Abstract
Nonalcoholic fatty liver disease (NAFLD) is a kind of liver lipid synthesis and degradation imbalance related with metabolic syndrome. Celecoxib shows the function of ameliorating NAFLD, but the underlying mechanisms remain unknown. Here, we discuss the possible mechanisms of celecoxib alleviating NAFLD by restoring autophagic flux. Lipids were accumulated in L02 cells treated with palmitate as well as SD rats fed with high-fat diet. Western blot showed that LC3 II/I was higher and p62 was lower on the early stage of steatosis while on the late stage both of them were higher, indicating that autophagic flux was activated on the early stage of steatosis, but blocked on the late stage. Rapamycin alleviated steatosis with activating autophagic flux while chloroquine aggravated steatosis with inhibiting autophagic flux. COX-2 siRNA and celecoxib were used to inhibit COX-2. Western blot and RFP-GFP-LC3 double fluorescence system indicated that celecoxib could ameliorate steatosis and restore autophagic flux in L02 cells treated with palmitate as well as SD rats fed with high-fat diet. In conclusion, celecoxib partially restores autophagic flux via downregulation of COX-2 and alleviates steatosis in vitro and in vivo.
Similar content being viewed by others
Introduction
Due to the modern sedentary and food-abundant lifestyle, nonalcoholic fatty liver disease (NAFLD) has become one of the most common liver diseases around world1. Prevalence of NAFLD ranges from 5% to 40% in Asia, among which China has become one of the highest prevalence regions2. It is widely acknowledged that the spectrum of NAFLD extends from nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH) and liver cirrhosis3,4. Furthermore, NAFLD can progress to liver cancer without fibrosis. Apart from lifestyle changes, effective treatment of NAFLD is still elusive. Recent studies suggest that celecoxib plays an important role in the treatment of NAFLD5.
Three types of cyclooxygenase exist, namely COX-1, COX-2, and COX-36,7, among which COX-2 is widely upregulated in NAFLD animal models and steatosis hepatocytes8,9. Recent studies reported that the upregulated expression of COX-2 has intertwined relationship with metabolic disorders including obesity, diabetes mellitus, and NAFLD. Furthermore, COX-2 activity not only has influence on insulin sensitivity10, but also acts as pro-inflammatory mediator during the progression of NAFLD11. Recently, it has gained increasing attention on the mechanisms by which celecoxib protects against the development of NAFLD. Many studies suggested that celecoxib could attenuate liver steatosis and inflammation in NAFLD5. Celecoxib, a selective COX-2 inhibitor, may protect cells from palmitate (PA) -induced insulin signaling Akt phosphorylation10,12,13. Celecoxib may also alleviate liver inflammation through regulation of NF-kB pathway as well as steatosis via suppression of non-canonical Wnt signaling pathway14. In addition, celecoxib may inhibit hepatocytes apoptosis through Akt/p53 signal pathway in NAFLD12.
Autophagy, a self-digestion process, plays an important role in the development of NAFLD15,16,17. In fact, apart from the tricarboxylic acid cycle in mitochondria, autophagy is also involved in lipid metabolism namely the lipophagy18,19. Lipophagy is a complex multi-step process which refers to the degradation of extra lipid by autolysosomes in cells16. Thus, autophagy is quite essential for the balance of lipid metabolism. Recent studies suggested that impaired autophagic flux is associated with the progress of NAFLD20,21. Activating autophagy with rapamycin (mTOR inhibitor) will attenuate steatosis, fibrosis, inflammation, ER stress, while treating with chloroquine will make steatosis and liver injury worsen22. Although many studies proposed different mechanisms of celecoxib on alleviating NAFLD, none of them have explored whether autophagy participates in this effect. Further studies are needed to clarify the underlying mechanisms of celecoxib on alleviating NAFLD which may provide us a better understanding of the pathogenesis and treatment of NAFLD.
In this study, we try to figure out whether celecoxib attenuates hepatic steatosis by regulating autophagic flux both in vivo and in vitro.
Results
PA induced lipid accumulation in hepatocytes
Oil Red O staining and TG kit were used to examine the levels of lipid accumulation in L02 cells after treating with PA. When compared to the control, intracellular lipid droplets were elevated in L02 cells in a time and dose-dependent manner (Fig. 1a). After treating with different concentrations and time of PA, the TG levels were also remarkably increased in L02 cells (Fig. 1b,c). We examined the effects of PA or celecoxib on cell viability, indicating that PA significantly reduced cell viability from 200 μM (Fig. 1d), while celecoxib had little effect on cell viability within 0–20 μM (Fig. 1e).
PA induces lipid accumulation in hepatocytes. (a–c) L02 hepatic cells exposed to different concentrations (200 μM, 400 μM) of PA for different time (12 h, 24 h). Lipid droplets accumulation and TG levels were determined by Oil Red O staining (400X) and TG assay respectively (*P < 0.05, **P < 0.01). (d) L02 cells exposed to different concentrations (100–500 μM) of PA for different time (12 h, 24 h). CCK-8 assay was used to detect cell viability (*P < 0.05, **P < 0.01). (e) L02 cells were treated with celecoxib at different concentrations (5–40 μM) for 24 h. CCK-8 assay was used to detect cell viability (*P < 0.05, **P < 0.01).
Effects of PA on autophagic flux and COX-2
To investigate the effects of PA on autophagic flux in L02 cells, LC3 II/I and p62 expression, which had been widely acknowledged as hallmarks of autophagic flux, were detected by western blot. Protein expression of LC3 II/I and p62 was increased in L02 cells treated with PA within 200–400 μM for 24 h (Fig. 2a and Supplementary Fig. S1). As shown in Fig. 2b and Supplementary Fig. S2, a remarkable increase of LC3 II/I and reduction of p62 were found in L02 cells treated with 200 μM PA at 4 h and 8 h. While at 24 h and 36 h, both of LC3 II/I and p62 were increased, which indicated that autophagic flux was activated on the early stage of steatosis but blocked at the late hour. The COX-2 expression was also noticeablely increased in L02 cells treated with PA (Fig. 2c,d and Supplementary Fig. S3).
Effects of PA on autophagic flux and COX-2. (a) L02 cells exposed to different concentrations (100–400 μM) of PA for 24 h. PA induced upregulation of LC3 II/I and p62 protein levels as indicated by western blot. (b) L02 cells were treated with PA (200 μM) for different time (0–36 h). PA induced upregulation of LC3 II/I and downregulation of p62 protein levels at 4 h, 8 h while upregulation of LC3 II/I and p62 protein levels at 24 h, 36 h indicating that autophagic flux was activated on the early stage but blocked at the late hour. (c,d) L02 cells were treated with PA of different concentrations (100–400 μM) for different time (12–36 h). PA induced upregulation of COX-2 protein levels. The value under the band is the ratio of the blot and normalized to control.
Autophagy played an important role in steatosis
We used rapamycin (autophagy activator) and chloroquine (autophagy inhibitor) to explore the effect of autophagy on lipid accumulation in steatosis L02 cells induced by PA. Rapamycin, a mammalian target of mTOR inhibitor, has been widely used as autophagy inducer. Rapamycin treatment enhanced the expression of LC3 II/I and reduced the expression of p62 in PA treated L02 cells (Fig. 3a and Supplementary Fig. S4). Meanwhile, lipid droplets accumulated in L02 cells treated with PA as well as TG content displayed a distinct lower level in combination of rapamycin and PA treatment groups than those in PA treatment groups (Fig. 3c,d). Chloroquine is widely acknowledged as a classical autophagy inhibitor with the effect to disrupt the fusion of autophagosome with lysosome and raise of lysosome pH. We found that chloroquine treatment resulted in increase of p62 in PA treated L02 cells (Fig. 3b and Supplementary Fig. S5). Furthermore, lipid droplets accumulation and TG content were remarkably increased in combination of chloroquine and PA treatment groups than those in PA treatment groups (Fig. 3c,d).
Autophagy plays an important role in steatosis. (a,b) L02 cells exposed to PA (200 μM) with different concentrations of rapamycin (Rapa) or chloroquine (CQ) for 24 h. PA induced higher protein expression of LC3 II/I and p62 compared with control as indicated by western blot. Rapamycin combined with PA treatment induced higher LC3 II/I protein levels and lower p62 protein levels compared with PA treatment. Chloroquine combined with PA treatment induced higher p62 protein levels compared with PA treatment. The value under the band is the ratio of the blot and normalized to control. (c,d) L02 cells exposed to PA (200 μM) with rapamycin (20 ng/ml) or chloroquine (0.5 μM) for 24 h. Intracellular lipid droplets accumulation and TG levels were determined by Oil Red O staining (400X) or TG assay respectively (*P < 0.05, **P < 0.01). L02 steatosis was decreased with rapamycin treatment while increased with chloroquine treatment.
Downregulation of COX-2 induced autophagic flux in vitro
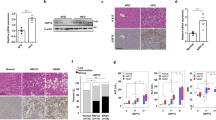
To investigate whether an increased expression of COX-2 in steatosis L02 cells induced by PA had an effect on autophagy, we performed siRNA to silence COX-2 expression. As displayed, the mRNA and protein expression of COX-2 were lower in COX-2 siRNA transfected cells than control cells (Fig. 4a,b). When COX-2 was silenced, LC3 II/I was increased and p62 was decreased in L02 cells treated with PA (Fig. 4c and Supplementary Fig. S7).
Downregulation of COX-2 induces autophagic flux in vitro. (a,b) COX-2 was knocked down with COX-2 siRNA transfection (si-1 or si-2) in L02 cells, then RT-PCR and western blot were performed for confirmation. (c) After treated with PA (200 μM) for 24 h, western blot of COX-2 siRNA transfected cells showed higher protein expression of LC3 II/I and lower protein expression of p62 than negative control (NC). The value under the band is the ratio of the blot and normalized to control.
Celecoxib alleviated steatosis partially by restoring autophagic flux in vitro
Recent studies have shown that celecoxib may protect against the development of NASH by attenuating liver steatosis and inflammation. To determine whether celecoxib alleviates steatosis by regulating autophagic flux in vitro, L02 cells were treated with 200 μM PA for 24 h combined with celecoxib. As shown in Fig. 5a and b, protein levels of COX-2 and p62 were remarkably reduced and LC3 II/I was increased. Autophagic flux was further evaluated in RFP-GFP-LC3 lentivirus transfected L02 cells. PA treatment increased the amount of yellow dots. However, PA treatment in combination with celecoxib displayed increased amount of both yellow and red dots indicating autophagic flux was restored partially (Fig. 5c). To further determine the effect of celecoxib on alleviating steatosis, Oil Red O staining and TG kit were used to examine the levels of lipid accumulation in steatosis L02 cells treated with celecoxib. The amount of lipid droplets accumulated in L02 cells and TG content were remarkably decreased in combination of celecoxib and PA treatment groups than those in PA treatment groups (Fig. 5d,e).
Celecoxib alleviates steatosis by restoring autophagic flux in vitro. (a) L02 cells exposed to PA (200 μM) with different concentrations of celecoxib (Cel, 5–40 μM) for 24 h. Celecoxib decreased protein expression of COX-2 compared with control as indicated by western blot. (b) L02 cells were treated with PA (200 μM) and celecoxib (20 μM) for 24 h. Celecoxib combined with PA treatment induced higher protein expression of LC3 II/I and lower protein expression of p62 compared to PA treatment. The value under the band is the ratio of the blot and normalized to control. (c) RFP-GFP-LC3 double fluorescence lentivirus tranfected L02 cells were treated with PA (200 μM) and celecoxib (20 μM) for 24 h. Representative confocal images of GFP-LC3 puncta (green, autophagosomes) and RFP-LC3 puncta (red, autolysosomes), as well as overlay images were shown (1000X). (d,e) L02 cells exposed to PA (200 μM) with celecoxib (20 μM) for 24 h. Intracellular lipid droplets accumulation and TG levels were determined by Oil Red O staining (400X) or TG assay respectively (*P < 0.05, **P < 0.01).
Celecoxib ameliorated NAFLD partially by restoring autophagic flux in vivo
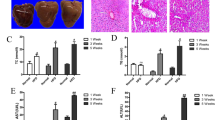
SD rats were fed with HFD and then treated with celecoxib. As shown in Fig. 6a, the liver HE staining and Oil Red O staining exhibited widespread lipid droplets deposited inside the liver parenchyma cells in HFD rats groups compared with the control groups. However, celecoxib treatment groups exhibited less lipid accumulation. Body weight, liver weight/body weight, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), triglyceride (TG), and the liver tissue TG level in celecoxib-treated rats were commonly decreased than control groups (Fig. 6b–h). As presented in Fig. 6i and Supplementary Fig. S9, the expression of COX-2, LC3 II/I and p62 all increased in HFD rats compared with control groups. However, COX-2 and p62 expression was decreased, LC3 II/I expression was increased in celecoxib treatment groups.
Celecoxib ameliorates NAFLD by restoring autophagic flux in vivo. SD rats were fed with normal chow diet (NC, n = 10) or high fat diet (HFD, n = 20) for 8 weeks. At the end of week 4, HFD groups were then randomly intragastricly administrated with celecoxib (HFD + Cel, 20 mg/kg/day, n = 10) or normal saline (n = 10) for another 4 weeks, while the NC groups were intragastricly administrated with normal saline. (a) Liver histology was determined by H&E and Oil Red O staining (200X). (b–h) Body weight, liver weight/body weight, serum levels of ALT, AST, TG, TC, and intrahepatic TG level in HFD + Cel groups were lower than HFD groups (*P < 0.05, **P < 0.01). (i) HFD induced upregulation of LC3 II/I, p62 and COX-2 levels compared with negative control as indicated by western blot. HFD + Cel groups liver showed higher protein expression of LC3 II/I and lower protein expression of p62 and COX-2 compared with HFD groups. The value under the band is the ratio of the blot and normalized to control.
Discussion
NAFLD is a complex multifactorial chronic disease which is charactered by an excessively high accumulation of fat deposits in the liver resulting from causes other than chronic alcohol abuse and drugs23. NAFLD is often accompanied by obesity, diabetes, and hyperlipidemia which terribly trouble health. And the pathogenesis of this disease is still unclear. It is generally acknowledged that the theory of “two hits” may provide us a new sight into the process of NAFLD. As is known, NAFLD is closely associated with insulin resistance and lipid metabolism dysfunction, both of which can lead to the accumulation of lipid droplets in hepatocytes (the first hit). Also, lipid oxidation, peroxidation, endoplasmic reticulum stress, inflammation, and endotoxins can further aggravate this disease (the second hit). During the process of the second hit, NAFLD is likely to develop into NASH, cirrhosis, and even cancer. But till today, there is no special medical treatment for NAFLD.
For that reason, it is urgent to find out new ways for NAFLD therapy. In this study, steatosis L02 cells and HFD-fed rats are used as NAFLD models to identify the beneficial effects and underlying mechanisms of celecoxib. We found that celecoxib alleviated lipid accumulation both in vitro and in vivo. In addition, we found that celecoxib regulated NAFLD partially by restoring autophagic flux.
Autophagy is a protect response to stress resulting from internal and external stimuli in all cells24,25. It is generally acknowledged that autophagy plays an important role in maintaining homeostatic state and normal function in cells26,27. In the development of NAFLD, autophagy serves as another way for lipid metabolism apart from tricarboxylic acid cycle18,28. The process is widely known as lipophagy. A study has demonstrated a relationship between SREBP-2 intracelluar trafficking and impaired autophagic flux in NAFLD. Downregulation of SREBP-2 may result in improved autophagic flux as well as lower accumulation of lipid droplets20. Other studies have indicated that inhibition of lipophagy may result in larger number and bigger size of lipid droplets in starved mouse embryonic fibroblasts, while degradation of lipid droplets in mitochondria only requires lipolysis activation29. In starved livers, autophagy marker LC3 along with perilipins has been found in lipid droplets and lysosomes30. It is likely that a cross-talk between lipolysis and lipophagy plays an important role in the accumulation of lipid droplets in NAFLD. It is also demonstrated that lipid accumulation in hepatocytes blocks autophagic flux. In return, impaired autophagic flux promotes the progress of NAFLD31.
We found that autophagic flux was activated on the early stage of steatosis induced by PA in L02 hepatic cells as steatosis maybe a stimuli to cell homeostatic state. Autophagic flux (lipophagy) may act as an attempt to promote lipid turnover. But at the late hour autophagic flux was blocked due to homeostatic imbalance. In addition, rapamycin (autophagy activator) could alleviate lipid accumulation in hepatocytes through activating impaired autophagic flux. Meanwhile, chloroquine (autophagy inhibitor) could aggravate steatosis through blocking autophagic flux. However, it is still unknown how autophagic flux regulates steatosis. Thus it still needs efforts to find out whether autophagic flux regulates lipolysis and lipogenic genes or promotes lipid droplets turnover through autolysosomes.
COX-2, a key enzyme in the process of prostaglandin synthesis, is highly expressed in many diseases, such as cancer, T2DM and NAFLD32,33. Celecoxib is a type of non-steroidal anti-inflammatory drug (NSAID) which directly inhibits COX-2. Studies have shown that celecoxib may have functions on cancer, kidney injury as well as NAFLD34,35. A study indicated that celecoxib induced and activated autophagy in human prostate cancer PC3 cells in a time and dose-dependent manner. Further study found that autophagy activated by celecoxib played a cytoprotective role in apoptosis33. In our study, we also found that COX-2 was overexpressed during steatosis induced by PA in L02 hepatic cells. Both COX-2 siRNA and celecoxib could alleviate lipid accumulation. Then, whether autophagy plays a role in the treatment of celecoxib on alleviating NAFLD is to be answered. We did find out celecoxib could improve NAFLD at least partially by restoring autophagic flux both in vitro and in vivo. However, it is still unknown how celecoxib regulates autophagic flux. More attention should be paid to find out the underlying mechanisms of celecoxib on regulating autophagy associated gene and the fusion of autophagosome and lysosome.
In conclusion, our present study showed that celecoxib alleviated NAFLD by restoring autophagic flux both in vitro and in vivo. These findings may help us better understand the mechanisms of NAFLD as well as developing novel therapy strategies.
Materials and Methods
Animals
Thirty male Sprague-Dawley (SD) rats were obtained from Beijing Vital River Laboratory Animal Technology (Beijing, China). Six-week-old rats were maintained at 22 ± 2 °C with 60% ± 5% relative humidity under a 12 h light and dark cycle with free access to water and regular chow diet. After one week acclimatization, rats were randomly divided into two groups: control group and HFD (high-fat diet) group. Rats of control group were fed with a normal chow diet (12% kcal fat, 66% kcal carbohydrate, 22% kcal protein) (#1022, from SD rats agent as above) (n = 10). Rats of HFD group were fed with high fat diet (60% kcal fat, 20% kcal carbohydrate, 20% kcal protein) (#D12492, from SD rats agent as above) (n = 20). At the end of week 4, the HFD group rats were then randomly intragastricly administrated with celecoxib (Pfizer, USA) (20 mg/kg/day, n = 10) or normal saline (n = 10) for another 4 weeks along with HFD, while the control group rats were intragastricly administrated with normal saline. At the end of week 8, rats were sacrificed for 12 h fasting. Blood samples were collected and livers were removed and weighed for further study. Animal experiments were approved by Ethics Committee of Tongji Hospital and all experiments were performed in accordance with relevant guidelines and regulations for care and use of laboratory animals.
Biochemical analyses
Blood biochemical parameters, including serum ALT, AST, TC, and TG were measured by kits (#C009-2, #C010-2, #A111-1, and #A110-1, Jiancheng Bioengineering Institute, Nanjing, China). Liver TG was determined by kit (#E1013, Applygen Technologies, Beijing, China).
Cell line, culture, and treatment
The human hepatic cell line L02 was maintained at Institute of liver diseases, Tongji Hospital, Wuhan, China. The L02 cells were cultured in RPMI-1640 medium containing 10% fetal calf serum (#10100147, Invitrogen Gibco, Carlsbad, CA, USA) and incubated in a 5% CO2 incubator at 37 °C. Celecoxib, rapamycin, and chloroquine (HY-14398, HY-17589, and HY-10219, respectively, MedChemExpress, Monmouth Junction, NJ, USA) were added into the culture at indicated concentrations and timepoints.
Preparation of palmitate
0.2 mmol sodium palmitate (PA) (#FIZ6I-PJ, Tokyo Chemical Industry, Japan) was mixed in 0.1 M 2 ml NaOH at 70 °C. 5% bovine serum albumin (BSA) was prepared in 38 ml phosphate-buffered saline (PBS). The solution of PA was conjugated to 5% BSA in a 50 °C water bath for 2 h. The stock concentration of PA is 5 mM. Finally, the PA solution filtered and stored at −20 °C.
Oil Red O staining
The lipid accumulation in cells or liver is detected by Oil Red O staining kit (#D027, Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
Cell viability assay
Cells (5 × 103 cells/well) were seeded in 96-wells plate. After treatment, cells were incubated with a mixed content (10 μl CCK8 reagent +90 μl RPMI-1640 medium) (#P5090, Promoter, Wuhan, China) at 37 °C for 2 h. Finally, the value was measured at 450 nm OD.
Transient transfection
L02 cells were transfected with RFP-GFP-LC3 double fluorescence lentivirus (#GPL2001, Genechem, Shanghai, China) at 20–30% confluence according to the manufacturer’s protocol. We got the stable expression transfectant by adding puromycin. Then the L02 cells were treated with PA or celecoxib. To knockdown COX-2, siRNAs were transfected into cells using Lipofectamine2000 (#11668-019, Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The siRNA sequences (#1299001, Invitrogen, Carlsbad, CA, USA) used as follow: si-1 5′-GAAGCCUUCUCUAACCUCUCCUAUU-3′ and si-2 5′-AUCAGUAUUAGCCUGCUUGUCUGGA-3′.
Quantitative real-time PCR
Total RNA was extracted by TRIzol reagent (#15596018, Invitrogen, Carlsbad, CA, USA). Reverse-transcribed complementary DNA was synthesized using the PrimeScript RT reagent kit (#RR036A, TaKaRa, Otsu, Japan). Real-time PCR was performed using SYBR Premix ExTaq (#RR820A, TaKaRa, Otsu, Japan) on an ABI StepOne Real-Time PCR System (Applied Biosystem, Carlsbad, CA, USA). Difference between samples was determined by the 2−ΔΔCt method. Primers were designed using NCBI Primer-BLAST. COX-2, Forward Primer 5′-CTGGCGCTCAGCCATACAG-3′ and Reverse Primer 5′-CGCACTTATACTGGTCAAATCCC-3′.
Western blot
Total protein from cells and liver were extracted, concentration was quantitated using BCA protein quantification kit (#P5026, Promoter, Wuhan, China). Protein in PVDF membranes were incubated with primary antibody at 4 °C overnight, LC3 I/II and p62 (#4108, #5114, both 1:1000, Cell Signaling Technology, Boston, USA), COX-2 and β-actin (#P1023-01, #P0015, 1:1000 and 1:4000, Promoter, Wuhan, China) followed by anti-rabbit IgG (#P0009, 1:4000, Promoter, Wuhan, China) for 1 h at room temperature. Immunoreactive bands were visualized with ECL kit (#P5876, Promoter, Wuhan, China) and pictured by GeneGnome5 Chemiluminescence Series Image Capture (Syngene, Frederick, MD, USA) according to manufacturers’ instructions. All the western blot experiments were repeated at least two times. Blot densitometric analysis was done by ImageJ software (NIH).
Statistical analysis
Results were presented as mean ± SD. For comparisons between two groups, Student’s t-tests were used. For multiple groups, ANOVA analysis with or without repeated measures was used. P < 0.05 was considered significant.
References
Loomba, R. & Sanyal, A. J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 10, 686–690 (2013).
Amarapurkar, D. N. et al. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J. Gastroenterol. Hepatol. 22, 788–793 (2007).
Cohen, J. C., Horton, J. D. & Hobbs, H. H. Human fatty liver disease: old questions and new insights. Science. 332, 1519–1523 (2011).
Liu, W., Baker, R. D., Bhatia, T., Zhu, L. & Baker, S. S. Pathogenesis of nonalcoholic steatohepatitis. Cell Mol. Life Sci. 73, 1969–1987 (2016).
Chen, J. et al. Celecoxib attenuates liver steatosis and inflammation in non-alcoholic steatohepatitis induced by high-fat diet in rats. Mol. Med. Rep. 4, 811–816 (2011).
Smith, W. L., DeWitt, D. L. & Garavito, R. M. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 69, 145–182 (2000).
Botting, R. M. Mechanism of action of acetaminophen: is there a cyclooxygenase 3? Clin. Infect. Dis. 31, 202–210 (2000).
Motino, O. et al. Cyclooxygenase-2 expression in hepatocytes attenuates non-alcoholic steatohepatitis and liver fibrosis in mice. Biochim. Biophys. Acta 1862, 1710–1723 (2016).
Cheng, Q. et al. Cyclooxygenase-2 promotes hepatocellular apoptosis by interacting with TNF-alpha and IL-6 in the pathogenesis of nonalcoholic steatohepatitis in rats. Dig. Dis. Sci. 58, 2895–2902 (2013).
Chen, X. et al. Comparative Proteomic Study of Fatty Acid-treated Myoblasts Reveals Role of Cox-2 in Palmitate-induced Insulin Resistance. Sci. Rep. 6, 21454 (2016).
Yu, J. et al. COX-2 induction in mice with experimental nutritional steatohepatitis: Role as pro-inflammatory mediator. Hepatology. 43, 826–836 (2006).
Wu, J. et al. Suppressing cyclooxygenase-2 prevents nonalcoholic and inhibits apoptosis of hepatocytes that are involved in the Akt/p53 signal pathway. Biochem. Biophys. Res. Commun. 469, 1034–1040 (2016).
Tsujimoto, S. et al. Nimesulide, a cyclooxygenase-2 selective inhibitor, suppresses obesity-related non-alcoholic fatty liver disease and hepatic insulin resistance through the regulation of peroxisome proliferator-activated receptor gamma. Int. J. Mol. Med. 38, 721–728 (2016).
Tian, F., Zhang, Y. J., Li, Y. & Xie, Y. Celecoxib ameliorates non-alcoholic steatohepatitis in type 2 diabetic rats via suppression of the non-canonical Wnt signaling pathway expression. PLoS One. 9, e83819 (2014).
Lavallard, V. J. & Gual, P. Autophagy and non-alcoholic fatty liver disease. Biomed. Res. Int. 2014, 120179 (2014).
Amir, M. & Czaja, M. J. Autophagy in nonalcoholic steatohepatitis. Expert Rev. Gastroenterol. Hepatol. 5, 159–166 (2011).
Mao, Y., Yu, F., Wang, J., Guo, C. & Fan, X. Autophagy: a new target for nonalcoholic fatty liver disease therapy. Hepat. Med. 8, 27–37 (2016).
Martinez-Lopez, N. & Singh, R. Autophagy and Lipid Droplets in the Liver. Annu. Rev. Nutr. 35, 215–237 (2015).
Liu, C., Liao, J. Z. & Li, P. Y. Traditional Chinese herbal extracts inducing autophagy as a novel approach in therapy of nonalcoholic fatty liver disease. World J. Gastroenterol. 23, 1964–1973 (2017).
Deng, X. et al. Regulation of SREBP-2 intracellular trafficking improves impaired autophagic flux and alleviates endoplasmic reticulum stress in NAFLD. Biochim. Biophys. Acta. 1862, 337–350 (2017).
Gonzalez-Rodriguez, A. et al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death. Dis. 5, e1179 (2014).
Chen, R. et al. Protective role of autophagy in methionine-choline deficient diet-induced advanced nonalcoholic steatohepatitis in mice. Eur. J. Pharmacol. 770, 126–133 (2016).
Day, C. P. & James, O. F. Steatohepatitis: a tale of two “hits”? Gastroenterology. 114, 842–845 (1998).
Flores-Toro, J. A., Go, K. L., Leeuwenburgh, C. & Kim, J. S. Autophagy in the liver: cell’s cannibalism and beyond. Arch. Pharm. Res. 39, 1050–1061 (2016).
Liu, L., Liao, J. Z., He, X. X. & Li, P. Y. The role of autophagy in hepatocellular carcinoma: friend or foe. Oncotarget. 8, 57707–57722 (2017).
Ueno, T. & Komatsu, M. Autophagy in the liver: functions in health and disease. Nat. Rev. Gastroenterol. Hepatol. 14, 170–184 (2017).
Gracia-Sancho, J., Guixé-Muntet, S., Hide, D. & Bosch, J. Modulation of autophagy for the treatment of liver diseases. Expert Opin. Investig. Drugs. 23, 965–977 (2014).
Singh, R. et al. Autophagy regulates lipid metabolism. Nature. 458, 1131–1135 (2009).
Rambold, A. S., Cohen, S. & Lippincott-Schwartz, J. Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev. Cell. 32, 678–692 (2015).
Kaushik, S. & Cuervo, A. M. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell. Biol. 17, 759–770 (2015).
Madrigal-Matute, J. & Cuervo, A. M. Regulation of Liver Metabolism by Autophagy. Gastroenterology. 150, 328–339 (2016).
Chung, M. Y. et al. Green Tea Lowers Hepatic COX-2 and Prostaglandin E2 in Rats with Dietary Fat-Induced Nonalcoholic Steatohepatitis. J. Med. Food. 8, 648–655 (2015).
Zhu, X. et al. Autophagy activated by the c-Jun N-terminal kinase-mediated pathway protects human prostate cancer PC3 cells from celecoxib-induced apoptosis. Exp. Ther. Med. 13, 2348–2354 (2017).
Luo, B. et al. Endoplasmic reticulum stress eIF2alpha-ATF4 pathway-mediated cyclooxygenase-2 induction regulates cadmium-induced autophagy in kidney. Cell Death. Dis. 7, e2251 (2016).
Gradilone, A. et al. Celecoxib induces MRP-4 in lung cancer cells: therapeutic implications. J. Clin. Oncol. 25, 4318–4320 (2007).
Acknowledgements
This work was supported by National Natural Science Foundation of China, No. 81372663 and No. 81672392; Natural Science Foundation of Hubei Province, No. 2017CFB457; State Key Laboratory of Cancer Biology (Fourth Military Medical University), No. CBSKL201720.
Author information
Authors and Affiliations
Contributions
C.L., L.L. and H.-D.Z. conducted the experiments and wrote the manuscript. J.-Q.S., X.-L.W., and X.-X.H. analyzed the data. D.-A.T., J.-Z.L. and P.-Y.L. designed the study and revised the manuscript. All authors discussed and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, C., Liu, L., Zhu, HD. et al. Celecoxib alleviates nonalcoholic fatty liver disease by restoring autophagic flux. Sci Rep 8, 4108 (2018). https://doi.org/10.1038/s41598-018-22339-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-22339-0
This article is cited by
-
Toxicity and Hepatoprotective Effects of ZnO Nanoparticles on Normal and High-Fat Diet-Fed Rat Livers: Mechanism of Action
Biological Trace Element Research (2024)
-
Celecoxib activates autophagy by inhibiting the mTOR signaling pathway and prevents apoptosis in nucleus pulposus cells
BMC Pharmacology and Toxicology (2022)
-
Rapamycin Enhanced Sensitivity of HT-29 Cells to 5-Fluororacil by Promoting Autophagy
Bulletin of Experimental Biology and Medicine (2022)
-
Endoplasmic Reticulum Stress and Autophagy in the Pathogenesis of Non-alcoholic Fatty Liver Disease (NAFLD): Current Evidence and Perspectives
Current Obesity Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.