Abstract
The Li+ ion diffusion characteristics of V- and Nb-doped LiFePO4 were examined with respect to undoped LiFePO4 using muon spectroscopy (µSR) as a local probe. As little difference in diffusion coefficient between the pure and doped samples was observed, offering DLi values in the range 1.8–2.3 × 10−10 cm2 s−1, this implied the improvement in electrochemical performance observed within doped LiFePO4 was not a result of increased local Li+ diffusion. This unexpected observation was made possible with the µSR technique, which can measure Li+ self-diffusion within LiFePO4, and therefore negated the effect of the LiFePO4 two-phase delithiation mechanism, which has previously prevented accurate Li+ diffusion comparison between the doped and undoped materials. Therefore, the authors suggest that µSR is an excellent technique for analysing materials on a local scale to elucidate the effects of dopants on solid-state diffusion behaviour.
Similar content being viewed by others
Introduction
Spin-polarised muons can be used as a local probe to investigate the solid-state diffusion behaviour of Li-ion battery materials. The diffusive processes within these materials are not always well understood and muons can provide detailed insight into the Li+ diffusion mechanisms1,2,3. The sensitivity of the embedded muon to local magnetism (through the time-evolution of its spin polarisation) has been utilised to investigate many properties of solid state materials, such as hydrogen diffusion, magnetism and radical chemistry1,2,3. Similarly, Li+ diffusion within a sample, perturbs embedded muons and the Li+ diffusion coefficient can be extracted from analysis of this perturbation. This technique has successfully determined the Li-ion diffusion coefficients in LiCoO24, bulk LiFePO45,6,7, nano-LiFePO48, and the Li6.5Al0.25La2.92Zr2O12 solid-state electrolyte material9. Indeed, µSR has successfully determined consistent experimental diffusion coefficients of Li+ in pure LiFePO4 in the range 10−10 to 10−9 m2 s−1, in good agreement with theoretical studies, but to the authors’ knowledge has not been extended to V- or Nb-doped LiFePO4 systems previously5,6,7,8.
LiFePO4 (and doped variants) have been extensively investigated as a cathode material for Li-ion batteries, as it offers a more sustainable alternative to cobalt-based cathodes such as LiCoO2 and LiNi x Mn y Co z O210. Historically, LiFePO4 initially suffered from poor Li insertion/extraction kinetics11; efforts to nanosize12,13, carbon-coat14,15,16, and dope the material have often improved the attainable storage capacity, particularly at high charge/discharge rates. In particular, aliovalent doping of LiFePO4 with transition metal ions such as V3+ and Nb5+ has been a successful strategy for improving the resulting electrochemical performance17,18, although there is a lack of consensus on the precise reasons for this. Many authors have observed distortions of the unit cell19,20,21,22, and widening of the 1D diffusion channels in the material, which was suggested as a mechanism for lowering the activation energy for Li-ion diffusion. There is also some argument as to whether the dopants create defects in the material (such as Li vacancies) that boost diffusion20. Furthermore, the dopant may alter the electronic conductivity and consequently improve performance18. The subtle effects of dopants on atomic structure can be difficult to observe in great detail using standard lab based analytical techniques, and therefore, alternative methods must be sought to fully understand the effects of doping on electrode materials.
Herein, we report our investigations into Li+ diffusion within LiFePO4, Nb- and V-doped LiFePO4. The two doped LiFePO4 samples both displayed enhanced cycling performance at high discharge rates in comparison with the undoped LiFePO4 sample21,23, and the µSR results allowed the unambiguous comparison of Li-ion mobility on a local level within these samples. This increased insight into Li+ diffusion processes present the future possibility of optimising doped compositions to give improved Li-ion battery performance.
Methods
The synthesis of pure LiFePO4, Nb- and V-doped LiFePO4 materials have been described in detail in previous publications21,23. Briefly, these carbon-coated lithium iron phosphate samples (where the C is amorphous) were synthesized using a pilot-scale continuous hydrothermal flow synthesis (CHFS) reactor, described in detail in the Supporting Information and elsewhere24. These samples were heat-treated at 700 °C for 3 h (5 °C min−1 ramp rate) to graphitize the carbon coatings. Undoped LiFePO4 was selected for muon analysis as a control as well as the optimal performing dopant compositions of LiFe0.99Nb0.01PO4 and LiFe0.95V0.05PO4, which were named δLFP, δLFNP(1.0) and δLFVP(5), respectively (the δ term denotes these samples were heat-treated).
The µSR experiments were conducted at the ISIS pulsed muon and neutron source on the EMU instrument25. The data were analysed using the Windows Muon Data Analysis (WiMDA) program26. These samples were prepared for analysis by transferring ca. 1 g into Ti cavities with a Ti foil window. Ti was chosen as a sample holder material because it has negligible internal magnetic fields and therefore gave a simple background feature which could be easily subtracted in the analysis.
Spin-polarised positive muons were implanted into the δLFP, δLFNP(1.0) and δLFVP(5) samples, where they occupied interstitial sites for a mean lifetime of 2.2 µs before decaying. The muon spin direction was affected by the local magnetic field or diffusing species near the implantation site. The asymmetry in the count rate of the positrons, A(t), was measured in two arrays of detectors on opposite sides of the sample. While the implanted muons are almost 100% polarised, their three-body decay into a positron and two neutrinos, as well detector geometry constraints, limited the positron count rate asymmetry to ca. 25% on the EMU instrument. In order to probe the lithium diffusion behaviour in the three samples, measurements were in the temperature range 100 to 400 K for all samples. At each temperature, measurements were made at multiple magnetic fields (applied along the initial muon spin direction). These gave a way of comparing the applied field to the internal fields experienced by muons in the sample and constrained the model used for analysing the data more rigorously than could have been done with a single measurement. The Li+ diffusion was investigated in this study with µSR with zero applied field (ZF) and varying strengths of applied longitudinal field (LF) at 5, 10 and 20 G. Representative muon decay asymmetry spectra at 290 K for sample LFP at 0 and 20 G are shown in Fig. 1.
The spectra arose from a combination of a rapid interaction with the paramagnetic iron moments, and a slower interaction with the 6Li, 7Li and 31P nuclear magnetic moments. This allowed Li+ diffusion to be extracted in a similar manner to previous µSR studies of LiFePO47,8. The data sets were fitted using four different parameters. Firstly, Keren’s analytic generalization of the Abragam function was applied, which has previously been altered to describe fluctuations due to Li+ or µ+ diffusion (assuming a Gaussian distribution of local fields)7. This function was chosen due to the increased relative speed of computation of the Keren function compared to the Kubo-Toyabe function used in previous studies5. Secondly, an exponential relaxing function was used, accounting for the rapid interaction with iron electronic magnetic moments. Thirdly, a baseline asymmetry was used to account for weak interactions with Ti and C present in the sample holder and sample, respectively. Finally, an additional exponentially decaying function was added as a separate term, to account for interactions with minor ferric impurities. These were not observed by XRD, so are assumed to be very minor, or amorphous. By fitting with these parameters, the muon fluctuation rate (vLi) due to Li+ diffusion and the local field distribution (Δ), could be extracted.
Results and Discussion
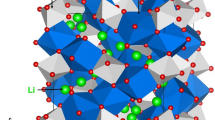
X-Ray diffraction analysis of the δLFP, δLFNP(1.0) and δLFVP(5) samples, confirmed each crystallised in the Pnma space group of the olivine structure (Fig. 2). The high-quality XRD revealed a minor impurity peak in δLFNP(1.0) (at 2θ~13.8°), which is consistent with Fe2P2O7 and has been observed previously in heat-treated carbon-coated olivines (Figure S2)27. The lattice parameters were extracted from Rietveld analysis using MAUD (Material Analysis Using Diffraction) software28, and are displayed in Table 1 and plots of the refinements are displayed in Figures S3–S5. The dopants had a minor effect on the lattice parameters, with a small contraction of the b-axis and lengthening of the c-axis, consistent with previous studies of doped samples20,21. This crystallographic change is primarily due to the different ionic radii of V3+ (0.64 Å) and Nb5+ (0.64 Å) occupying the Fe2+ (0.78 Å) site. Occupation of V on the Fe site with a Li vacancy as a charge-compensation mechanism in LiFePO4 was confirmed by the authors previously for δLFVP(5) with a combined Extended X-Ray Absorption Spectroscopy (EXAFS) and Density Functional Theory (DFT) study21, with the V:Fe ratio quantified as 5:95 by ICP-AES analysis. In addition, the authors confirmed an even dispersion of Nb within Nb-doped LiFePO4 samples23, with no Nb-containing impurity phases observed. The proportion of Nb within LiFePO4 was found to approximately match the stoichiometry of the precursors (Figures S6 and S7).
X-Ray diffraction patterns of δLFP, δLFNP(1.0) and δLFVP(5) with an LiFePO4 standard (PDF Card No. 01-070-6684), with the Fe2P2O7 minor impurity phase peak (PDF Card No. 00-076-1672, present in 1.5 vol% from Rietveld refinement) highlighted with an asterisk. A more detailed figure of the impurity phase peak is shown in Figure S2.
The behaviour of Δ was similar to that reported previously by others for undoped LiFePO4, i.e. a steady decrease with increasing temperature, although the values for Δ were consistently lower for the doped samples (Fig. 3). The relative reduction in Δ seen in the doped samples herein cannot be definitively attributed, but could originate from an altered occupation of muon stopping sites, increased Li vacancies or changes in the muon mobility within the sample.
All samples showed a characteristic increase and then decrease in vLi with temperature, although the magnitude of vLi was about 10% of that reported in the literature for undoped LiFePO4 samples previously (Fig. 4a–c)6,7,8. This may have been due to the significant embedding of muons in the carbon shell (range of 3 to 9 wt% carbon within the three samples, Table S1, corresponding to 1:2 and 1:1 molar ratios of C:LiFePO4), which would have detracted from the overall measured diffusion rate, but would not be expected to contribute to the observed fluctuation rate. For δLFP and δLFNP(1.0), an increase in vLi with increasing T in the range ca. 180–250 K and a decrease thereafter was observed (Fig. 4a,b). In contrast, δLFVP(5) displayed a rapid increase of vLi in the range 170–210 K, followed by a rapid decay above 210 K to a lower value of vLi (0.03 MHz, Fig. 4c). This behaviour indicated there may be some observable difference in diffusion behaviour of δLFVP(5) and the other samples in this temperature range. However, given the relative error of the data points, further experiments are necessary to confirm the existence of any deviation from normal diffusion behaviour in vanadium-doped LiFePO4.
The hopping rate of Li+ can be converted to a diffusion coefficient according to Equation 1, where N i is the number of Li sites in the ith path, Zv,i is the vacancy fraction, and s i is the hopping distance29. Therefore, as Li can diffuse in either direction along the 1D LiFePO4 diffusion tunnels, n = 2, where the number of vacant sites in each direction is 1, i.e. N1 = N2 = 1. For diffusion to occur, there must be a vacancy in the neighbouring sites, so Z1 = Z2 = 1. Therefore, Equation 1 simplifies to Equation 2. As Li+ diffusion in LiFePO4 has been shown to occur exclusively along the b-axis30, the hopping length is approximately b/2, and therefore DLi can be estimated from b2vLi/4, and extrapolating vLi against 1/T can determine a value for the Li-ion diffusion coefficient at room temperature (Fig. 4d–f). As the carbon coating contains no mobile lithium, it was assumed that the diffusion distance was accurately described by b/2.
Diffusion coefficients of approximately 2 × 10−10 cm2 s−1 were estimated for δLFP, δLFNP(1.0) and δLFVP(5), respectively, which were similar within error (Table 2). As a comparison, the diffusion coefficient values obtained here were consistent with those obtained by µSR for undoped LiFePO4 previously, pointing to the reliability of this technique for determining the diffusion properties of off-stoichiometry olivines5,7,8. The activation energies of Li+ diffusion (calculated from the gradient of diffusion coefficient against 1/T) were also consistent with previous analyses; Ea was in the range 70–100 meV for all samples5,7,8. For example, Baker et al. found DLi values in the range 4–20 × 10−10 m2 s−1 and Ea values in the range 80–130 meV for the lithium-deficient olivines, Li1−xFePO4 (where 0 ≤ x ≤ 0.2)7. Indeed, Baker et al. found greater values of Ea for their Li-deficient samples, which suggests that Li deficiencies present in doped LiFePO4 (Table S2) may be responsible for any difference observed between samples in the temperature range 170–210 K. However, no quantitative difference in diffusion coefficient was observed between the undoped and doped samples when the low-temperature data was extrapolated to room temperature. This suggested that the intrinsic Li+ diffusion hopping rate at room temperature was not affected by doping, and that any enhancement of electrochemical performance observed must be due to other factors, such as increased electronic conductivity, or stabilisation of the Li1−xFePO4 and Li x FePO4 solid solutions. Such an observation could not be made via conventional techniques, such as impedance spectroscopy, as the two-phase delithiation mechanism of LiFePO4 prevents accurate probing of Li+ diffusion.
Conclusions
Li+ diffusion within V- and Nb-doped LiFePO4 samples, made via a continuous hydrothermal process31, have been characterised with muon spectroscopy for the first time. The calculated Li+ diffusion coefficients were close to the values previously reported for bulk and nanometric undoped LiFePO4. Thus, this report highlights the versatility of the μSR technique to analyse families of materials made by a variety of synthesis techniques. Within experimental error, the Li+ diffusion data suggested that electrochemical enhancements due to doping are not a result of improved local Li+ diffusion. Rather, the authors suggest that other factors, such as increased electronic conductivity or stabilisation of the Li1−xFePO4 and Li x FePO4 solid solutions may account for these enhancements. Therefore, these results have indicated the utility of μSR to provide key insights into the diffusive behaviour of doped LiFePO4, and could be applied to further battery materials in the future.
References
Blundell, S. J. Spin-polarized Muons in Condensed Matter Physics. Contemp. Phys. 40, 175–192 (1999).
Lee, S. L., Cywinski, R. & Kilcoyne, S. H. Muon Science: Muons in Physics, Chemistry and Materials. (Proceedings of the 50th Scottish University Summer School in Physics, A Nato Advanced Study Institute, vol. 51, Institute of Physics, 1998).
Yaouanc, A. & Dalmas de Reotier, P. Muon Spin Rotation, Relaxation and Resonance: Applications to Condensed Matter Vol. 147.
Sugiyama, J. et al. Li Diffusion in Li x CoO2 Probed by Muon-Spin Spectroscopy. Phys. Rev. Lett. 103, 147601 (2009).
Sugiyama, J. et al. Magnetic and Diffusive Nature of LiFePO4 Investigated by Muon Spin Rotation and Relaxation. Phys. Rev. B 84, 1–9 (2011).
Sugiyama, J. et al. Diffusive Behavior in LiMPO4 with M = Fe, Co, Ni Probed by Muon-spin Relaxation. Phys. Rev. B 85, 54111 (2012).
Baker, P. J. et al. Probing Magnetic Order in LiMPO4 (M = Ni, Co, Fe) and Lithium Diffusion in Li x FePO4. Phys. Rev. B 84, 174403 (2011).
Ashton, T. E. et al. Muon Studies of Li+ Diffusion in LiFePO4 Nanoparticles of Different Polymorphs. J. Mater. Chem. A 2, 6238–6245 (2014).
Amores, M., Ashton, T. E., Baker, P. J., Cussen, E. J. & Corr, S. A. Fast Microwave-assisted Synthesis of Li-stuffed Garnets and Insights into Li Diffusion from Muon Spin Spectroscopy. J. Mater. Chem. A 4, 1729–1736 (2016).
Zou, Y. et al. Multishelled Ni-Rich Li(Ni x Co y Mn z )O2 Hollow Fibers with Low Cation Mixing as High-Performance Cathode Materials for Li-Ion Batteries. Adv. Sci. 4, 1–8 (2017).
Padhi, A. K., Nanjundaswamy, K. S. & Goodenough, J. B. Phospho-olivines as Positive Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 144, 1188–1194 (1997).
Delacourt, C., Poizot, P., Levasseur, S. & Masquelier, C. Size Effects on Carbon-Free LiFePO4 Powders. Electrochem. Solid-State Lett. 9, A352 (2006).
Rui, X. et al. Olivine-Type Nanosheets for Lithium Ion Battery Cathodes. ACS Nano 7, 5637–5646 (2013).
Oh, S. W. et al. Double Carbon Coating of LiFePO4 as High Rate Electrode for Rechargeable Lithium Batteries. Adv. Mater. 22, 4842–4845 (2010).
Zou, Y. et al. Suppressing Fe–Li Antisite Defects in LiFePO4/Carbon Hybrid Microtube to Enhance the Lithium Ion Storage. Adv. Energy Mater. 6 (2016).
Wang, Y., Wang, Y., Hosono, E., Wang, K. & Zhou, H. The Design of a LiFePO4/Carbon Nanocomposite with a Core-shell Structure and its Synthesis by an in situ Polymerization Restriction Method. Angew. Chem. Int. Ed. Engl. 47, 7461–7465 (2008).
Wen, Y., Zeng, L., Tong, Z., Nong, L. & Wei, W. Structure and Properties of LiFe0.9V0.1PO4. J. Alloys Compd. 416, 206–208 (2006).
Chung, S.-Y., Bloking, J. T. & Chiang, Y.-M. Electronically Conductive Phospho-olivines as Lithium Storage Electrodes. Nat. Mater. 1, 123–128 (2002).
Omenya, F. et al. Can Vanadium be Substituted into LiFePO4? Chem. Mater. 23, 4733–4740 (2011).
Omenya, F., Chernova, N. A., Wang, Q., Zhang, R. & Whittingham, M. S. The Structural and Electrochemical Impact of Li and Fe Site Substitution in LiFePO4. Chem. Mater. 25, 2691–2699 (2013).
Johnson, I. D. et al. Pilot-scale Continuous Synthesis of a Vanadium-doped LiFePO4/C Nanocomposite High-rate Cathodes for Lithium-ion Batteries. J. Power Sources 302, 410–418 (2016).
Chen, M.-S., Wu, S. & Pang, W. K. Effects of Vanadium Substitution on the Cycling Performance of Olivine Cathode Materials. J. Power Sources 241, 690–695 (2013).
Johnson, I. D. et al. High Power Nb-doped LiFePO4 Li-ion Battery Cathodes; Pilot-scale Synthesis and Electrochemical Properties. J. Power Sources 326, 476–481 (2016).
Gruar, R., Tighe, C. & Darr, J. Scaling-up a Confined Jet Reactor for the Continuous Hydrothermal Manufacture of Nanomaterials. Ind. Eng. Chem. Res. 52, 5270–5281 (2013).
Giblin, S. R. et al. Optimising a Muon Spectrometer for Measurements at the ISIS Pulsed Muon Source. Nucl. Instruments Methods Phys. Res. Sect. A 751, 70–78 (2014).
Pratt, F. L. WIMDA: A Muon Data Analysis Program for the Windows PC. Phys. B Condens. Matter 289–290, 710–714 (2000).
Johnson, I. D., Loveridge, M., Bhagat, R. & Darr, J. A. Mapping Structure-Composition-Property Relationships in V and Fe Doped LiMnPO4 Cathodes for Lithium-Ion Batteries. ACS Comb. Sci. 18, 665–672 (2016).
Lutterotti, L., Matthies, S. & Wenk, H.-R. MAUD (Material Analysis Using Diffraction): a User Friendly Java Program for Rietveld Texture Analysis and more. In Twelfth International Conference on Textures of Materials (ICOTOM-12) 1599 (1999).
Borg, R. J. & Dienes, G. J. An Introduction to Solid State Diffusion.
Nishimura, S. et al. Experimental Visualization of Lithium Diffusion in Li x FePO4. Nat. Mater. 7, 707–711 (2008).
Darr, J. A., Zhang, J., Makwana, N. M. & Weng, X. Continuous Hydrothermal Synthesis of Inorganic Nanoparticles: Applications and Future Directions. Chemical Reviews 117(17), 11125–11238 (2017).
Acknowledgements
JAD would like to thank the EPSRC for funding (EP/R023662/1; The JUICED Hub [Joint University Industry Consortium for Energy (Materials) and Devices Hub]). EPSRC are thanked by JAD, TEA, EB and ML for funding the ELEVATE project (EP/M009394/1) and by IDJ for funding the Centre for Doctoral Training in Molecular Modelling & Materials Science (EP/G036675/1, UCL, U.K.) which supports a studentship for IDJ. A Star (Singapore) is thanked for supporting a studentship for ML. STFC are thanked for the provision of muon beamtime and the ISIS staff are thanked for their support.
Author information
Authors and Affiliations
Contributions
IDJ wrote the paper, performed the synthesis and XRD characterisation, with EB assisting with the latter. IDJ, TEA, GJS and PJB collected and fitted the muon data. ML and SAC contributed to the discussion and editing of the manuscript. JAD is the supervising scientist of the research team at University College London and co-developed the synthesis technology for the nanomaterials that were measured herein.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Johnson, I.D., Ashton, T.E., Blagovidova, E. et al. Mechanistic insights of Li+ diffusion within doped LiFePO4 from Muon Spectroscopy. Sci Rep 8, 4114 (2018). https://doi.org/10.1038/s41598-018-22435-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-22435-1
This article is cited by
-
Understanding the sluggish and highly variable transport kinetics of lithium ions in LiFePO4
Science China Chemistry (2023)
-
Investigation on electrochemical performance at the low temperature of LFP/C-P composite based on phosphorus doping carbon network
Ionics (2020)
-
Optimization of LiFePO4 cathode material based on phosphorus doped graphite network structure for lithium ion batteries
Ionics (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.