagulants20. In the UK, house mice resistant to the first-generation anticoagulants are widespread and both Leu128Ser and Tyr139Cys mutations have been found in the VKORC1 gene of resistant mice13. In France a mutation Trp59Gly in exon 2 of the VKORC1 gene was found to be associated with warfarin resistant mice21.
Of the 50 mice samples tested in this study eight (16%, 95% CI: 7.2% to 29.1%) showed no sequence variation in the VKORC1 gene in comparison with the Mus musculus VKORC1 reference sequence (NM_178600.2). In the remaining 84% (95% CI: 70.9% to 92.8%), one or both of the two point mutations Leu128Ser and Tyr139Cys were detected. There was no evidence of the linked substitutions found in mice in Germany and Switzerland or of the substitution Trp59Gly found in mice in France. Twenty two mice tested (44%, 95% CI: 30.0% to 58.7%) were heterozygous for the point mutation Leu128Ser and 7 (14%, 95% CI: 5.8% to 26.7%) were homozygous (Table 3). Sixteen mice (32%, 95% CI: 19.5% to 46.7%) were heterozygous for Tyr139Cys with seven (14%, 95% CI: 5.8% to 26.7%) being homozygous. None of the mice tested were homozygous for both Leu128Ser and Tyr139Cys mutations.
The occurrence of two mutations associated with resistance to anticoagulants rodenticides is indicative of the occurrence of resistance to anticoagulant rodenticides in house mice in at least the Eastern region of the island of Ireland. In similar sized European studies resistance was present in >70–80% of tested mice populations22,23. This compares well with the values obtained in this study, in which 78% (95% CI: 56.3% to 92.5%) of mice from the Dublin region had mutations indicative of resistance (based on n = 23 mice), while for the Eastern region of Ireland the value was found to be 84% (95% CI: 70.9% to 92.8%, based on n = 50 mice).
Conclusions
Although the number of samples available for analysis (65) was less than planned, no genetic evidence was found of the occurrence and distribution of mutations associated with Norway rat resistance to anticoagulant rodenticides in the Eastern region of Ireland. Having regard to comparable European studies with similar sample numbers, the absence of genetic evidence of resistance in the 65 samples taken in the Eastern part of Ireland, suggests that there is a high probability that these are true negative findings or that the prevalence is very low.
The data generated confirmed the widespread presence of mutations that result in the two most commonly found amino acid substitutions, Leu128Ser and Tyr139Cys, associated with house mice resistance to anticoagulant rodenticides in the Eastern region of Ireland, but these were not present in samples from all counties in which samples were collected (see Supplementary Information Table). Of the 6 mutations for which screening was conducted (Table 1), only Leu128Ser and Tyr139Cys were detected.
No conclusions can be drawn concerning the occurrence of those mutations in the rest of the island of Ireland. Biological testing would be required to establish the extent to which those mutations have conferred field resistance to the house mouse populations concerned, however it is highly likely that such field resistance is present in house mouse populations in at least the Eastern Region of Ireland.
The probability remains that field resistance to anticoagulant rodenticides is present in populations of house mice in at least the Eastern region of Ireland. The use of anticoagulants in attempts to control rodent pests that have developed resistance to them unnecessarily exposes wildlife to poisoning with rodenticides.
Rodent pests are vectors or carry vectors of many serious human and animal diseases, cause significance spoilage, losses of food and feed as well as damage to buildings and infrastructure. There are around 200 rodenticide products authorised for use in Ireland24, and in excess of 60 companies provide pest control services in Ireland25. There is a clear need for further research to establish the extent and distribution of anticoagulant resistance in populations of rodent pests on the island of Ireland. Given the challenges identified in collecting sufficient numbers of samples from farms, industrial and urban areas on a goodwill basis, it is recommended that any future study be funded such that relevant voluntary organisation personnel can be trained in both sample collection and handling under the supervision of a PhD student selected to lead the project. Since a key goal of acquiring such information is to allow assessment of the extent of resistant populations of rodents and consequently of the extent of unnecessary exposure of non-target wildlife to rodenticides though both primary and secondary poisoning, the preferred approach is to utilise samples acquired along rural to urban gradients as per the initial strategy utilised in this study.
References
Guidelines on good plant protection practice. Rodent control for crop protection and on farms. Bull OEPP/EPPO Bull 25, 709–736 (1995).
Boyle, C. M. Case of apparent resistance of Rattus norvegicus Berkenhout to anticoagulant poisons. Nature 188, 517 (1960).
Kerins, G. M., Dennis, N., Atterby, H., Gill, J. E. & MacNicoll, A. D. Distribution of resistance to anticoagulant rodenticides in the Norway rat (Rattus nurvegicus Berk.) in England 1995–98. In Advances in Vertebrate Pest Management (Eds Pelz, H. J., Cowan, D. P., and Feare, C. J.), 149–160 (Filander Verlag, Fürth 2001).
Lodal, J. Distribution and levels of anticoagulant resistance in rats (Rattus norvegicus) in Denmark. In Advances in Vertebrate Pest Management (Eds Pelz, H. J., Cowan, D. P., & Feare, C. J.), 149–160 (Filander Verlag, Fürth 2001).
Pelz, H. J., Hänish, D. & Lauenstein, G. Resistance to anticoagulant rodenticides in Germany and future strategies to control Ratus norvegicus. Pesticide Science 43, 61–67 (1995).
Baert, K., Stuyck, J., Breyne, P., Maes, D. & Casaer, J. Distribution of anticoagulant resistance in the brown rat in Belgium. Belg J Zool 142, 39–48 (2012).
Dodsworth, E. Mice are spreading despite such poisons as warfarin. Munic Engng Lond 3746, 1668 (1961).
Atterby, H., Kelly, M. J., MacNicoll, A. D. Difenacoum resistance in rats is not a consequence of increased metabolism and excretion. In Advances in Vertebrate Pest Management (Eds Pelz, H. J., Cowan, D. P. and Feare, C. J.), 149–160 (Filander Verlag, Fürth 2001).
Oldenburg, J., Marinova, M., Müller-Reible, C. & Watzka, M. The vitamin K cycle. Vitamins and Hormones 78, 33–62 (2008).
Thijssen, H. H. W. Warfarin-based rodenticides: mode of action and mechanism of resistance. Pesticide Science 43, 73–78 (1995).
Rost, S. et al. Mutations in VKORC1 cause warfarin resistance and multiple factor deficiency type 2. Nature 427, 537–541 (2004).
Tie, J. K., Nicchita, C., Heijne, G. & Stafford, D. W. Membrane topology mapping of vitamin K epoxide reductase by in vitro translation/cotranslation. J Biol Chem 280, 16410–16416 (2005).
Pelz, H. J. et al. The genetic basis of resistance to anticoagulants in rodents. Genetics 170, 1839–1847 (2005).
Rost, S. et al. Novel mutations in the VKORC1 gene of wild rats and mice – a response to 50 years of selection pressure by warfarin. BMC Genet 10, 4 (2009).
Pelz, H. J. Extensive distribution and high frequency of resistance to anticoagulant rodenticides in rat populations from northwestern Germany. In Advances in Vertebrate Pest Management (Eds Pelz, H. J., Cowan, D. P. and Feare, C. J., 149–160 (Filander Verlag, Fürth 2001).
Gill, J. E., Kerins, G. M. & MacNicoll, A. D. Inheritance of low grade brodifacoum resistance in the Norway Rat. J of Wildlife Management 56, 809–816 (1992).
Census 2011 Population Classified by Area, Government Publications Sales Office, Sun Alliance House, Molesworth Street, Dublin 2, http://www.cso.ie/en/media/csoie/census/documents/census2011vol1andprofile1/Census_2011_-_Population_Classified_by_Area.pdf. Accessed 27th December2017.
Source BioScience, 1 Orchard Place, Nottingham Business Park, Nottingham NG8 6PX, England https://www.sourcebioscience.com/company/about-us/. Accessed 27th December 2017.
Prescott, C. V., Buckle, A. P., Gibbings, J. G., Allan, N. W. & Stuar, A. M. Anticoagulant resistance in Norway rats (Rattus norvegicus Berk.) in Kent – a VKORC1 Single nucleotide polymorphism, tyrosine139phenylalanine), new to the UK. Internat J Pest Management 57, 61–65 (2011).
Pelz, H. J. et al. Distribution and Frequency of VKORC1 sequence variants conferring resistance to anticoagulants in Mus musculus. Pest Manag Sci 68, 254–259 (2012).
Lasseur, L., Grandemange, A., Longin-Sauvageon, C., Berny, P. & Benoit, E. Heterogeneity of the Coumarin Anticoagulant targeted Vitamin K Epoxide Reduction System. Study of Kinetic Parameters in Susceptible and Resistant Mice (Mus musculus domesticus). J Biochem Mol Toxicol 20, 221–229 (2006).
Pelz, H.-J., Hanisch, D. & Lauenstein, G. Resistance to Anticoagulant Rodenticides in Germany and Future Strategies to Control. Rattus norvegicus. Pestic. Sci. 43, 61–67 (1995).
Goulois, J., Lambert, V., Legros, L., Benoit, E. & Lattard, V. Adaptative evolution of the Vkorc1 gene in Mus musculus domesticus is influenced by the selective pressure of anticoagulant rodenticides. Ecol Evol. 7, 2767–2776 (2017).
PRCD Biocidal Products Register – Authorised Products, 8 July 2016 - http://www.pcs.agriculture.gov.ie/registers/biocidalproductregisters/biocidalproductregister-authorisedproducts/. Accessed 27th December 2017.
CRRU Ireland Supporters’ Club members http://www.crru.ie/crru-supporters/. Accessed 27th December 2017.
Acknowledgements
The authors would like to acknowledge the efforts of those individual Rodent Pest Control Officers who provided samples that facilitated the analytical work completed. The authors would also like to thank David Rymer of the University of Reading who provided laboratory strain rat tail samples used in validating laboratory methodologies used.
Author information
Authors and Affiliations
Contributions
The text of the paper submitted was prepared by Jean Mooney and Mark R. Lynch. Colin V. Prescott provided advice regarding sampling of rodent pests and sample handling. Tracy Clegg provided statistical advice and calculated the 95% confidence limits provided in Tables 2 and 3. Michael Loughlin, Bernard Hannon, Colm Moore and Richard Faulkner recruited commercial Pest Control Officers to collect samples and instructed them in the procedures to be followed.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mooney, J., Lynch, M.R., Prescott, C.V. et al. VKORC1 sequence variants associated with resistance to anticoagulant rodenticides in Irish populations of Rattus norvegicus and Mus musculus domesticus. Sci Rep 8, 4535 (2018). https://doi.org/10.1038/s41598-018-22815-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-22815-7
This article is cited by
-
Investigation of anticoagulant rodenticide resistance induced by Vkorc1 mutations in rodents in Lebanon
Scientific Reports (2022)
-
VKORC1 mutations in rodent populations of a tropical city-state as an indicator of anticoagulant rodenticide resistance
Scientific Reports (2022)
-
Comparative pharmacokinetics of difethialone stereoisomers in male and female rats and mice: development of an intra- and inter-species model to predict the suitable formulation mix
Archives of Toxicology (2022)
-
Efficacy of rodenticide baits with decreased concentrations of brodifacoum: Validation of the impact of the new EU anticoagulant regulation
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.