Abstract
The eye provides an opportunistic “window” to view the microcirculation. There is published evidence of an association between retinal microvascular calibre and renal function measured by estimated glomerular filtration rate (eGFR) in individuals with diabetes mellitus. Beyond vascular calibre, few studies have considered other microvascular geometrical features. Here we report novel null findings for measures of vascular spread (vessel fractal dimension), tortuosity, and branching patterns and their relationship with renal function in type 2 diabetes over a mean of 3 years. We performed a nested case-control comparison of multiple retinal vascular parameters between individuals with type 2 diabetes and stable (non-progressors) versus declining (progressors) eGFR across two time points within a subset of 1072 participants from the GoDARTS study cohort. Retinal microvascular were measured using VAMPIRE 3.1 software. In unadjusted analyses and following adjustment for age, gender, systolic blood pressure, HbA1C, and diabetic retinopathy, no associations between baseline retinal vascular parameters and risk of eGFR progression were observed. Cross-sectional analysis of follow-up data showed a significant association between retinal arteriolar diameter and eGFR, but this was not maintained following adjustment. These findings are consistent with a lack of predictive capacity for progressive loss of renal function in type 2 diabetes.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus is a disease characterised by micro- and macrovascular complications in 30–50% of people with the condition1 and is the commonest cause of end-stage renal disease (ESRD), accounting for approximately 45% of incident and 38% of prevalent ESRD in the United States2. High rates of incident chronic kidney disease (CKD) have been reported in both type 1 and type 2 diabetes based on established renal function cut-offs (estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2), with up to 29% of those with newly diagnosed type 2 diabetes likely to develop CKD over 15 years of follow-up3, with similar rates also reported in type 1 diabetes4,5.
Pathological microvascular changes that manifest themselves in diabetes are important contributory mechanisms leading to increased prevalence of CKD in those with the condition6,7,8. The retina enables accessible visualisation of tissue vascularisation and quantitative evaluation of retinal microvascular parameters (RVP) and specific retinal microvascular changes have been associated with a range of vascular-related conditions, including hypertension9, coronary heart disease10, stroke11 and diabetes12. Retinal microvascular parameters have also been suggested to reflect systemic microvascular damage resulting from renal dysfunction13, and in population-based studies, reduced eGFR has been associated with both narrower retinal arteriolar14,15,16,17,18,19,20,21 and venular calibre14,16,22,23, but to date the evidence has been limited and the findings reported, inconsistent.
A wide range of perturbed metabolic pathways are associated with vascular injury in diabetes. Advanced glycation end products are formed, microRNA profiles become attenuated, nitric oxide bioavailability is reduced, oxidative damage is increased and there is a proliferation of inflammatory mediators such as interleukin-6 (IL-6) and c-reactive protein (CRP)8,24. Several of these biochemical changes are associated with alterations in retinal vascular calibre, e.g. IL-6 and CRP25,26, serum glucose26, and nitric oxide inhibition27. CKD has also been associated with vascular changes linked to hypertension, dyslipidaemia, endothelial dysfunction, accelerated atherosclerosis, inflammation and abnormal bone mineral metabolism28,29. While calcification may influence vascular morphology, hypertension has established associations with narrower retinal arteriolar calibre30. The combined vascular effects of type 2 diabetes and renal impairment may correlate with earlier retinal changes which, if identified during routine screening, may enable detection and stratification of those at increased risk of progressive CKD, facilitating earlier clinical intervention to slow CKD progression.
The complexity of the retinal vasculature can be estimated by its fractal dimension. Lower fractal dimension has been reported in those with CKD18,23 in contrast to increased fractal dimension in type 2 diabetes31. Given the divergent effects of type 2 diabetes and CKD on retinal microvascular fractal dimension, it is unclear if this parameter is associated with diabetes, CKD or both. Increased retinal vascular tortuosity has also been reported in association with longer diabetes duration32 but evidence on the impact of change in renal function and retinal vascular tortuosity in diabetes is scarce. The aim of this study therefore was to examine the prognostic potential of RVP to predict eGFR decline in a large prospective cohort of type 2 diabetes.
Results
Overall the sample population had a mean age of 63.0 years (standard deviation (SD) = 7.6) and 49% were female. Mean follow-up time was 3.01 years (SD = 0.35). The study sample had a mean glycated haemoglobin (HbA1c) of 7.41% (SD = 1.39), and a mean systolic blood pressure (SBP) of 138 mmHg (SD 13). A total of 570 participants met the group 1 definition and were designated non-progressors, 335 participants met the group 2 criteria and were designated progressors. There were no significant differences in blood pressure, HbA1c or diabetic retinopathy status between progressors and non-progressors at baseline. Baseline eGFR was significantly higher in progressors compared to non-progressors (98.6 ml/min/1.73 m2, SD = 21.3 vs. 91.3 ml/min/1.73 m2, SD = 14.3, p < 0.001) (Table 1).
Over the mean follow up time of 3.01 yrs, mean change in eGFR was −27.73 ml/min/1.73 m2 (SD = 14.31 ml/min/1.73 m2) in progressors versus +2.74 ml/min/1.73 m2 (SD = 10.85 ml/min/1.73 m2) in non-progressors, p < 0.001. SBP and diastolic blood pressure (DBP) fell in both groups, but decreased significantly more in progressors than in non-progressors (SBP decreased by 2.49 mmHg (16.26) and 0.23 mmHg (12.94) for progressors and non-progressors respectively, p = 0.04; DBP decreased by 2.61 mmHg (9.36) and 1.15 mmHg (7.69) respectively in progressors and non-progressors, p = 0.02). There was no significant change in HbA1C in both groups between time-points (+0.13% (1.42), and +0.04% (1.37) for progressors and non-progressors respectively, p = 0.41). In both groups a non-significant decrease in arteriolar and venular calibre of approximately 1% was observed between time-points but there was no significant difference in the rate of vascular narrowing between groups. No significant differences in the other parameters measured (fractal dimension, tortuosity, or number of first branches) were detected between progressors and non-progressors (Table 2).
In unadjusted and adjusted logistic regression models controlling for age, sex, SBP, and HbA1c, none of the baseline RVP were significantly associated with greater odds of being a progressor. For instance, per unit increase in central retinal arteriolar calibre (CRAE) the odds of being a progressor were multiplied by 1.02 (OR = 1.02, 95% CI = 0.97, 1.08) in the adjusted analysis but this was not statistically significant (p = 0.46). Similarly, per unit increase in central retinal venular calibre (CRVE), the odds of being a progressor were multiplied by 1.03 (OR = 1.03, 95% CI = 0.99, 1.07) in the adjusted analysis but this was also not statistically significant (p = 0.18). Further adjustment for diabetic retinopathy and fellow vessel calibre also failed to identify any significant associations between RVP and likelihood of decline in eGFR. No associations were identified between baseline RVP and odds of being a progressor in this type 2 diabetes cohort (Table 3).
Lower CRAE at follow-up was significantly associated with follow-up eGFR in unadjusted linear regression analysis (β = −0.47, 95% CI = −0.87, −0.07, p = 0.02), with CRVE (β = −0.30, 95% CI = −0.60, 0.00, p = 0.05) and arteriolar fractal dimension (β = −18.41, 95% CI = −36.92, 0.10, p = 0.05) approaching statistical significance. After adjustment for age, gender, follow-up SBP and HbA1c, the associations between RVP and eGFR at follow-up were no longer statistically significant (Table 4). Associations were not materially altered following additional adjustment for diabetic retinopathy or fellow vessel calibre (data not shown).
Discussion
Routine investigation of retinal biomarkers has improved through advances in digital imaging systems and routine eye screening programmes, wider availability through high street opticians and software improvements capable of quantifying multiple RVP with improved accuracy.
In this longitudinal, case-control study of individuals with type 2 diabetes, the prognostic value of a wide range RVP for the identification of those at increased risk of eGFR decline was considered. No association between baseline RVP and change in eGFR between two time points approximately 3 years apart was found, suggesting a lack of potential for RVP as predictive biomarkers of eGFR decline in this cohort of type 2 diabetes over the limited time-period considered.
Several studies have previously reported associations between renal disease and RVP in both type 1 and type 2 diabetes. Cross-sectional studies in type 1 diabetes found associations between narrower retinal arterioles and prevalent diabetic nephropathy (DN)33,34. In contrast, prospective studies of type 1 diabetes have reported wider venular calibre in association with albuminuria35,36 and renal insufficiency35 over 16 years of follow-up, while narrower retinal arterioles36 and sparser vasculature37 have also been reported in association with proteinuria. These previous findings in type 1 diabetes contrast to the present study which found no significant associations with eGFR decline in type 2 diabetes. Reductions in eGFR can occur in the presence or absence of albuminuria38 and may have entirely independent associations with retinal vascular morphology. Population differences between type 1 and type 2 diabetes are also worthy of further consideration. Age is a well-established confounder of RVP and type 1 diabetes populations are typically younger. The population of the Danish Cohort of Pediatric Diabetes36 had a mean age of 21 years, far younger than the 63 year average age of the population of this study. Age-related variation leads to greater “noise” within the data due to associations with retinal vascular changes and other risk factors. In particular, older age is associated with reduced vessel calibre39 in contrast to the increased venular calibre reported previously in association with DN. Older age is also strongly associated with a reduction in eGFR resulting in increased prevalence of CKD in older populations which may be sufficient to obscure any changes in retinal vascular morphology40. Although most studies adjust for the effects of age, the potential for confounding in cross-study comparisons, exists. Furthermore, although we adjusted for glycated haemoglobin, the potential confounding of insulin control may also influence variation in RVP between type 1 and type 2 diabetes.
Previously reported associations between RVP and renal outcomes in type 2 diabetes have not always been consistent. Data from the Wisconsin Epidemiological study of Diabetic Retinopathy (WESDR) reported an association between wider venular calibre and DN incidence over 14 years of follow-up41. In contrast, our data failed to support the findings from WESDR, but do support other cross sectional studies in type 2 diabetes42, and prospective data with similar 2-year follow-up duration43 suggesting RVP may not predict change in eGFR over a short time period. Inclusion of proteinuria or albuminuria measures with eGFR may prove more informative, given previous reported associations with RVP in both type 1 and type 2 diabetes33,34,35,36,37,41.
The majority of previous investigations have been limited to the analysis of vessel calibre, with only a single study considering fractal dimensions with regard to albuminuria in a young type 1 diabetes population37. We report novel findings for measures of vascular spread (retinal vessel fractal dimension), tortuosity, and branching patterns in type 2 diabetes. Fractal dimension and tortuosity were not associated with eGFR decline over the 3-year time-period in this type 2 diabetes cohort.
This study had several strengths. The prospective design allowed the predictive capacity of RVP to be examined over a 2–4 year period. Participant recruitment was clinically driven and electronic data record linkage provided an extensive range of variables on most participants. Our study provided novel data on the predictive utility of RVP and eGFR in type 2 diabetes and included wide range RVP previously under-reported in this context (i.e. fractal dimension, tortuosity, and number of first arteriolar branches). RVP were found to have no predictive value for 3-year change in eGFR in type 2 diabetes in our cohort with a mean age of 63 years. Our cohort originated from a population of type 2 diabetes from Tayside, Scotland, with healthcare record linkage which reduced the likelihood of bias. As a result, the findings are likely to be generalisable to other type 2 diabetes populations. The population was however almost entirely white and therefore, this sample is likely to be most closely generalisable to other predominantly white populations with type 2 diabetes of a similar age, given known associations of diabetes and renal disease with ethnicity. There is also evidence to suggest ethnic differences in retinal microvascular parameters20, perhaps in part due to factors associated with iris colour, retinal pigmentation and/ or underlying genetic influences44.
The limitations of this study include the case-control design which increased the likelihood of regression toward the mean, although the risk of regression toward the mean was reduced through the use of median eGFR values calculated from all available measurements recorded within 6 months either side of the date of each retinal photograph. The definition of progressors as those with eGFR < 60 ml/min/1.73 m2 at follow-up, or a reduction in eGFR of at least 15% between baseline and follow up, risked the inclusion of participants with limited reduction in renal function (e.g. eGFR 60 ml/min/1.73 m2 at baseline, and 59 ml/min/1.73 m2 at follow-up). This proved not to be an issue as only two participants had eGFR < 60 at follow-up combined with reductions in eGFR smaller than −15%, and each of these had reductions in eGFR from baseline exceeding 14% (data not shown).
Another limitation was the 3-year duration between baseline and follow-up measures, which may have been insufficient to detect associations between RVP and change in eGFR. As both retinal calibre and eGFR decline over time, the age (mean age 63 years, SD 7.6) of the population may also have limited the sensitivity to detect such associations with RVP. A study with longer follow-up, including participants entering at a younger age may be required to detect such associations.
A more comprehensive assessment of renal function (such as albumin / creatinine ratio (ACR)) and/or appropriate GFR estimating equations in ‘at risk’ individuals may have improved the sensitivity of our approach and comparability with other studies. Unfortunately, proteinuria/ACR data was not available for the earlier phase of GoDARTS recruitment. Although, HbA1C was included in regression models, duration of diabetes was not, and may have a confounding influence. The suitability and size of our sample may also have limited our capacity to detect meaningful associations. Inclusion of additional individuals with baseline eGFR at the lower end of CKD stage 2 (60–70 ml/min/1.73 m2) may have provided more meaningful clinical significance as they transition from CKD stage 2 to stage 3.
The algorithms used by the retinal vessel measurement platform have been validated against the “gold standard” method of manual vessel tracing45,46. However, establishing the ground truth for validation of retinal vessel measurements is challenging because of the time intensive nature of the manual work and relatively poor inter-grader reliability that arises from manual assessment through expert disagreement on challenging issues related to defining vessel boundaries47,48. In contrast, the semi-automatic vessel assessment platform used in this study shows excellent inter-operator reliability49. Nevertheless, results may vary from associations based on manually assessed retinal vascular parameters. Furthermore, measures of vascular geometry in other locations of the retina, such as the macula, may be of particular interest. However, summary measures of vessel calibre within an annulus encircling the optic disc avoid problems created by variations in branching patterns between individuals, by including all of the largest microvessels of the eye50.
The results of this study suggest that retinal vascular calibre, fractal dimension, tortuosity, and number of first vascular branches surrounding the optic disc are not predictive of eGFR decline over a 3 year follow-up in this white population with type 2 diabetes.
Methods
A nested longitudinal case-control design was undertaken in participants (n = 1072) from the Genetics of Diabetes Audit and Research in Tayside Scotland (GoDARTS) study cohort (ClinicalTrials.gov Identifier: NCT02783469). The GoDARTS cohort comprises 9,439 participants with type 2 diabetes and 8,187 individuals with similar demographics but without type 2 diabetes at the time of recruitment. The study was granted approval from the Tayside
Research Ethics Committees in Scotland and was carried out in accordance with the Declaration of Helsinki, and has been described elsewhere51. Briefly, participants in GoDARTS were identified from a central database of all patients registered with a general practitioner from the Tayside region of Scotland. Diagnosis of type 2 diabetes was made by physicians and participants provided informed consent and agreed to electronic healthcare record linkage. All electronic medical record data was processed and provided in an anonymised form for research through robust information governance procedures approved by local NHS Caldicott Guardians through the Health Informatics Centre at the University of Dundee.
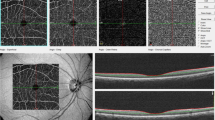
For inclusion in the present study, GoDARTS participants had to meet the following inclusion criteria: presence of type 2 diabetes, eGFR >60 ml/min/1.73 m2 at baseline, available digital retinal fundus images of sufficient quality for analysis at two time points (2–4 years apart) with corresponding clinical serum creatinine measurements within 6 months of each retinal image. Serum creatinine measurements were obtained from centralised Blood Sciences Laboratory records and retinal fundus images were obtained through routine diabetic retinopathy screening52. The earliest available digital fundus image of suitable quality for analysis was selected for the right eye with a follow-up image captured 2–4 years later from the same eye. Retinal fundus images were analysed using semi-automated software, Vessel Assessment and Measurement Platform for Images of the REtina (VAMPIRE; VAMPIRE group, University of Dundee, Dundee, Scotland) version 3.153,54, by trained graders blinded to participant data. VAMPIRE 3.1 measures RVP within predefined annular zones: CRAE, CRVE, arteriovenous ratio (AVR), number of first vessel branches within a pre-defined zone C, fractal dimension and vessel tortuosity (Fig. 1). Intragrader reliability of retinal vascular measurements was measured using the intraclass correlation coefficient, assessed in four sessions of 20 retinal images at regular intervals over the course of the measurement period. Mean intraclass correlation coefficient for these sessions was calculated as 0.936 for CRAE and 0.950 for CRVE, respectively, indicating excellent operator alignment. Diabetic retinopathy status (presence/absence) was obtained from medical records.
Retinal fundus photograph indicating vessels and zones of measurement. Figure 1 shows a retinal image centred on the optic disc. Line R indicates the optic disc radius, line B indicates the annulus 1 to 1.5 optic disc diameters from the centre of the optic disc (Zone B); line C indicates the annulus 1 to 2.5 disc diameters from the centre of the optic disc (Zone C). Zone B is the area of measurement for central retinal arteriolar equivalent and central retinal venular equivalent. Zone C is the area of measurement for fractal dimension. The dark and light lines in this greyscale reproduction indicate arterioles (light) and venules (dark) recognised by VAMPIRE vessel assessment software and corrected by a trained operator.
The median serum creatinine values, HbA1c, SBP and DBP were calculated from all available measurements recorded within 6 months either side of the date of each retinal photograph. eGFR values were calculated from median serum creatinine values using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation55.
Participants were divided into two groups based on change in eGFR between both time points. Group 1, “non-progressors”, included participants with stable renal function or a reduction in eGFR <10% between both time points. Group 2, “progressors”, included participants with an eGFR of <60 ml/min/1.73 m2 at follow-up or a reduction in eGFR of at least 15% between baseline and follow-up.
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Statistical analyses were performed using IBM SPSS v24 (Chicago, Illinois, USA). Participants with missing data were excluded from the analyses. Continuous variables were reported using means and SDs. Categorical variables were reported as percentages. Between and within-group comparisons were made using t-tests (two-sided significance reported) for continuous variables, and Chi-squared tests for proportions. The relationship between RVP and eGFR was assessed using logistic and linear regression models. Logistic regression models were used to test for association between baseline RVP and progression of renal disease. The independent variables were RVP and progressor/non-progressor designation as a binary dependent variable. Logistic regression models were carried out unadjusted, and adjusted for important known confounding variables; age, gender, baseline SBP, and baseline HbA1c. Additional models were used to further adjust for covariates related to RVP; diabetic retinopathy and fellow vessel calibre (for models including retinal vascular calibre as the independent variable). Associations involving continuous outcome variables were assessed using multiple linear regression. Linear regression models were used to test for cross-sectional association between renal function and RVP at follow-up. The independent variables were RVP, the dependent variable was eGFR. Linear regression models controlled for age, gender, follow-up SBP and HbA1c. Additional models adjusted for diabetic retinopathy and fellow vessel calibre (where retinal vascular calibre was the independent variable). Tortuosity variables were log transformed before linear regression to produce normal distributions and to conform to the assumptions of the analysis. All significance values reported are two-sided.
References
Rahman, S., Rahman, T., Ismail, A. A.-S. & Rashid, A. R. A. Diabetes-associated macrovasculopathy: pathophysiology and pathogenesis. Diabetes Obes. Metab. 9, 767–780 (2007).
USRDS. United States Renal Data System Incident and Prevalent Counts by Quarter. USRDS, Available at: https://www.usrds.org/qtr/default.aspx (Accessed: 5th May 2017) (2017).
Retnakaran, R., Cull, C. A., Thorne, K. I., Adler, A. I. & Holman, R. R. Risk Factors for Renal Dysfunction in Type 2 Diabetes. Diabetes 55, 1832–1839 (2006).
Molitch, M. E. et al. Development and Progression of Renal Insufficiency With and Without Albuminuria in Adults With Type 1 Diabetes in the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications Study. Diabetes Care 33, 1536–1543 (2010).
Costacou, T., Ellis, D., Fried, L. & Orchard, T. J. Sequence of Progression of Albuminuria and Decreased GFR in Persons With Type 1 Diabetes: A Cohort Study. Am. J. Kidney Dis. 50, 721–732 (2007).
Rask-Madsen, C. & King, G. L. Vascular Complications of Diabetes: Mechanisms of Injury and Protective Factors. Cell Metab. 17, 20–33 (2013).
Forbes, J. M. & Cooper, M. E. Mechanisms of Diabetic Complications. Physiol. Rev. 93, 137–188 (2013).
Paneni, F., Beckman, J. A., Creager, M. A. & Cosentino, F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy. Eur. Heart J. 34, 2436–2443 (2013). part I.
Ding, J. et al. Retinal vascular caliber and the development of hypertension: a meta-analysis of individual participant data. J. Hypertens. 32, 207–215 (2014).
McGeechan, K. et al. Meta-analysis: retinal vessel caliber and risk for coronary heart disease. Ann. Intern. Med. 151, 404–413 (2009).
McGeechan, K. et al. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am. J. Epidemiol. 170, 1323–1332 (2009).
Sabanayagam, C. et al. Retinal microvascular calibre and risk of diabetes mellitus: a systematic review and participant-level meta-analysis. Diabetologia 58, 2476–2485 (2015).
Wong, T. Y. Retinal Microvascular Abnormalities and Renal Dysfunction: The Atherosclerosis Risk in Communities Study. J. Am. Soc. Nephrol. 15, 2469–2476 (2004).
Bao, S. et al. Retinal Vessel Diameter and Chronic Kidney Disease in Rural China: A Cross-Sectional Study. Medicine (Baltimore) 94, e2076 (2015).
Sabanayagam, C. et al. Retinal microvascular caliber and chronic kidney disease in an Asian population. Am. J. Epidemiol. 169, 625–632 (2009).
Daien, V. et al. Retinal vascular caliber is associated with renal function in apparently healthy subjects. Acta Ophthalmol. (Copenh.) 91, e283–288 (2013).
Sabanayagam, C. et al. Retinal arteriolar narrowing increases the likelihood of chronic kidney disease in hypertension. J. Hypertens. 27, 2209–2217 (2009).
Sng, C. C. A. et al. Fractal analysis of the retinal vasculature and chronic kidney disease. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. - Eur. Ren. Assoc. 25, 2252–2258 (2010).
Ooi, Q. L. et al. Microvascular Disease After RenalTransplantation. Kidney Blood Press. Res. 40, 575–583 (2015).
Yau, J. W. Y. et al. Retinal arteriolar narrowing and subsequent development of CKD Stage 3: the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 58, 39–46 (2011).
Lim, L. S. et al. Structural changes in the retinal microvasculature and renal function. Invest. Ophthalmol. Vis. Sci. 54, 2970–2976 (2013).
Ooi, Q. L. et al. The microvasculature in chronic kidney disease. Clin. J. Am. Soc. Nephrol. CJASN 6, 1872–1878 (2011).
Grunwald, J. E. et al. Retinopathy and chronic kidney disease in the Chronic Renal Insufficiency Cohort (CRIC) study. Arch. Ophthalmol. Chic. Ill 1960(130), 1136–1144 (2012).
Sun, Y.-M., Su, Y., Li, J. & Wang, L.-F. Recent advances in understanding the biochemical and molecular mechanism of diabetic nephropathy. Biochem. Biophys. Res. Commun. 433, 359–361 (2013).
Klein, R. Are Inflammatory Factors Related to Retinal Vessel Caliber?: The Beaver Dam Eye Study. Arch. Ophthalmol. 124, 87 (2006).
Wong, T. Y. et al. Retinal Vascular Caliber, Cardiovascular Risk Factors, and Inflammation: The Multi-Ethnic Study ofAtherosclerosis (MESA). Investig. Opthalmology Vis. Sci. 47, 2341 (2006).
Dorner, G. T. et al. Nitric oxide regulates retinal vascular tone in humans. Am. J. Physiol. - Heart Circ. Physiol. 285, H631–H636 (2003).
Schiffrin, E. L., Lipman, M. L. & Mann, J. F. E. Chronic Kidney Disease: Effects on the Cardiovascular System. Circulation 116, 85–97 (2007).
Schlieper, G., Schurgers, L., Brandenburg, V., Reutelingsperger, C. & Floege, J. Vascular calcification in chronic kidney disease: an update. Nephrol. Dial. Transplant. 31, 31–39 (2016).
McGowan, A. et al. Evaluation of the Retinal Vasculature in Hypertension and Chronic Kidney Disease in an Elderly Population of Irish Nuns. PLOS ONE 10, e0136434 (2015).
Aliahmad, B., Kumar, D. K., Sarossy, M. G. & Jain, R. Relationship between diabetes and grayscale fractal dimensions of retinal vasculature in the Indian population. BMC Ophthalmol. 14, 152 (2014).
Sasongko, M. B. et al. Retinal Vessel Tortuosity and Its Relation to Traditional and Novel Vascular Risk Markers in Persons with Diabetes. Curr. Eye Res. 41, 551–557 (2016).
Grauslund, J. et al. Retinal vessel calibre and micro- and macrovascular complications in type 1 diabetes. Diabetologia 52, 2213–2217 (2009).
Klein, R. et al. Retinal vascular abnormalities in persons with type 1 diabetes: the Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVIII. Ophthalmology 110, 2118–2125 (2003).
Wong, T. Y., Shankar, A., Klein, R. & Klein, B. E. Retinal vessel diameters and the incidence of gross proteinuria and renal insufficiency in people with type 1 diabetes. Diabetes 53, 179–184 (2004).
Broe, R. et al. Retinal vessel calibers predict long-term microvascular complications in type 1 diabetes: the Danish Cohort of Pediatric Diabetes 1987 (DCPD1987). Diabetes 63, 3906–3914 (2014).
Broe, R. et al. Retinal vascular fractals predict long-term microvascular complications in type 1 diabetes mellitus: the Danish Cohort of Pediatric Diabetes 1987 (DCPD1987). Diabetologia 57, 2215–2221 (2014).
Moresco, R. N., Sangoi, M. B., De Carvalho, J. A. M., Tatsch, E. & Bochi, G. V. Diabetic nephropathy: Traditional to proteomic markers. Clin. Chim. Acta 421, 17–30 (2013).
Sun, C., Wang, J. J., Mackey, D. A. & Wong, T. Y. Retinal Vascular Caliber: Systemic, Environmental, and Genetic Associations. Surv. Ophthalmol. 54, 74–95 (2009).
Schaeffner, E. S. et al. Two Novel Equations to Estimate Kidney Function in Persons Aged 70 Years or Older. Ann. Intern. Med. 157, 471 (2012).
Klein, R., Klein, B. E. K., Moss, S. E. & Wong, T. Y. Retinal Vessel Caliber and Microvascular and Macrovascular Disease in Type 2 Diabetes: XXI: The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology 114, 1884–1892 (2007).
Frydkjaer-Olsen, U., Hansen, R. S., Pedersen, K., Peto, T. & Grauslund, J. Retinal Vascular Fractals Correlate With Early Neurodegeneration in Patients With Type 2 Diabetes MellitusEarly Vascular and Neurogenic Changes in Diabetes. Invest. Ophthalmol. Vis. Sci. 56, 7438–7443 (2015).
Frydkjaer-Olsen, U. et al. Correlation between Retinal Vessel Calibre and Neurodegeneration in Patients with Type 2 Diabetes Mellitus in the European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Ophthalmic Res. 56, 10–16 (2016).
Rochtchina, E. et al. Ocular Pigmentation Confounds the Measurements of Retinal Vessel Calibre: The Sydney Childhood Eye Study. Invest. Ophthalmol. Vis. Sci. 48, 3079–3079 (2007).
MacGillivray, T. J., Patton, N., Doubal, F. N., Graham, C. & Wardlaw, J. M. Fractal analysis of the retinal vascular network in fundus images. in 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society 6455–6458, https://doi.org/10.1109/IEMBS.2007.4353837 (2007).
Azegrouz, H., Trucco, E., Dhillon, B., MacGillivray, T. & MacCormick, I. J. Thickness dependent tortuosity estimation for retinal blood vessels. in 2006 International Conference of the IEEE Engineering in Medicine and Biology Society 4675–4678, https://doi.org/10.1109/IEMBS.2006.260558 (2006).
Patton, N. et al. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 206, 319–348 (2005).
Trucco, E. et al. Validating Retinal Fundus Image Analysis Algorithms: Issues and a Proposal. Invest. Ophthalmol. Vis. Sci. 54, 3546–3559 (2013).
MacGillivray, T. J. et al. Suitability of UK Biobank Retinal Images for Automatic Analysis of Morphometric Properties of the Vasculature. PLOS ONE 10, e0127914 (2015).
Knudtson, M. D. et al. Revised formulas for summarizing retinal vessel diameters. Curr. Eye Res. 27, 143–149 (2003).
Hébert, H. L. et al. Cohort Profile: Genetics of Diabetes Audit and Research in Tayside Scotland (GoDARTS). Int. J. Epidemiol. https://doi.org/10.1093/ije/dyx140.
Scotland, G. S. et al. Cost-effectiveness of implementing automated grading within the national screening programme for diabetic retinopathy in Scotland. Br. J. Ophthalmol. 91, 1518–1523 (2007).
Trucco, E. et al. Morphometric Measurements of The Retinal Vasculature in Fundus Images with Vampire. in BiomedicalImage Understanding(eds. Lim, J.-H., Ong, S.-H. & Xiong, W.) 91–111, https://doi.org/10.1002/9781118715321.ch3 (John Wiley & Sons, Inc, 2015).
Lisowska, A., Annunziata, R., Loh, G. K., Karl, D. & Trucco, E. An experimental assessment of five indices of retinal vessel tortuosity with the RET-TORT public dataset. in 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society 5414–5417, https://doi.org/10.1109/EMBC.2014.6944850 (2014).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009).
Acknowledgements
The authors wish to acknowledge the participation of the GoDARTS study, NHS Tayside, the providers of the original data source, and the Health Informatics Centre (HIC), University of Dundee, for managing the anonymised/pseudo-anonymised datasets. The project was funded by the Medical Research Council UK, grant number MR/K003364/1, the Northern Ireland Health and Social Care Research and Development Office (STL/4936/14), and the Department for Education and Learning, Northern Ireland.
Author information
Authors and Affiliations
Contributions
Gareth J. McKay, Alexander P. Maxwell: obtained funding, undertook data analysis, drafted manuscript. Thomas J. MacGillivray, Emanuele Trucco, Alexander S. Doney: extracted data, undertook data analysis, drafted manuscript. Euan N. Paterson, Christopher C. Cardwell: undertook data analysis and drafted manuscript. Ruixuan Wang, Stephen Hogg: undertook retinal measurements and drafted manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McKay, G.J., Paterson, E.N., Maxwell, A.P. et al. Retinal microvascular parameters are not associated with reduced renal function in a study of individuals with type 2 diabetes. Sci Rep 8, 3931 (2018). https://doi.org/10.1038/s41598-018-22360-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-22360-3
This article is cited by
-
Retinal microvascular parameters are not associated with diabetes in the Northern Ireland Cohort for the Longitudinal Study of Ageing
Irish Journal of Medical Science (1971 -) (2022)
-
Retinal microvascular parameters are not significantly associated with mild cognitive impairment in the Northern Ireland Cohort for the Longitudinal Study of Ageing
BMC Neurology (2021)
-
Association of reduced retinal arteriolar tortuosity with depression in older participants from the Northern Ireland Cohort for the Longitudinal Study of Ageing
BMC Geriatrics (2021)
-
Retinal microvascular signs and risk of diabetic kidney disease in asian and white populations
Scientific Reports (2021)
-
Association of retinal venular tortuosity with impaired renal function in the Northern Ireland Cohort for the Longitudinal Study of Ageing
BMC Nephrology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.