Abstract
As an image-based single-cell transcriptomics approach, multiplexed error-robust fluorescence in situ hybridization (MERFISH) allows hundreds to thousands of RNA species to be identified, counted and localized in individual cells while preserving the native spatial context of RNAs. In MERFISH, RNAs are identified via a combinatorial labeling approach that encodes RNA species with error-robust barcodes followed by sequential rounds of single-molecule FISH (smFISH) to read out these barcodes. The accuracy of RNA identification relies on spatially separated signals from individual RNA molecules, which limits the density of RNAs that can be measured and makes the multiplexed imaging of a large number of high-abundance RNAs challenging. Here we report an approach that combines MERFISH and expansion microscopy to substantially increase the total density of RNAs that can be measured. Using this approach, we demonstrate accurate identification and counting of RNAs, with a near 100% detection efficiency, in a ~130-RNA library composed of many high-abundance RNAs, the total density of which is more than 10 fold higher than previously reported. In parallel, we demonstrate the combination of MERFISH with immunofluorescence in expanded samples. These advances increase the versatility of MERFISH and will facilitate its application to a wide range of biological problems.
Similar content being viewed by others
Introduction
In situ imaging-based approaches to single-cell transcriptomics allow not only the expression profile of individual cells to be determined but also the spatial positions of individual RNA molecules to be localized. These approaches provide a powerful means to map the spatial organizations of RNAs inside cells and the transcriptionally distinct cells in tissues1. Multiplexed fluorescence in situ hybridization (FISH)2,3,4,5,6,7 and in situ sequencing8,9 have been used to profile the expressions of a large number of (ranging from ~10 to substantially more) RNA species in single cells. In particular, MERFISH, a massively multiplexed form of smFISH, enables RNA imaging at the transcriptomic scale with high accuracy and detection efficiency7. By imaging single RNA molecules, smFISH provides the precise copy number and spatial distribution of individual RNA species in single cells10,11. MERFISH multiplexes smFISH measurements by labeling RNAs combinatorically with oligonucleotide probes which contain error-robust barcodes and then measuring these barcodes through sequential rounds of smFISH imaging. Using this approach, we demonstrated simultaneous imaging of hundreds to a thousand of RNA species in individual cells using barcoding schemes capable of detecting and/or correcting errors7. Recently, we have increased the measurement throughput of MERFISH to tens of thousands of cells per single-day-long measurement12. In addition, we developed a matrix-imprinting-based sample clearing approach that substantially reduces the fluorescence background and increases the signal-to-background ratio by anchoring RNA molecules to a polymer matrix and removing other cellular components that give rise to fluorescence background13. This clearing approach enabled high-quality MERFISH measurement of tissue samples13.
In order to accurately identify RNA molecules, MERFISH, as well as other multiplexed smFISH-based RNA profiling methods, requires non-overlapping signals from individual RNAs. However, when two molecules are sufficiently close to each other, the signal from one molecule will overlap with that from the other molecule, diminishing our ability to identify these RNAs and, thus, limiting the density of RNAs that can be profiled. Indeed, in MERFISH experiments, we often find this density limit a major limiting factor in our choice of genes to profile, both in terms of the total number of genes and their RNA expression levels. This problem could potentially be mitigated by super-resolution optical imaging14,15, by analysis methods to address partially overlapping fluorophores16,17,18,19, or by sample expansion20,21. In particular, since neighboring RNA molecules may physically overlap in space, expansion microscopy (ExM), which uses sample expansion to effectively increase the distances between neighboring molecules20, may provide an especially effective means to increase the RNA density limit accessible by MERFISH. In ExM, the desired signal is conjugated to an expandable polyelectrolyte gel, and then the gel is physically expanded by changing the ionic strength of the buffer20. ExM has recently been combined with smFISH to help better resolve highly expressed RNAs, with either single-round or multiple rounds of smFISH to measure one or several genes21,22. In addition, RNAs have been anchored to a polyacrylamide matrix to facilitate sample clearing and background removal in RNA FISH13,23,24 and improve the signal-to-background ratio in MERFISH measurements13. Thus, we reason that ExM should also be compatible with MERFISH and may help substantially increase the RNA density measurable by MERFISH.
In this paper, we report an approach that combines MERFISH and ExM to greatly increase the molecular density of RNA libraries accessible to MERFISH. We anchor mRNAs to an expandable polyelectrolyte gel via acrydite-modified poly(dT) locked nucleic acid (LNA) probe hybridized to the poly(A) tail of mRNAs. We demonstrate the efficacy of this approach by imaging a high-abundance RNA library, which contains ~130 RNA species with a total RNA abundance that is 14-fold higher than what has been previously demonstrated with MERFISH imaging12,13, in cultured human osteosarcoma (U-2 OS) cells. Unlike our previous MERFISH measurements of lower-density RNA libraries, in which we demonstrated 80–90% detection efficiency, the RNAs in this high-density library are not well resolved and hence are detected with a low detection efficiency of ~20% without gel expansion. In contrast, individual RNA molecules become well resolved in expanded samples, leading to a substantial increase in their detection efficiency. Comparison with smFISH and bulk sequencing results demonstrate that these RNAs in the expanded sample are detected with high accuracy and near 100% detection efficiency. In addition, we also demonstrate the ability to perform simultaneous MERFISH RNA imaging and immunofluorescence imaging of proteins in these expanded samples. This combination can be used to provide cellular context for the RNA expression analysis, and can potentially enable simultaneous profiling of transcriptomic and proteomic expression.
Results
Effect of RNA density on the detection efficiency of MERFISH measurements
To illustrate the effect of RNA density on multiplexed smFISH measurements, we measured a high-abundance, ~130-RNA library using our previously published 16-bit modified Hamming distance 4 (MHD4) binary code, which allows error detection and correction7,12,13. This code includes 140 unique barcodes and we used 129 of them to encode RNAs and kept 11 of them as blank controls that did not correspond to any RNA. Among the 129 targeted RNAs, 106 RNAs were in the abundance range of 40–250 copies per cell to increase the total abundance of the library, and the remaining 23 spanned an abundance range of 1–1000 copies per cell to quantify performance across different abundances. The total abundance of RNAs in this library was 14-fold higher than the 130-RNA libraries that we have previously measured using the MHD4 code with ~80–90% detection efficiency7,12,13.
As in our previous MERFISH measurements7,12,13, we labeled the RNAs with two sets of oligonucleotide probes. In the first step, each cellular RNA was hybridized with a complex library of oligonucleotide probes termed encoding probes, each encoding probe containing a targeting sequence that binds the cellular RNA and multiple readout sequences; the collection of readout sequences associated with the cellular RNA determine the barcode of this RNA. In the second step, the readout sequences, and hence the barcodes, were detected through a series of smFISH measurements, each round with one or more readout probes complementary to one or more readout sequences. We carried out MERFISH measurements in U-2 OS cells using the matrix-imprinting-based clearing method to reduce the fluorescence background as previously published13. Briefly, the cells were fixed, permeabilized, labeled simultaneously with encoding probes for the 129 RNA species as well as acrydite-modified poly(dT) LNA probes that target polyadenylated RNAs. We then embedded the cells in a non-expandable, polyacrylamide gel and the poly(A)-containing mRNAs were anchored to the gel through the poly(dT) probes. Next, we removed cellular proteins and lipids by Proteinase K digestion and detergent extraction to remove fluorescence background and clear the samples. After this clearing, eight rounds of two-color smFISH measurements were performed to read out the 16-bit barcode for each RNA. Because the heights of the cells were greater than the depth of a single optical section, we imaged the sample with multiple z-sections to ensure that >90% of RNA molecules within the cells were detected.
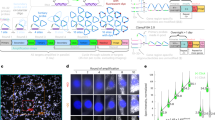
Because of the high molecular density associated with this RNA library, a substantial fraction of RNA molecules generated spatially overlapping smFISH signals in each round of imaging (Fig. 1a,b). As a result, only a small fraction of the RNA molecules was decodable (Fig. 1c). This observation was in contrast to our previous MERFISH measurements on lower-abundance RNA libraries, where the vast majority of RNAs were spatially separated and decodable7,12,13. The average copy number per cell detected for the RNAs in this high-abundance library by MERFISH correlated with the RNA abundance measured by bulk RNA sequencing with a Pearson correlation coefficient (r) of 0.6 (Fig. 1d), which is lower than the ~0.8 correlation coefficient value that we observed previously with lower-abundance libraries in this and other cell lines7,12,13. To determine the detection efficiency, we measured the RNA abundance of 12 genes in this library individually with smFISH in matrix-imprinted-and-cleared samples. Comparison of the RNA copy numbers determined for these genes by MERFISH with those determined by smFISH showed that MERFISH only detected 21% ± 4% (average ± s.e.m., N = 129 RNA species) or 16% (median) of the RNA copies determined by smFISH (Fig. 1e), which contrasts with the ~80–90% detection efficiency that we obtained previously for lower-abundance RNA libraries by the same MERFISH-smFISH comparison7,12,13. Thus, as expected, we find that the ability of MERFISH to identify and quantify RNAs drops when the abundance of the measured RNAs is increased to a level that leads to substantial overlap in the signals from neighboring RNAs.
MERFISH measurements of a high-abundance RNA library in unexpanded U-2 OS samples. (a) Image of gel-embedded and cleared U-2 OS sample stained with encoding probes for 129 RNA species and a Cy5-labeled readout probe that detects one of the bits of the RNA barcodes at one focal plane of the z-scan. The yellow solid lines mark the segmentation boundaries separating neighboring cells, which do not necessarily represent the physical edges of cells. The yellow dashed lines mark the DAPI-stained cell nuclei. (b) Fluorescence images of all 8 rounds of two-color readout imaging for the orange boxed region in (a). Images were deconvolved and Gaussian filtered. Magenta and green represent the Cy5 and Alexa 750 channels, respectively. (c) The localizations of all decoded RNAs in (a) colored according to their measured binary barcodes. Decoded RNAs across all z-sections are displayed. The black solid lines mark the segmentation boundaries separating neighboring cells and the black dashed lines marks the DAPI-stained cell nuclei. (d) The average RNA copy numbers per cell for the 129 RNA species determined by MERFISH vs. the abundances as determined by RNA-seq. The Pearson correlation coefficient (r) between the log10 values of MERFISH-determined copy number per cell and RNA-seq determined FPKM value is 0.6. ~1,200 cells were measured in MERFISH experiments. (e) The average RNA copy numbers per cell determined by MERFISH (~1,200 cells) vs. those by smFISH (~1,000 cells per gene) for 12 of the 129 RNA species. The Pearson correlation coefficient (r) between the log10 values of MERFISH-determined and smFISH-determined copy numbers is 0.75. The average ratio of the copy number values determined by MERFISH to that determined by smFISH is 0.21 ± 0.04 (s.e.m., N = 12 RNA species) and the median ratio is 0.16. The scale bar in (a) represents 10 µm; the scale bars in (b) represent 1 µm.
High-RNA-density MERFISH measurements with expansion microscopy
We reasoned that the reduced MERFISH detection efficiency due to overlapping single-molecule signals could be alleviated by sample expansion, which will increase the distance between molecules and reduce overlap. To test this idea, we fixed and permeabilized the cells, and labeled them with the encoding probes and poly(dT) anchoring probes as described above. Then we embedded the cells in an expandable polyelectrolyte gel using a protocol modified from the published ExM methods20,21. Afterwards, cells were digested by Proteinase K and treated with detergent to remove proteins and lipids, clearing the sample and facilitating gel expansion. The gel was then expanded in a low salt buffer and finally embedded again in a non-expandable polyacrylamide gel to stabilize it in the expanded state (Fig. 2a)20,21. There are several notable differences between our protocol and the previous ExM protocol used for smFISH21. First, in the previous protocol, RNAs were randomly modified with a chemical cross-linker, LabelX, that allowed them to be covalently linked to the expandable gel. To ensure that the majority of RNAs contain at least one anchor, Poisson statistics will dictate that a substantial portion of RNAs will be labeled with more than one anchor. Thus, it is possible that RNAs are connected to the gel at multiple points and stretched during expansion. Here, we anchored each RNA to the gel at a single location—the poly(A) tail. This anchoring geometry should allow the separation between molecules during expansion without concurrent stretching of the molecules, and could potentially improve separation of the nearby RNA molecules. Second, we found that MERFISH encoding probes did not reliably penetrate the gel after expansion and stabilization, perhaps due to the increased length of these probes compared to those used for conventional smFISH. Thus, we stained the samples with encoding probes before embedding in the gel. By contrast, we found that the short MERFISH readout probes penetrated this gel without a problem, allowing the sequential rounds of smFISH to be performed in expanded samples. Third, to stabilize the already bound encoding probes on RNAs, we utilized a low salt buffer (0.5× saline-sodium citrate (SSC)), instead of water, for the expansion of the gel. Our measurement of the gel volume showed that dialysis in 0.5× SSC produced 2.3-fold expansion in each dimension and 12-fold expansion in volume, which was smaller than that reported previously for expansion in water20,21. We note that as long as the expansion is sufficient to separate neighboring RNA molecules, a lower expansion factor has the advantage of allowing faster imaging.
MERFISH measurements of a high-abundance RNA library in expanded U-2 OS cells. (a) Schematic of the gel embedding and expansion for MERFISH imaging. (b) Image of an expanded U-2 OS sample stained with encoding probes for 129 RNA species and a Cy5-labeled readout probe that detects one of the bits of the RNA barcodes at one focal plane of the z-scan. (c) Fluorescence images of all 8 rounds of two-color readout imaging for the orange boxed region in (b). (d) The localizations of all decoded RNAs in (b) colored according to their measured binary barcodes. Decoded RNAs across all z-sections are displayed. (e) The average RNA copy numbers per cell for the 129 RNA species determined by MERFISH (~750 cells) vs. the abundances determined by RNA-seq. The Pearson correlation coefficient (r) for the log10 values of RNA abundances is 0.83. (f) The average RNA copy numbers per cell for the 129 RNA species detected in one expanded sample (~750 cells) vs. a replicate sample (~250 cells). The Pearson correlation coefficient (r) is 0.94. (g) The average RNA copy numbers per cell for the 129 RNA species determined by expansion MERFISH (~1,000 cells) vs. those by MERFISH in unexpanded samples (~1,200 cells). The Pearson correlation coefficient (r) is 0.88. The copy numbers per cell detected in expanded samples were 7.5 ± 0.3 fold (average ± s.e.m., N = 129 RNA species) or 6.6 fold (median) of those detected in unexpanded samples. (h) The average RNA copy numbers per cell determined by MERFISH in expanded samples (~1,000 cells) vs. those by smFISH measurements in unexpanded samples (~1,000 cells per gene) for 12 of the 129 RNA species. The Pearson correlation coefficient (r) is 0.96. The average ratio between MERFISH and smFISH results is 1.05 ± 0.09 (average ± s.e.m., N = 12 RNA species) and the median ratio is 1.11. The scale bars in (b) and (c) represent 10 µm and 1 µm, respectively, with the grey scale bars representing the scales in the expanded sample and the orange scale bars representing the scales calculated back to sizes before expansion.
Notably, in the expanded samples, individual RNA molecules became substantially better resolved (Fig. 2b,c) and many more molecules were successfully decoded (Fig. 2d). Compared to the results from unexpanded samples, the average copy number per cell detected for these RNAs by MERFISH correlated better with the RNA abundance measured by RNA-seq with an increased Pearson correlation coefficient of 0.83 (Fig. 2e). The copy number per cell results are highly reproducible between replicates of MERFISH experiments (Fig. 2f).
To quantify the improvement in detection efficiency with expansion, we compared the average MERFISH counts for individual RNA species per cell between expanded and unexpanded samples. We found that the copy numbers per cell for the 129 RNA species detected in expanded samples correlated with those in the unexpanded samples with a high Pearson correlation coefficient of 0.88 (Fig. 2g). However, the copy numbers per cell detected in expanded samples were 7.5 ± 0.3 fold (average ± s.e.m., N = 129 RNA species) or 6.6 fold (median) higher than those detected in unexpanded samples for these 129 RNA species, suggesting that the detection efficiency for the expanded sample is substantially higher compared to the unexpanded sample for this high-abundance library. Indeed, comparison with smFISH measurements showed that the copy numbers per cell determined by MERFISH in the expanded samples was 105% ± 9% (average ± s.e.m., N = 129 RNA species) or 111% (median) of those determined by smFISH (Fig. 2h), indicating that the detection efficiency after expansion is close to 100%. Compared to the 16% median value described earlier for the unexpanded samples, the 111% median value obtained here for the expanded samples suggests a 6.9-fold increase in the detection efficiency, which is consistent with the 6.6-fold increase in the median copy numbers detected in expanded samples as compared to unexpanded samples. We do not make such comparison for the average values because, for broad distributions, the arithmetic mean values of ratios do not propagate mathematically.
Our ability to quantify the copy numbers of RNAs in a high-density library also allowed us to characterize the cell-to-cell variability in the expression levels of these RNAs. We determined the variance in RNA copy number and the Fano factor, defined as the ratio of the variance to the mean RNA copy number, for the 129 genes in our library. Both the variance and the Fano factor increased with the RNA expression level (Supplementary Fig. 1), consistent with previously findings25,26,27.
Because MERFISH is an image-based RNA profiling method, our MERFISH images naturally provides the information on the physical sizes of individual cells in addition to the RNA copy number information. By determining the volumes of individual cells, we found a strong positive correlation between the total RNA copy number of the 129 genes measured in each cell and the cell volume (Supplementary Fig. 2). The observed linear correlation suggests that the concentration of RNA molecules remains largely constant from cell to cell despite variation in the cell volume. This observation is consistent with a previous study which examined 26 genes28.
Incorporating immunofluorescence imaging into MERFISH experiments of expanded samples
Cells are comprised of different structures and compartments, and immunofluorescence is a powerful technique to visualize specific subcellular structures and compartments. The ability to combine immunofluorescence imaging with MERFISH could provide important cellular context of the RNA molecules imaged. We thus tested whether combination of immunofluorescence and MERFISH imaging is possible in the expanded samples. It has been previously demonstrated that ExM can be used to image immunostained samples, using oligo-conjugated antibodies and complementary probes with a methacryloyl group that can be crosslinked to the gel20 or via direct crosslinking of protein targets or antibodies to the gel29,30,31. When the oligo-labeling approach is utilized, the positions of the protein targets are detected by hybridization of fluorophore-labeled complementary oligos, which we reason can be incorporated into our MERFISH readout measurements.
To demonstrate this ability, we performed immunostaining of the protein targets with primary antibody and oligo-labeled secondary antibody after hybridization of the cells with the MERFISH encoding probes and poly(dT) anchor probes, and then embedded the labeled sample in an expandable gel. We added acrydite modification to the oligo on the antibodies so that it can be incorporated into the polymer gel during the embedding step. After digestion, gel expansion and second embedding in a non-expandable gel as described in the previous section, we performed the MERFISH readout procedure to first detect the readout sequences in the RNA encoding probes and then with an additional round of FISH detection to read out the oligo sequence representing the protein target.
For demonstration purposes, we immunostained cadherin in cultured U-2 OS cells, which were also stained for the same high-abundance RNA library described in the earlier sections. We observed both specific staining of cadherin on the cell periphery (Fig. 3a) as well as clearly resolved smFISH spots during each readout round in the same cells (Fig. 3b,c). The combination with immunofluorescence did not affect MERFISH imaging quality. We observed the same high correlation of MERFISH results and the RNA-seq results with a Pearson correlation coefficient of 0.83 (Fig. 3d). The average copy numbers per RNA species per cell detected in immunostained samples correlated strongly with those in detected samples not subjected to immunostaining, with a Pearson correlation coefficient of 0.95 (Fig. 3e). On average, the ratio of copy numbers of individual RNA species per cell between immunostained and non-immunostained samples was 0.99 ± 0.03 (average ± s.e.m., N = 129 RNA species), indicating negligible impairment in the performance of MERFISH when combined with immunofluorescence. The staining of cadherin in expanded and MERFISH labeled samples also appeared similar to cadherin staining in control U-2 OS samples immunostained directly after fixation without any gel-embedding, expansion or MERFISH RNA labeling (Fig. 3f). Quantitatively, we compared the cadherin intensity per unit length on the cell periphery (normalized to the actual length before expansion) in expanded MERFISH samples versus the control samples not subjected to any MERFISH RNA labeling and gel embedding, and obtained quantitatively similar results (Fig. 3g).
Combining immunofluorescence and MERFISH imaging in expanded samples. (a) Image of an expanded U-2 OS sample stained with MERFISH encoding probes for 129 RNA species, cadherin primary antibodies, oligo-conjugated secondary antibodies and visualized with a Cy5-conjugated complementary probe that detects the secondary antibodies. (b) Image of the same expanded sample visualized with a Cy5-labeled readout probe that detects one of the bits of the RNA barcodes at one focal plane for the white boxed region in (a). (c) Fluorescence images of all 8 rounds of two-color readout imaging for the orange boxed region in (b). (d) The average RNA copy numbers per cell for the 129 RNA species determined by MERFISH (~1,100 cells) vs. the abundances as determined by RNA-seq. The Pearson correlation coefficient (r) based on the log10 values of RNA abundances is 0.83. (e) The average RNA copy numbers per cell for the 129 RNA species determined by MERFISH in immunostained samples (~1,100 cells) vs. those in samples that are not immunostained (~1,000 cells). The Pearson correlation coefficient (r) is 0.95. The average ratio between the RNA copy numbers determined in the two experiments is 0.99 ± 0.03 (average ± s.e.m., N = 129 RNA species) and the median ratio is 0.94. (f) Image of unembedded U-2 OS cells stained with the same cadherin primary antibodies and oligo-conjugated secondary antibodies as well as a Cy5-conjugated complementary probe without any MERFISH RNA labeling and gel embedding. (g) Box plot of cadherin intensity per unit length of cell periphery (normalized to the actual length before expansion) in expanded MERFISH samples and in control immunostained samples that have not been subject to any MERFISH RNA labeling or gel embedding. The red lines indicate the median, the boxes mark the 25th to 75th percentiles, the whiskers extend to the most extreme data points not including outliers, and the outliers are plotted using the ‘+’ symbol. The P value is derived from a two-side Kolmogorov-Smirnov test (N = 20 areas each). The scale bars in (a-c) are as defined in Fig. 2. The white scale bar in (f) represents 10 µm.
Discussion
In this paper, we have demonstrated an approach to combine MERFISH and ExM to measure high-abundance RNA libraries. We showed that when the total RNA density is high, the overlap between signals from nearby RNA molecules substantially reduced the MERFISH detection efficiency and that sample expansion can overcome this overlapping problem and greatly increase the detection efficiency. Specifically, for an RNA library with total RNA abundance that is 14-fold higher than our previously measured libraries, the MERFISH detection efficiency dropped to <20% in unexpanded samples, and expansion recovered the near 100% detection efficiency of MERFISH.
Each mammalian cell can express thousands of different RNA species. We have previously demonstrated MERFISH imaging of up to 1,000 RNA species in single cells7. To increase the number of RNA species that can be simultaneously imaged in single cells while maintaining a similar number of imaging rounds, the density of RNAs imaged per round will increase and will become an important limiting factor in the number of RNAs that can be imaged. Likewise, our ability to profile RNAs with high expression levels will also be limited by this factor. We anticipate that the expansion MERFISH approach reported here should facilitate a substantial increase in the number of RNA species and the total RNA abundance level that can be measured in single cells. In this work, we measured 129 RNA species, the total abundance of which is ~13,000 molecules per cell, with ~100% detection efficiency in expanded U2-OS cell with a 12-fold volume expansion factor. It is possible that a substantial increase in the total RNA copy number will lead to a reduction in detection efficiency even in expanded samples. To assess this limitation, we simulated MERFISH images of RNA molecules at higher total abundance based on our experimental measured RNA spot size and pixel size. The simulation results suggest that an RNA density of ~3–4 molecules per µm2 will lead to significant signal overlap between neighboring molecules that results in a 50% reduction in MERFISH detection efficiency. This molecular density, with an imaging depth of ~1 µm per optical section, corresponds to ~70,000–100,000 molecules per cell after expansion based on the measured U2-OS cell cytoplasm volume. For a measurement of a randomly chosen 1,000 RNA species, there is only a ~2–5% probability that their total copy number will reach this density range. We thus expect our current approach to combine MERFISH and ExM to be adequate for many applications of MERFISH. However, a typical mammalian cell expresses ~50,000 to 300,000 of RNA molecules in total when all genes are considered32 and, in addition, certain subcellular regions can have higher RNA density than others. Hence, we anticipate that further improvements are still needed for some applications that involve a very large number of genes and/or very high-abundance RNAs. This could potentially be achieved through decreasing the ionic strength of the expansion buffer or using multiple rounds of expansion33 to further increase the volume expansion factor or combining expansion-MERFISH with other super-resolution imaging methods14,15.
Here, we linked RNAs with a poly(A) tail to the gel matrix using an acrydite-labeled LNA poly(dT) anchoring probes. For the RNAs measured in this work, the poly(A) tail lengths (between 30–100 nt)34,35 are expected to be sufficiently long to ensure high capture efficiency36, and we did not observe a significant effect of the poly(A) tail length on the RNA detection efficiency. However, for RNAs with too short a poly(A) tail or without a poly(A) tail, alternative anchoring strategies will need to be used. Potential alternative strategies include using acrydite-modified oligos with complementary sequences to the RNAs of interest to link these RNAs to the gel matrix, or directly linking the RNAs to the gel through cross-linking molecules such as LabelX21. Additionally, one can also explore protocols to expand the gel without strong proteinase digestion29,30,31, in which case RNAs can be linked to the gel through proteins by chemicals such as EDC24.
In addition to enabling the measurement of high-density RNA libraries, we also demonstrated the ability to combine MERFISH with immunofluorescence in expanded samples. Such combination can substantially expand the scope of MERFISH applications. For example, immunofluorescence allows us to measure cellular structures and compartments, such as endoplasmic reticulum, mitochondria, and microtubules, among others, which can provide important information on the cellular context for the measured RNAs. In addition, although individual cells in the system studied in this work can be clearly segmented just based on the total RNA image itself, it is possible that the total RNA signal alone is not adequate for cell segmentation in some other systems. In such cases, immunofluorescence imaging of cell surface markers could be helpful. Moreover, because the expression levels of mRNA and proteins are not always correlated in a simple manner, but could be complicated by post-transcriptional regulation, a combined RNA and protein expression profiling of single cells can provide useful insights that may not be deciphered from mRNA profiling alone. In conventional immunofluorescence with fluorophore-conjugated antibodies, the number of spectrally-distinct fluorophores limits the number of protein targets that can be studied simultaneously. The use of oligo-conjugated antibodies potentially allows visualization of many proteins in sequential rounds of hybridization using just one or a few spectrally distinct fluorophores37,38,39,40. We thus envision such combination of MERFISH and immunofluorescence imaging may allow a combined transcriptomic and proteomic measurements simultaneously in single cells.
Methods
Design of the encoding probes
The MERFISH encoding probes were designed using the same 16-bit Hamming-weight-4 Hamming-distance-4 code with 140 possible barcodes as previously published7,12. In this encoding scheme, all barcodes used are separated by a Hamming distance of at least 4, and hence at least four bits must be read incorrectly to change one valid barcode to another. A constant Hamming weight (i.e. the number of “1” bits in each barcodes) of 4 is used to avoid potential measurement bias due to differential rates of “1” to “0” and “0” to “1” errors7,12. The encoding probe set that we used contained 92 encoding probes per RNA. Each encoding probe was comprised of a 30-nt target region designed using a pipeline published previously12, flanked by two 20-nt readout sequences randomly selected out of the four ones assigned to each RNA, one 20-nt priming region at the 5′ end and another 20-nt priming region using the reverse complement of the T7 promoter at the 3′ end. Additional adenosine nucleotide spacers were added between readout sequences and target regions to prevent target regions from combining with Gs from adjacent sequences to form G quadruplets. The priming region at the 5′ end had a thymine at the end, which was put at the junction of the priming regions at the 5′ end and the encoding region. This was designed to incorporate a uracil on the forward primer used in the reverse transcription step of probe construction, which can be cleaved by Uracil-Specific Excision Reagent (USER) Enzyme41. The cleavable primer design together with using the reverse complement of the T7 promoter as the second primer allowed us to remove these priming regions from the final probes, producing encoding probes with a length of 72 nt compared to 112 nt using previous approaches7,12,13. The reduction in probe length may facilitate penetration of probes and reduce non-specific binding. To include some abundant but relatively short RNAs in our library, instead of designing probes targeting distinct regions of RNAs, we allowed probes to share up to 20 nt with another probe. 92 target regions per RNA were selected randomly out of all potential target regions of an RNA. By allowing up to a 20-nt overlap between neighboring encoding probes, this design allowed RNAs as short as 1,200 nt to be targeted by 92 encoding probes with a 30-nt targeting sequence. Because a given cellular RNA is typically bound by less than one third of the 92 encoding probes (determined by comparing the fluorescence signals from individual cellular RNA molecules with the fluorescence signals from single dye molecules), we reason that the encoding probes with overlapping targeting regions would not substantially interfere with each other but would partially compensate for reduced binding due to local inaccessible regions on the target RNA (e.g. secondary structure) or loss of probes during synthesis. The same 20-nt, three-letter readout sequences were used as previously published12.
Construction of the encoding probes
The encoding probe set was amplified from complex oligonucleotide pools. Briefly, we amplified the oligopools (CustomArray) via limited-cycle PCR to make in vitro transcription templates, converted these templates into RNA via in vitro transcription (New England Biolabs), and converted the RNA back to DNA via reverse transcription (Maxima RT H-, Thermo Fisher Scientific) as previously published12. The probes were then digested by USER Enzyme41 (New England Biolabs) at a dilution of 1:30 (vol/vol) incubated at 37 °C for 24 h to cleave off the priming region at the site of a uracil between a priming region at the 5′ end and the target region. After that, DNA was purified via alkaline hydrolysis to remove RNA and column purification (Zymo Research). The final probes were resuspended in RNAase-free water and stored at −20 °C.
Construction of the readout probes
The three-letter readout sequences were designed as published previously12,42. Readout probes conjugated to the desired dye via a disulfide linkage were synthesized and purified by Bio-synthesis, Inc., resuspended immediately in Tris-EDTA (TE) buffer, pH 8 (Thermo Fisher) to a concentration of 100 μM and stored at −20 °C. To reduce the number of freeze-thaw cycles, 1-μM aliquots were made in TE buffer and stored at −20 °C.
Oligo conjugation to secondary antibodies
Oligonucleotides containing the complementary sequence of the desired readout probe were conjugated to secondary antibodies via a combination of NHS-ester and copper-free click chemistries similar to a published method43. First, secondary antibodies were labeled with a copper-free click crosslinking agent using NHS-ester chemistry. Specifically, azide preservative was removed from the unconjugated Donkey Anti-Rabbit secondary antibodies (Thermo Fisher Scientific) using a spin-column based dialysis membrane (Amicon, 100 kDa molecular weight cut off) according to the manufacturer’s instructions. DBCO-PEG5-NHS Ester (Kerafast) was diluted to a concentration of 10 μM in anhydrous dimethyl sulfoxide (DMSO) (Thermo Fisher Scientific). 2 μL of the solution was then combined with 100 μL of 2 mg/mL of the antibody in 1× phosphate-buffered saline (PBS). This reaction was incubated at room temperature for 1 hour and then terminated via a second round of purification using the Amicon columns as described above. The average number of DBCO crosslinkers per antibody was determined via the relative absorption of the sample at 280 nm (antibody) and 309 nm (DBCO). On average the procedure described above produced ~7 DBCO per antibody.
Oligonucleotide probes containing the desired sequence as well as a 5′-acrydite, to allow cross-linking to the polymer gel, and a 3′-azide, to allow cross-linking to the DBCO-labeled antibodies, were ordered from IDT and suspended to 100 μM in 1× PBS. 20 μL of the appropriate oligonucleotide was then added to 100 μL of the DBCO-labeled antibodies at a final concentration of ~2 mg/mL. This reaction was incubated at 4 C for at least 12 hours. Labeled antibodies were not further purified as residual oligonucleotides, not conjugated to antibodies, were readily washed away from samples.
Cell culture and fixation
U-2 OS cells (ATCC) were cultured with Eagle’s Minimum Essential Medium (ATCC) containing 10% (vol/vol) fetal bovine serum (FBS) (Thermo Fisher Scientific). Cells were plated on 40-mm-diameter, no.1.5 coverslips (Bioptechs) at 350,000 cells per coverslip and were incubated in Petri dishes at 37 °C with 5% CO2 for 48 h. Cells were fixed for 15 min in 4% (vol/vol) paraformaldehyde (PFA) (Electron Microscopy Sciences) in 1× PBS at room temperature, washed three times with 1× PBS, permeabilized for 10 min with 0.5% (vol/vol) Triton X-100 (Sigma) in 1× PBS at room temperature, and washed once with 1× PBS.
Encoding probe staining
Permeabilized cells were incubated for 5 min in encoding wash buffer comprising 2× saline-sodium citrate (SSC) (Ambion) and 30% (vol/vol) formamide (Ambion). Then 30 μL of ∼300 μM encoding probes and 3.3 μM of poly(dT) LNA anchor probe (a 20-nt sequence of alternating dT and thymidine-locked nucleic acid (dT+) with a 20-nt reverse complement of a readout sequence and a 5′-acrydite modification (Integrated DNA Technologies)) in encoding hybridization buffer was added to the surface of Parafilm (Bemis) and was covered with a cell-containing coverslip. Encoding hybridization buffer was composed of encoding wash buffer supplemented with 0.1% (wt/vol) yeast tRNA (Life Technologies), 1% (vol/vol) murine RNase inhibitor (New England Biolabs), and 10% (wt/vol) dextran sulfate (Sigma). Samples were incubated in a humid chamber inside a hybridization oven at 37 °C for 40 h. Cells then were washed with encoding wash buffer and incubated at 47 °C for 30 min; this washing step was repeated once.
Immunostaining
For immunofluorescence only, cells were fixed and permeabilized as described in the “Cell culture and fixation” Section. Samples were first blocked at room temperature for 30 min in blocking buffer consisted of 4% (wt/vol) UltraPure BSA (Thermo Fisher Scientific) in 2× SSC supplemented with 3% (vol/vol) RNasin Ribonuclease inhibitor (Promega), 6% (vol/vol) murine RNAase inhibitor and 1 mg/ml yeast tRNA. Samples were then incubated with primary antibodies (anti-pan Cadherin, Abcam) in blocking buffer at a concentration of 2 μg/ml for 1 h at room temperature, and washed three times with 2× SSC for 10 min each. Samples were then incubated with oligo-labeled secondary antibodies in blocking buffer at a concentration of 3.75 μg/ml for 1 h at room temperature, then washed with 2× SSC three times for 10 min each. Samples were fixed again with 4% (vol/vol) PFA in 2× SSC for 10 min and washed three times with 2× SSC.
To combine MERFISH with immunofluorescence, samples were first stained with encoding probes and washed as described in the “Encoding probe staining” section above. To stabilize these probes, samples were then briefly post-fixed with 4% (vol/vol) PFA in 2× SSC at room temperature for 10 min and washed three times with 2× SSC. Samples were then stained for immunofluorescence as described above.
Gel embedding, digestion and clearing for unexpanded samples
Stained samples on silanized coverslips treated as in “Silanization of coverslips for unexpanded samples” Section (see below), were first incubated for 5 min with a de-gassed polyacrylamide solution, consisted of 4% (vol/vol) of 19:1 acrylamide/bis-acrylamide (BioRad), 60 mM Tris⋅HCl pH 8 (Thermo Fisher Scientific), 0.3 M NaCl (Thermo Fisher Scientific), 0.2% (vol/vol) Tetramethylethylenediamine (TEMED) (Sigma) and a 1:25,000 dilution of 0.1-µm-diameter carboxylate-modified orange fluorescent beads (2% solids, Life Technologies). The beads served as fiducial markers for the alignment of images taken across multiple rounds of smFISH imaging. The polyacrylamide solution was kept on ice and further supplemented with ammonium persulfate (Sigma) at a final concentration of 0.2% (wt/vol).
50 µL of this gel solution was added to the surface of a glass plate (TED Pella) that had been pretreated for 5 min with 1 mL GelSlick (Lonza) so as not to stick to the polymer gel. Samples were aspirated, dried quickly with KimWipes (Kimtech) from the edge of the coverslips, and gently inverted onto this 50-µL droplet to form a thin layer of solution between the coverslip and the glass plate. The solution was then allowed to polymerize for 2 h at room temperature in a home-built chamber filled with nitrogen. The coverslip and the glass plate were then gently separated, and the PA film was washed once with a digestion buffer consisted of 2% (wt/vol) Sodium dodecyl sulfate (SDS) (Thermo Fisher Scientific), 0.5% (vol/vol) Triton X-100 in 2× SSC. Our digestion buffer is different from published previously13,20,21. We used 2% (wt/vol) SDS to facilitate lipid removal. After the wash, the gel was covered with digestion buffer supplemented with 1% (vol/vol) Proteinase K (New England Biolabs). The sample was digested in this buffer for >12 h in a humidified, 37 °C incubator and then washed three times with 2× SSC for 15 min each on a rocker. MERFISH measurements were either performed immediately or the sample was stored in 2× SSC supplemented with 0.1% (vol/vol) murine RNase inhibitor at 4 °C for no longer than 48 h.
Silanization of coverslips for unexpanded samples
To stabilize the polymer film, coverslips were silanized as published previously13,44. Briefly, 40-mm-diameter #1.5 coverslips (Bioptechs) were washed for 30 min via immersion in a 1:1 mixture of 37% (vol/vol) hydrochloric acid (Sigma) and methanol (Sigma) at room temperature. Coverslips were then rinsed three times in deionized water and once in 70% (vol/vol) ethanol. Coverslips were blown dry with nitrogen gas and then immersed in 0.1% (vol/vol) triethylamine (Millipore) and 0.2% (vol/vol) allyltrichlorosilane (Sigma) in chloroform for 30 min at room temperature. Coverslips were washed once each with chloroform and ethanol and then blow dry with nitrogen gas. Silanized coverslips were then stored at room temperature in a desiccated chamber overnight before use to dehydrate the silane layer.
Gel embedding, digestion and clearing, and expansion for expanded samples
The embedding and expansion protocol was modified based on previously published methods20,21. Monomer solution consisted of 2 M NaCl, 7.7% (wt/wt) sodium acrylate (Sigma), 4% (vol/vol) of 19:1 acrylamide/bis-acrylamide and 60 mM Tris-HCl pH 8 was prepared and frozen in aliquots at −20 °C. Monomer solution was thawed, degassed and cooled to 4 °C on ice before use. TEMED with a final concentration of 0.2% (vol/vol) and a 1:5,000 dilution of 0.1-µm-diameter carboxylate-modified orange fluorescent beads was added to the solution. The beads served as fiducial markers for the alignment of images taken across multiple rounds of smFISH imaging. Stained samples were incubated in the solution for 5 min at room temperature. The solution was then kept on ice and further supplemented with ammonium persulfate at a final concentration of 0.2% (wt/vol).
The casting of a thin polymer film and polymerization was performed the same as described in the “Gel embedding, digestion and clearing for unexpanded samples” Section. After polymerization, the coverslip and the glass plate were gently separated. The gel film on the coverslip was washed once with the digestion buffer and trimmed to desired sizes using a razor blade. Digestion was performed the same as described in the “Gel embedding, digestion and clearing for unexpanded samples” Section. The gel would expand ~1.5 fold during digestion.
After digestion, samples were expanded in 0.5× SSC buffer supplemented with 0.2% (vol/vol) Proteinase K at room temperature. Proteinase K was added to maintain samples in an RNAase free environment and to digest away any newly exposed proteins. The buffer was changed every 30 min until samples no longer expanded (typically ~2 h). Expanded gels were re-embedded in polyacrylamide gel to stabilize the gel for sequential rounds of readout probe hybridization and imaging. Briefly, samples were incubated in re-embedding solution composed of 4% (vol/vol) of 19:1 acrylamide/bis-acrylamide with 30–75 mM NaCl, 6–15 mM Tris ⋅HCl pH 8 and 0.2% (vol/vol) of TEMED for 20 min at room temperature on a rocker. The re-embedding solution was then kept on ice and further supplemented with ammonium persulfate at a final concentration of 0.2% (wt/vol). Gels were placed on a bind-silane-treated coverslip prepared using the protocol described in “Bind-silane treatment of coverslips” section (see below), rinsed with the solution and dried quickly with KimWipes. Coverslips with gels were put in a home-built nitrogen chamber, covered a glass plate and allowed to polymerize at room temperature for 1 h. The salt concentrations in the buffers utilized for expansion and re-embedding were determined so that the encoding probes were maintained on the RNA during these processes. The salt concentration may need to be adjusted when different probe sets are used.
Bind-silane treatment of coverslips
40-mm-diameter, no.1.5 coverslips (Bioptechs) were sonicated in 1 M potassium hydroxide for 30 min, wash three times with deionized water and sonicated again in 70% (vol/vol) ethanol for 30 min. Coverslips were silanized using a modified version of published protocols21,45. Briefly, coverslips were immersed in a solution composed of 5% (vol/vol) glacial acetic acid (Sigma) and 0.38% (vol/vol) bind-silane (GE Healthcare) in 99% (vol/vol) ethanol for 1 h at room temperature. After being quickly washed with 70% (vol/vol) ethanol three times, coverslips were put into a 60 °C oven until dried completely. Coverslips can be stored in seal containers with desiccants for up to a month.
Sequential rounds of readout probe staining and automatic buffer exchange
To facilitate choosing the right focal plane for imaging, embedded samples were hybridized in dish with the first pair of two-color readout probes in hybridization buffer composed of 2× SSC, 5% (vol/vol) ethylene carbonate (Sigma), 0.1% (vol/vol) murine RNase inhibitor in nuclease-free water, and 3 nM of the appropriate readout probes, for 30 min (expanded samples) or 10 min (unexpanded samples) at room temperature and washed for 20 min (expanded samples) or 7 min (unexpanded samples) in wash buffer composed of 2× SSC and 10% (vol/vol) ethylene carbonate in nuclease-free water. Samples were then washed with 2× SSC once, stained with 4′,6-Diamidino-2-Phenylindole, Dihydrochloride (DAPI) (Thermo Fisher Scientific) at 10 µg/ml in 2× SSC for 10 min, and washed 3 times in 2× SSC for 5 min each.
Samples were then mounted into a flow chamber and buffer exchange through this chamber was controlled via a home-built fluidics system composed of three computer-controlled eight-way valves (Hamilton) and a computer-controlled peristaltic pump (Gilson) as published previously7,12. Flow and incubation time was increased for expanded samples (besides the hybridization and wash time as described in the previous section, tris(2-carboxyethyl)phosphine hydrochloride (TCEP) (Sigma) incubation time was increased to 30 min and all buffer exchange time to 7 min) to allow diffusion to reach equilibrium inside of the gel. After each MERFISH imaging round, the fluorescence of the readout probes was extinguished by incubating the sample in a reductive cleavage buffer composed of 2 × SSC and 50 mM TCEP.
MERFISH Imaging
In order to accurately compare the counts per cell between different samples, we performed z-scanning to image more than 90% of target RNAs in a cell. Specifically, we performed optical sectioning at discrete imaging planes across the cell with a step size of 0.5 µm and quantified distribution of smFISH signals as a function of z position. We found >90% of RNAs located in the first 5 µm-depth volume in unexpanded samples and in the first 12 µm-depth volume in expanded samples from the surface of coverslips. Thus, we performed z-stack imaging by scanning a 5 µm-depth volume for unexpanded samples and a 12 µm-depth volume for expanded samples with a step size of 1 µm. The scanning in z direction was controlled by a Nano-F200 nanopositioner (Mad City Labs).
Sequential probe imaging and signal removal was carried out on a high-throughput imaging platform as published previously12. Briefly, after hybridization of readout probes and exchange of imaging buffer, samples were imaged with a FOV area of 223 × 223 µm utilizing a 2,048 × 2,048 pixel, scientific complementary metal-oxide semiconductor (sCMOS) camera (Zyla 4.2; Andor) in combination with a high numerical aperture (NA = 1.3) and a high-magnification (60×) silicone oil objective (UPLSAPO 60 × S2; Olympus). The imaging (and corresponding fluidic exchange) process was repeated eight times for MERFISH-only samples and nine times for MERFISH + IF samples, with 405-nm DAPI channel imaged in conjunction with the first round of readout imaging.
100–400 FOVs were measured per sample. We measured a total of ~1,000 cells for expanded samples in two independent sample preparations and measurements (one containing ~750 cells and the other containing ~250 cells), and results from the two independent experiments were compared with each other in Fig. 2f. In addition, we measured a total of ~1,200 cells for unexpanded samples (Fig. 1) and a total of ~1,100 cells for expanded samples in combination with immunofluorescence (Fig. 3).
Image registration and decoding
Registration of images of the same FOV across imaging rounds as well as decoding of the RNA barcodes was conducted using a previously published analysis pipeline12. Briefly, z-stacks at each location from different imaging rounds were corrected for lateral offsets based on the location of fiducial beads. Each focal plane of the corrected z-stacks was high-pass filtered to remove background, deconvolved to tighten RNA spots, and then Gaussian-pass filtered to facilitate connecting signals from one imaging round to another. To correct for differences in the brightness between color channels, images were first normalized by equalizing their intensity histograms and refined further via iterative decoding trials to remove substantial variation in the fluorescence intensity between different bits. The set of 16 normalized intensity values (corresponding to 16 readout imaging) observed for each pixel in each FOV at each focal plane represented a vector in a 16-dimensional space. The pixel vector was normalized and compared to each of the 140 barcodes in the 16-bit MHD4 code. A pixel was assigned to a barcode if the Euclidean distance between the vector and a barcode was smaller than a given threshold defined by the distance of a single-bit error. Adjacent pixels in all focal planes of the z-stacks were combined into a single putative RNA using a 3-D connectivity array with maximal neighborhood connectivity.
Cells were segmented using a previously published approach12. Briefly, the segmentation boundaries separating cells were calculated using the watershed algorithm based on the inverted RNA density with DAPI stained regions as initial seeds. Because the neighboring cells were not necessarily in physical contact, the segmentation boundaries that separate cells do not necessary coincide with the physical boundaries of the cells, but might encompass slightly more area than that occupied physically by cells.
Computations were split between the Odyssey cluster supported by the FAS Division of Science, Research Computing Group at Harvard University and a desktop server that contained two 10-core Intel Xeon E5–2680 2.8 GHz CPUs and 256 GB of RAM.
Single-molecule FISH
Each smFISH probe contains a 30-nt target region, designed using a pipeline published previously12, and a 20-nt custom-designed readout sequence12. We designed 48 probes for each gene. The probes were synthesized from IDT, resuspended at 100 μM per probe in TE buffer, and pooled together for each gene. After permeabilization, cells on silanized coverslips treated as in “Silanization of coverslips for unexpanded samples” Section were incubated for 5 min in encoding wash buffer comprising 2× SSC and 30% (vol/vol) formamide. Then 30 μL of 2 μM smFISH probes and 3.3 μM of poly(dT) LNA anchor probe in encoding hybridization buffer was added to the surface of Parafilm and was covered with a cell-containing coverslip. Samples then were incubated in a humid chamber inside a hybridization oven at 37 °C for 24 h. Encoding hybridization buffer was the same as the one used for MERFISH. Cells then were washed with encoding wash buffer and were incubated at 47 °C for 30 min; this washing step was repeated once. Cells were then embedded and cleared using the matrix-imprinting-based clearing method described in “Gel embedding, digestion and clearing for unexpanded samples” Section. Embedded samples were hybridized in dish with a Cy5 20-nt readout probe in hybridization buffer, washed and stained with DAPI as described in “Sequential rounds of readout probe staining and automatic buffer exchange” Section. Images were collected scanning of a 5 µm-depth volume at a step size of 1 µm and 100 FOVs were collected for each smFISH probe set. smFISH spots were detected using the multi-emitter fitting routine 3D-DAOSTORM46 for each z section and spots appearing in adjacent z sections at the same x,y location were combined into one. ~1,000 cells per gene were measured in each smFISH measurement.
Simulation of the effect of RNA density on MERFISH detection efficiency
We performed simulation using parameters based on real MERFISH measurement conditions and encoding scheme. In each simulated image, the pixel size is 109 nm and the RNA spots have a Gaussian intensity profile with a standard deviation of 1.6 pixels. RNAs were distributed randomly across the field of view. The decoding of the simulated images was performed in the same way as the standard MERFISH decoding procedure described earlier and decoded results at different simulated RNA density were compared with the ground truth to determine the detection efficiency.
The estimation of copy numbers per cell for the randomly chosen 1,000 RNA species was done by computing the total FPKM of the randomly chosen RNA species multiplied by the ratio of copy number to FPKM value as calculated from expanded MERFISH measurements.
Data availability
The datasets that support the finding of this paper are available from the corresponding author upon request.
Code availability
The software used to analyze the datasets are available from the corresponding author upon request.
Change history
19 April 2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
Crosetto, N., Bienko, M. & van Oudenaarden, A. Spatially resolved transcriptomics and beyond. Nat Rev Genet 16, 57–66 (2015).
Levsky, J. M., Shenoy, S. M., Pezo, R. C. & Singer, R. H. Single-cell gene expression profiling. Science 297, 836–40 (2002).
Lubeck, E. & Cai, L. Single-cell systems biology by super-resolution imaging and combinatorial labeling. Nat Meth 9, 743–748 (2012).
Levesque, M. J. & Raj, A. Single-chromosome transcriptional profiling reveals chromosomal gene expression regulation. Nat Meth 10, 246–248 (2013).
Jakt, L. M., Moriwaki, S. & Nishikawa, S. A continuum of transcriptional identities visualized by combinatorial fluorescent in situ hybridization. Development 140, 216–225 (2013).
Lubeck, E., Coskun, A. F., Zhiyentayev, T., Ahmad, M. & Cai, L. Single-cell in situ RNA profiling by sequential hybridization. Nat Meth 11, 360–361 (2014).
Chen, K. H., Boettiger, A. N., Moffitt, J. R., Wang, S. & Zhuang, X. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 348, aaa 6090, https://doi.org/10.1126/science.aaa6090 (2015).
Ke, R. et al. In situ sequencing for RNA analysis in preserved tissue and cells. Nat. Methods 10, 857–860 (2013).
Lee, J. H. et al. Highly multiplexed subcellular RNA sequencing in situ. Science 343, 1360–1363 (2014).
Femino, A. M., Fay, F. S., Fogarty, K. & Singer, R. H. Visualization of single RNA transcripts in situ. Science 280, 585–590 (1998).
Raj, A., van den Bogaard, P., Rifkin, S. A., van Oudenaarden, A. & Tyagi, S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat. Methods 5, 877–879 (2008).
Moffitt, J. R. et al. High-throughput single-cell gene-expression profiling with multiplexed error-robust fluorescence in situ hybridization. Proc. Natl. Acad. Sci. 113, 11046–11051 (2016).
Moffitt, J. R. et al. High-performance multiplexed fluorescence in situ hybridization in culture and tissue with matrix imprinting and clearing. Proc. Natl. Acad. Sci. 113, 14456–14461 (2016).
Hell, S. W. Far-Field Optical Nanoscopy. Science 316, 1153–1158 (2007).
Huang, B., Babcock, H. & Zhuang, X. Breaking the Diffraction Barrier: Super-Resolution Imaging of Cells. Cell 143, 1047–1058 (2010).
Holden, S. J., Uphoff, S. & Kapanidis, A. N. DAOSTORM: an algorithm for high- density super-resolution microscopy. Nat. Methods 8, 279–80 (2011).
Zhu, L., Zhang, W., Elnatan, D. & Huang, B. Faster STORM using compressed sensing. Nat Meth 9, 721–723 (2012).
Small, A. & Stahlheber, S. Fluorophore localization algorithms for super-resolution microscopy. Nat Meth 11, 267–279 (2014).
Coskun, A. F. & Cai, L. Dense transcript profiling in single cells by image correlation decoding. Nat Meth 13, 657–660 (2016).
Chen, F., Tillberg, P. W. & Boyden, E. S. Expansion microscopy. Science 347, 543–548 (2015).
Chen, F. et al. Nanoscale imaging of RNA with expansion microscopy. Nat Meth 13, 679–684 (2016).
Tsanov, N. et al. smiFISH and FISH-quant – a flexible single RNA detection approach with super-resolution capability. Nucleic Acids Res. 44, e165, https://doi.org/10.1093/nar/gkw784 (2016).
Yang, B. et al. Single-Cell Phenotyping within Transparent Intact Tissue Through Whole-Body Clearing. Cell 158, 945–958 (2014).
Sylwestrak, E. L., Rajasethupathy, P., Wright, M. A., Jaffe, A. & Deisseroth, K. Multiplexed Intact-Tissue Transcriptional Analysis at Cellular Resolution. Cell 164, 792–804 (2016).
Dey, S. S., Foley, J. E., Limsirichai, P., Schaffer, D. V. & Arkin, A. P. Orthogonal control of expression mean and variance by epigenetic features at different genomic loci. Mol. Syst. Biol. 11, 806, https://doi.org/10.15252/msb.20145704 (2015).
Skupsky, R., Burnett, J. C., Foley, J. E., Schaffer, D. V. & Arkin, A. P. HIV promoter integration site primarily modulates transcriptional burst size rather than frequency. PLoS Comput. Biol. 6, 9, https://doi.org/10.1371/journal.pcbi.1000952 (2010).
Sanchez, A. & Golding, I. Genetic Determinants and Cellular Constraints in Noisy Gene Expression. Science 342, 1188–1193 (2013).
Padovan-Merhar, O. et al. Single mammalian cells compensate for differences in cellular volume and DNA copy number through independent global transcriptional mechanisms. Mol. Cell 58, 339–352 (2015).
Tillberg, P. W. et al. Protein-retention expansion microscopy of cells and tissues labeled using standard fluorescent proteins and antibodies. Nat Biotech 34, 987–992 (2016).
Chozinski, T. J. et al. Expansion microscopy with conventional antibodies and fluorescent proteins. Nat Meth 13, 485–488 (2016).
Ku, T. et al. Multiplexed and scalable super-resolution imaging of three-dimensional protein localization in size-adjustable tissues. Nat Biotech 34, 973–981 (2016).
Marinov, G. K. et al. From single-cell to cell-pool transcriptomes: Stochasticity in gene expression and RNA splicing. Genome Res. 24, 496–510 (2014).
Chang, J.-B. et al. Iterative expansion microscopy. Nat. Methods 14, 593–599 (2017).
Chang, H., Lim, J., Ha, M. & Kim, V. N. TAIL-seq: genome-wide determination of poly(A) tail length and 3′ end modifications. Mol. Cell 53, 1044–1052 (2014).
Subtelny, A. O., Eichhorn, S. W., Chen, G. R., Sive, H. & Bartel, D. P. Poly(A)-tail profiling reveals an embryonic switch in translational control. Nature 508, 66–71 (2014).
Meijer, H. A. et al. A novel method for poly(A) fractionation reveals a large population of mRNAs with a short poly(A) tail in mammalian cells. Nucleic Acids Res. 35, e132, https://doi.org/10.1093/nar/gkm830 (2007).
Schweller, R. M. et al. Multiplexed in situ Immunofluorescence via Dynamic DNA Complexes. Angew. Chem. Int. Ed. Engl. 51, 9292–9296 (2012).
Jungmann, R. et al. Multiplexed 3D cellular super-resolution imaging with DNA-PAINT and Exchange-PAINT. Nat. Methods 11, 313–318 (2014).
Wang, Y. et al. Rapid Sequential in Situ Multiplexing with DNA Exchange Imaging in Neuronal Cells and Tissues. Nano Lett. 17, 6131–6139 (2017).
Guo, S.-M. et al. Multiplexed confocal and super-resolution fluorescence imaging of cytoskeletal and neuronal synapse proteins. Preprint at https://www.biorxiv.org/content/early/2017/02/25/111625 (2017).
Jiang, D., Hatahet, Z., Melamede, R. J., Kow, Y. W. & Wallace, S. S. Characterization of Escherichia coli endonuclease VIII. J. Biol. Chem. 272, 32230–32239 (1997).
Zhang, Z., Revyakin, A., Grimm, J. B., Lavis, L. D. & Tjian, R. Single-molecule tracking of the transcription cycle by sub-second RNA detection. Elife 3, e01775, https://doi.org/10.7554/eLife.01775 (2014).
Gong, H. et al. Simple Method To Prepare Oligonucleotide-Conjugated Antibodies and Its Application in Multiplex Protein Detection in Single Cells. Bioconjug. Chem. 27, 217–225 (2016).
Buxboim, A., Rajagopal, K., Brown, A. E. X. & Discher, D. E. How deeply cells feel: methods for thin gels. J. Phys. Condens. Matter 22, 194116, https://doi.org/10.1088/0953-8984/22/19/194116 (2010).
Hoffecker, I. T., Guo, W. & Wang, Y. Assessing the spatial resolution of cellular rigidity sensing using a micropatterned hydrogel-photoresist composite. Lab Chip 11, 3538–3544 (2011).
Babcock, H., Sigal, Y. M. & Zhuang, X. A high-density 3D localization algorithm for stochastic optical reconstruction microscopy. Opt. Nanoscopy 1, 6, https://doi.org/10.1186/2192-2853-1-6 (2012).
Acknowledgements
We thank Edward S. Boyden (Massachusetts Institute of Technology) for helpful discussions regarding expansion microscopy.
Author information
Authors and Affiliations
Contributions
G.W., J.R.M., and X.Z. designed research; G.W. performed experiments; G.W. and J.R.M. analyzed data; and G.W., J.R.M., and X.Z. wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
X.Z., G.W., and J.R.M. are inventors on patents applied for by Harvard University that describe MERFISH related technologies.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, G., Moffitt, J.R. & Zhuang, X. Multiplexed imaging of high-density libraries of RNAs with MERFISH and expansion microscopy. Sci Rep 8, 4847 (2018). https://doi.org/10.1038/s41598-018-22297-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-22297-7
This article is cited by
-
scGIST: gene panel design for spatial transcriptomics with prioritized gene sets
Genome Biology (2024)
-
Spatial transcriptomics reveal neuron–astrocyte synergy in long-term memory
Nature (2024)
-
Spatial transcriptomics in development and disease
Molecular Biomedicine (2023)
-
HT-smFISH: a cost-effective and flexible workflow for high-throughput single-molecule RNA imaging
Nature Protocols (2023)
-
The expanding vistas of spatial transcriptomics
Nature Biotechnology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.