Abstract
Emerging evidences addressed an association between phosphate and muscle function. Because little attention was focused on this issue, the objective of our study was to explore the relationship of phosphate with muscle strength, dynapenia, and sarcopenia. From the National Health and Nutrition Examination Survey, a total of 7421 participants aged 20 years or older were included in our study with comprehensive examinations included anthropometric parameters, strength of the quadriceps muscle, and appendicular lean masses. Within the normal range of serum phosphate, we used quartile-based analyses to determine the potential relationships of serum phosphate with dynapenia, and sarcopenia through multivariate regression models. After adjusting for the pertinent variables, an inverse association between the serum phosphate quartiles and muscle strength was observed and the linear association was stronger than other anthropometric parameters. Notably, the significant association between phosphate and muscle strength was existed in >65 years old age group, not in 20–65 years old. The higher quartiles of phosphate had higher likelihood for predicting the presence of dynapenia rather than sarcopenia in entire population. Our study highlighted that higher quartiles of phosphate had significant association with lower muscle strength and higher risks for predicting the presence of dynapenia.
Similar content being viewed by others
Introduction
Aging is a complicated process that results in muscle weakness and disability due to variations in skeletal muscle quantity and quality1. The terms of “sarcopenia” is limited to its original definition of an age-related loss of skeletal muscle mass, and “dynapenia” is defined as the present the age-related loss of strength2. It has been proposed that muscle strength is a more useful index of the development of declines in mobility and disability than muscle mass3. Stenholm et al. demonstrated that low muscle strength could predict the risk of death in adult men and women with an additive pattern4. A threshold of muscle strength associated with an increased likelihood of MetS in men was identified in a large-scale study5. Muscle strength is included in the definition of frailty status, which is closely associated with HRQoL in elderly Taiwanese preventive health service users6.
Serum phosphate is absorbed through the gut, stored in the bone and reabsorbed through the proximal renal tubules7. The level of serum phosphate is regulated within a narrow range in healthy individuals because inorganic phosphate plays fundamental physiological roles in energy production, membrane transport, and signal transduction8. Inorganic phosphate remains relatively constant through the influence of multiple factors, such as parathyroid hormone, fibroblast growth factor 23, and vitamin D, on the kidneys, bone, and digestive system9. In patients with chronic kidney disease (CKD), the kidneys fail to excrete excessive phosphate, which leads to a hyperphosphatemic state10.
In a recent literature, Acevedo et al. demonstrated that high-phosphorus diet potentiated adverse skeletal muscle changes in long-term uremic rats11. Inorganic phosphate had been observed to play an important role in muscle contractile function12, and increased inorganic phosphate was involved in skeletal muscle fatigue13. The objects of our study were to examine the association between phosphate with muscle strength and whether the phosphate could predict the presence of dynapenia or sarcopenia based on population-based data analysis from the NHANES 1999–2002.
Results
Serum phosphate level and different anthropometric parameters
The associations of the serum phosphate level and different anthropometric parameters (MS, TC, CC, and ALM) were analyzed as shown in Table 1. Notably, the serum phosphate level in the normal range had significant negative relationships with MS, TC and CC (P < 0.05), but not in the hyperphosphatemic or hypophosphatemic individuals. The fully adjusted β coefficient for the serum phosphate with the MS, TC and CC were −28.37, −1.99 and −1.12 (95% confidence intervals (95%CI) = −52.23, −4.50, P < 0.05; 95%CI = −3.05, −0.93, P < 0.001; and 95%CI = −1.78, −0.47, P < 0.001, respectively). In other words, the subjects with higher serum phosphate tended to have lower MS, TC and CC in our findings.
Demographic characteristics of the eligible subjects
In Table 2, participants with frankly low (<0.81) and high (>1.49) phosphate levels and normal range divided into quartiles were categorized and the characteristics of these subgroups were subsequently summarized. The serum phosphate level was significantly associated with age, BMI, total cholesterol, serum high density lipoprotein, serum fasting glucose, serum calcium and serum creatinine. In normal range of serum phosphate, the muscle strength of participants in the higher quartiles were weaker than those in the lower quartiles. Besides, subjects with hyperphosphatemia had lower muscle strength than those with normal range and with hypophosphatemia.
Association between phosphate and muscle strength
The quartile-based analysis of not only normal range of serum phosphate but also abnormal range was conducted by multiple linear regression, and the results are presented in Table 3. In normal range, negative associations between phosphate with muscle strength and TC were noted among all models. The higher quartiles of serum phosphate had lower muscle strength and TC, and a dose-dependent response was noted. However, no significant differences were found between serum phosphate with all anthropometric parameters in the hyperphosphatemic or hypophosphatemic groups.
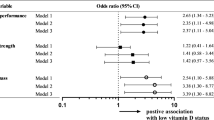
In Table 4, we further examine the association of muscle strength and serum phosphate level according to age. A significant relationship was observed in participants older than 65 years (P value, 0.011, 0.021 and 0.021 respectively).
The odd ratios for dynapenia and sarcopenia performed by the multivariable-adjusted logistic regression with phosphate quartiles analysis were provided in Table 5. In dynapenia, the higher quartiles of phosphate had higher risks for predicting the presence of dynapenia for all models in both 20–65 and >65 years old age groups. In sarcopenia, however, the same trend was only found in the unadjusted model for both age groups. The significant differences were existed in the highest quartile of phosphate in adjusted models with sarcopenia in 20–65 years old age group, but not in >65 years old.
Association between entire range of serum phosphate and muscle strength
In Supplementary Tables 1–5, we investigated the association of entire range of phosphate levels and muscle strength without excluding frankly low and high phosphate. After additionally adjusting for other covariates from Models 1 to 4, the inverse association between muscle strength and serum phosphate remained essentially unchanged. The similar results were consistent with the original findings in our study.
Gender difference in association between phosphate and muscle strength
In Supplementary Tables 6–11, gender difference was performed to examine the association of phosphate with muscle strength and dynapenia instead of age variable. In male, muscle strength had negative association with 3rd quartile of phosphate in Model 2 and Model 3 (Supplementary Table 10). In female, muscle strength was associated with 2nd and 4th quartiles of phosphate in Model 2 and Model 3. To predict the presence of dynapenia and sarcopenia, logistic regression was performed with phosphate divided into quartiles. In male, the higher quartiles of phosphate were significantly associated with the presence of dynapenia after model adjustment (Supplementary Table 11). In female, 4th quartile of phosphate had association with dynapenia in Model 2 and Model 3. However, the presence of sarcopenia was only predicted by 4th quartile of phosphate with model adjustment in female group.
Discussion
Based on this population-based, non-institutionalized sample of U.S. citizens, our study demonstrated an inverse association between serum phosphate and muscle strength. Furthermore, the higher levels of phosphate had higher risks to predict the presence of dynapenia in both age groups. To the best of our knowledge, this study is the first to provide epidemiological evidence regarding the relationship of serum phosphate and dynapenia.
Hypophosphatemia resulted in skeletal muscle damage with an elevation of the serum creatine phosphokinase and rhabdomyolysis14. Besides, alternation in renal acid–base status caused by hypophosphatemia via depressed proximal tubular reabsorption of bicarbonate and decreased buffer excretion due to low phosphate excretion15. Hypophosphatemia could contribute to muscle weakness and respiratory and heart failure16. In a study of mice model, muscle weakness could be explained by decreased muscle ATP synthetic flux which caused by hypophosphatemia17. Hyperphosphatemia could result from increased phosphate intake, decreased phosphate excretion, or a disorder that shifts intracellular phosphate to extracellular space. In the hyperphosphatemic mouse models, force decline of intact muscle was accompanied by increased inorganic phosphate and reduced tetanic myoplasmic Ca2+ 18.
For hyperphosphatemia, the possible underlying physiological functions of phosphate on muscle function could be divided into direct and indirect pathways. Emerging evidences had indicated that hyperphosphatemia led to vascular calcification with mineral deposition in vessel wall, leading to muscle dysfunction. It also caused related CVD mortalities including coronary artery disease and peripheral arterial disease (PAD) in CKD patients19,20. Patients with PAD manifested altered muscle control strategies and further strength deficits21. McDermott et al. represented that weaker plantar flexion strength, knee extension power, and hand grip strength were correlated with increased mortality in participants with PAD22. Phosphate was directly and independently correlated with inflammation23. Clinical studies had reported that serum phosphate was associated with serum inflammatory markers in CKD patients24 and phosphate overload directly induced systemic inflammatory and malnutrition in CKD rats25. High IL-6 and CRP levels were resulted in threefold greater risk of losing 40% of grip strength26. It was similar with our findings that elevated serum phosphate had significant associated with decline of muscle strength in participants with elevated CRP levels (Supplementary Table 14). It raised the possibility that that inflammatory processes mediated the association between serum phosphate and muscle strength. The possible mechanism was that inflammatory markers could induce alterations of muscle proteins independently of protein loss and result in diminished force production27. Several indirect consequences resulted in disturbance of phosphate homeostasis and caused hyperphosphatemia. Klotho-fibroblast growth factor system was thought to play an important role in the control of serum phosphate28. Hyperphosphatemia indirectly increased parathyroid hormone (PTH) secretion to reduce serum calcium by different possible pathways. First, increased serum phosphate contributed to calcium precipitation in soft tissues. Second, it might increase resistance of bone to PTH action by decreasing the efflux of calcium from bone. Third, it also impeded the 1 a-hydroxylase enzyme to reduce the levels of 1, 25(OH)2D3 (calcitriol)29. Low calcitriol levels resulted in decreased intestinal absorption of calcium and hypocalcemia30. Failure of sarcoplasmic reticulum Ca2+ release was proposed to be a major contributor to muscle fatigue12. In a recent study, hypocalcemia was reported to be one of the factors causing the decline in leg muscle strength in athletes31.
In terms of hypophosphatemia, the effect of hypophosphatemia on muscle function was examined in dog that reversible changes in the skeletal muscle composition and transmembrane potential was caused by moderate phosphorus depletion32. It was well known that a deficiency of vitamin D resulted in muscle weakness and a significant reduction in muscle force was noted when vitamin D deficiency was accompanied by hypophosphatemia33. Klotho and fibroblast growth factor were proposed that might play an important role in the maintenance of muscle property and function by interplaying vitamin D and phosphate in energy (ATP) and protein production34. Another association between muscle and hypophosphatemia was hypophosphatemic osteomalacia. Proximal muscle weakness and low serum phosphorus concentrations were common characteristic of these patients35.
In our study, higher levels of phosphate were significantly associated with the decline of muscle strength, but not the decrease of lean muscle mass. Higher quartiles of phosphate had higher risks of predicting the presence of dynapenia rather than sarcopenia. It seemed that phosphate might influence the decline of muscle strength via some potential pathways such as changes in neuromuscular transmission, muscle architecture, and the processes involved in E-C coupling instead of the change of muscle mass. It was generally accepted that reduction of muscle force occurred because phosphate was released from cross-bridges at a stage closely associated with force production so that elevation of phosphate accelerated the backward rate of this step and thus reduced force36,37. Neuromuscular fatigue onset was associated with an alteration of the mechanisms involved in force production which might be induced by a perturbation of the calcium movements, and accumulation of phosphate38. It was already proposed that increasing inorganic phosphate reduced cross-bridge force and Ca2+ sensitivity of the myofilaments39. Increasing the open probability of the SR Ca2+ release channels and slowing of the SR Ca2+ pump might be plausible causes of muscle fatigue40,41. It had long been known that cytoplasmic inorganic phosphate could cross into the SR and precipitate with the Ca2+ there. Precipitation of Ca2+ and phosphate in the SR would not only reduce the amount of Ca2+ available for release, but would also decrease the stimulatory effect of SR Ca2+ on the release channel42. However, no mechanistic explanation could be provided based on the available data.
There are still several limitations concerning the current study. First, the interpretations cannot be extended to causal inferences because this study was a cross-sectional observational analysis of an existing database. The long-term association between muscle strength decline and fluctuations in serum phosphate level was not investigated because repeated observations of these clinical variables were not recorded. Second, paucity of unmeasured variables such as parathyroid hormone and fibroblast growth factor-23 in NHANES 1999–2002 dataset might cause incomprehensive analysis. Because the data of serum vitamin D was only available in the NHANES 2001–2002, we tried to reanalysis our modeling hypothesis. After additional adjustment of serum vitamin D, the inverse association between serum phosphate and muscle Strength remained unchanged (Supplemental Tables 12 and 13). Third, data related to potentially confounding biomarkers such as IL-6 and TNF-alpha are not accessible in the NHANES database. If we could analyze these inflammatory biomarkers, which influence muscle strength as reported in previous studies, interesting findings could be uncovered. Finally, the complete pathway and mechanisms of increments in serum phosphate levels were not fully addressed.
Conclusion
Our study highlighted that being within the higher quartiles of phosphate levels within the normal range was inversely associated with muscle strength and had higher risks for predicting the presence of dynapenia. Hyperphosphatemia may result in impaired functions of the muscles that harbor a predisposing milieu for the decline of muscle strength and development of dynapenia. Although no prospective randomized controlled trial was assessable, our study provides epidemiologic evidence and a plausible pathophysiological mechanism for future studies to use to determine a strategy for the prevention of dynapenia and physical disabilities.
Methods
Ethics statement
The NHANES study protocol was conducted according to the National Center for Health Statistics (NCHS) Institutional Review Board (IRB). Before data collection and the health examinations, all informed consents had been obtained. All the experimental protocols were approved by NCHS IRB.
Study design and participants
All data were obtained from the National Health and Nutrition Examination Survey (NHANES), which was a cross-sectional survey of a representative sample of the U.S. population from 1999 to 2002 that was administered by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). The NHANES collected representative data on the health and nutritional statuses of non-institutionalized civilian residents and conducted more detailed laboratory analyses on a subset of the participants. These data are available for public download.
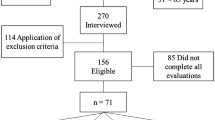
According to the flow chart of the study (Fig. 1), our study sample was composed of 7817 participants who were 20 years old and older. We excluded those with serum phosphate levels <0.81 mg/dL or >1.49 mg/dL to reduce any confounding effects and finally 7421 participants were analyzed in further stages. After examining the association between phosphate and anthropometric parameters, the decline of muscle strength was a better index which related to increased phosphate compared to other parameters. Normal phosphate levels were divided into quartiles to examine the association with muscle strength, thigh and calf circumference by multivariable model adjustment and observe whether the dose dependent effects were existed. Next, we examined the association of phosphate and muscle strength in 20–65 years old and >65 years old age groups respectively. Logistic regression was performed to analyze the odd ratios whether quartiles of phosphate predicted the presence of dynapenia and sarcopenia in different age groups in the final stage.
Measurement of serum phosphate
Serum phosphate was measured with a Hitachi model 737 multichannel analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN). Inorganic phosphate was reacted with ammonium molybdate in an acidic solution to construct ammonium phosphomolybdate, which was quantified in the ultraviolet range (340 nm) using a sample-blanked end point method43.
Assessment of anthropometric parameters
The calf and thigh circumference were measured by using an elastic tape and an elastic measuring tape was placed around the middle of calf and thigh44. We estimated the isokinetic strength of the quadriceps muscle to obtain the muscle strength. A Kin Com MP isokinetic dynamometer (Chattanooga, TN) was used to assess the maximum voluntary concentric muscle force of the right knee extensor muscle with an angular velocity goal of 60 degrees/second45. The appendicular and lower limb lean masses were determined for the whole body by dual-energy X-ray absorptiometry (DXA; Hologic QDR-4500A scanner; Hologic, Waltham, MA, USA). DXA supplied validated total and regional measures of fat mass, bone mass, and lean mass in all age groups46. The participants received whole-body scans while lying in the supine position on the DXA table with their limbs close to their body. Body compositions were determined with the Hologic software version 11.2:3 (Hologic) for Windows47.
Criteria of dynapenia and sarcopenia
To define dynapenia, the participants are divided into quartiles according to their sex-specific age, height-adjusted fat mass and leg strength residual values48. The isokinetic strength of the quadriceps muscle was used to determine the muscle strength. The patients in the lowest leg strength tertiles were defined as dynapenia.
For the definition of sarcopenia, the measure of DXA was performed to estimate the lean muscle mass in our study. The isokinetic strength of the quadriceps muscle was used to determine the muscle strength.
Covariates
The covariates included those taken from the analysis of the self-report demographic information including age and race or ethnicity. The body mass index (BMI) was obtained based on the formula in which the weight of subject in kilograms is divided by the square of the height in meters (kg/m2). The laboratory data analyzed in the study included levels of serum aspartate aminotransferase(AST), total cholesterol, serum high-density lipoprotein (HDL), serum fasting glucose, serum calcium and serum creatinine. All measurements were collected with standard methods as delineated in a CDC reference, and all information is accessible from the NHANES database. For the comorbidities related to physical function, a trained interviewer confirmed the smoking status of the participants by asking the question “Do you smoke cigarettes now?”. The presences of congestive heart failure, coronary heart disease, angina/ angina pectoris, heart attack, stroke and arthritis were defined according to whether the participants had ever been diagnosed with or told that they had these conditions. All protocols followed the standardized methods based on the CDC reference methods. All experimental methods were performed in accordance with the relevant guidelines and regulations of CDC.
Statistical Analysis
All statistical estimations were performed using by the Statistical Package for the Social Sciences, version18.0 (SPSS Inc., Chicago, IL, USA) for Windows. One-way analyses of variance and Pearson’s chi-square tests were performed to examine the differences between the groups in terms of demographic information, anthropometric parameters, laboratory data, and past histories. A two-sided p-value of ≤0.05 was regarded as the threshold for statistical significance. To prevent the influence of underlying comorbidities, we excluded all participants with abnormal serum phosphate levels and further divided the participants into quartiles based on their serum phosphate levels. When the participants in the lowest serum phosphate quartiles were regarded as the reference group, multivariate linear regression and logistic regression analyses were used. The following three-model approach was applied for covariate adjustment: Model 1 = unadjusted; Model 2 = age, gender, race, BMI, serum fasting glucose, serum cholesterol, AST, HDL, serum calcium and serum creatinine were included as covariates; and Model 3 = Model 2 + history of arthritis, congestive heart failure, coronary heart disease, stroke, and smoking were included as covariates.
References
Seene, T. & Kaasik, P. Muscle weakness in the elderly: role of sarcopenia, dynapenia, and possibilities for rehabilitation. European Review of Aging and Physical Activity 9, 109–117, https://doi.org/10.1007/s11556-012-0102-8 (2012).
Clark, B. C. & Manini, T. M. Sarcopenia =/= dynapenia. The journals of gerontology. Series A, Biological sciences and medical sciences 63, 829–834 (2008).
Newman, A. B. et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. The journals of gerontology. Series A, Biological sciences and medical sciences 61, 72–77 (2006).
Stenholm, S. et al. Obesity and muscle strength as long-term determinants of all-cause mortality–a 33-year follow-up of the Mini-Finland Health Examination Survey. International journal of obesity (2005) 38, 1126–1132, https://doi.org/10.1038/ijo.2013.214 (2014).
Senechal, M. et al. Cut points of muscle strength associated with metabolic syndrome in men. Med Sci Sports Exerc 46, 1475–1481, https://doi.org/10.1249/mss.0000000000000266 (2014).
Chang, Y. W. et al. Frailty and its impact on health-related quality of life: a cross-sectional study on elder community-dwelling preventive health service users. PLoS One 7, e38079, https://doi.org/10.1371/journal.pone.0038079 (2012).
Linglart, A. & Chaussain, C. Endocrine control of serum phosphate: from the discoveries of phosphatonins to novel therapies. Ann Endocrinol (Paris) 77(Suppl 1), S36–s42, https://doi.org/10.1016/s0003-4266(17)30076-8 (2016).
Weisinger, J. R. & Bellorin-Font, E. Magnesium and phosphorus. Lancet 352, 391–396, https://doi.org/10.1016/s0140-6736(97)10535-9 (1998).
Suki, W. N. & Moore, L. W. Phosphorus Regulation in Chronic Kidney Disease. Methodist DeBakey cardiovascular journal 12, 6–9, https://doi.org/10.14797/mdcj-12-4s1-6 (2016).
Moore, L. W., Nolte, J. V., Gaber, A. O. & Suki, W. N. Association of dietary phosphate and serum phosphorus concentration by levels of kidney function. Am J Clin Nutr 102, 444–453, https://doi.org/10.3945/ajcn.114.102715 (2015).
Acevedo, L. M. et al. High-phosphorus diet maximizes and low-dose calcitriol attenuates skeletal muscle changes in long-term uremic rats. Journal of applied physiology (Bethesda, Md.: 1985) 120, 1059–1069, https://doi.org/10.1152/japplphysiol.00957.2015 (2016).
Allen, D. G. & Westerblad, H. Role of phosphate and calcium stores in muscle fatigue. The Journal of physiology 536, 657–665 (2001).
Dahlstedt, A. J., Katz, A. & Westerblad, H. Role of myoplasmic phosphate in contractile function of skeletal muscle: studies on creatine kinase-deficient mice. The Journal of physiology 533, 379–388 (2001).
Knochel, J. P. et al. Hypophosphatemia and Rhabdomyolysis. J Clin Invest 62, 1240–1246 (1978).
Knochel, J. P. The pathophysiology and clinical characteristics of severe hypophosphatemia. Archives of internal medicine 137, 203–220 (1977).
Amanzadeh, J. & Reilly, R. F. Jr. Hypophosphatemia: an evidence-based approach to its clinical consequences and management. Nature clinical practice. Nephrology 2, 136–148, https://doi.org/10.1038/ncpneph0124 (2006).
Pesta, D. H. et al. Hypophosphatemia promotes lower rates of muscle ATP synthesis. The FASEB Journal 30, 3378–3387, https://doi.org/10.1096/fj.201600473R (2016).
Nocella, M., Cecchi, G. & Colombini, B. Phosphate increase during fatigue affects crossbridge kinetics in intact mouse muscle at physiological temperature. The Journal of physiology 595, 4317–4328, https://doi.org/10.1113/jp273672 (2017).
Hruska, K. A., Mathew, S., Lund, R., Qiu, P. & Pratt, R. Hyperphosphatemia of Chronic Kidney Disease. Kidney international 74, 148–157, https://doi.org/10.1038/ki.2008.130 (2008).
Karwowski, W., Naumnik, B., Szczepanski, M. & Mysliwiec, M. The mechanism of vascular calcification - a systematic review. Medical science monitor: international medical journal of experimental and clinical research 18, Ra1–11 (2012).
Schieber, M. N. et al. Muscle strength and control characteristics are altered by peripheral artery disease. Journal of vascular surgery 66, 178–186, https://doi.org/10.1016/j.jvs.2017.01.051 (2017). e112.
McDermott, M. M. et al. Calf muscle characteristics, strength measures, and mortality in peripheral arterial disease: a longitudinal study. Journal of the American College of Cardiology 59, 1159–1167, https://doi.org/10.1016/j.jacc.2011.12.019 (2012).
Navarro-Gonzalez, J. F., Mora-Fernandez, C., Muros, M., Herrera, H. & Garcia, J. Mineral metabolism and inflammation in chronic kidney disease patients: a cross-sectional study. Clinical journal of the American Society of Nephrology: CJASN 4, 1646–1654, https://doi.org/10.2215/cjn.02420409 (2009).
Lee, C. T. et al. Association between C-reactive protein and biomarkers of bone and mineral metabolism in chronic hemodialysis patients: a cross-sectional study. Journal of renal nutrition: the official journal of the Council on Renal Nutrition of the National Kidney Foundation 19, 220–227, https://doi.org/10.1053/j.jrn.2008.11.004 (2009).
Yamada, S. et al. Phosphate overload directly induces systemic inflammation and malnutrition as well as vascular calcification in uremia. American journal of physiology. Renal physiology 306, F1418–1428, https://doi.org/10.1152/ajprenal.00633.2013 (2014).
Schaap, L. A., Pluijm, S. M., Deeg, D. J. & Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. The American journal of medicine 119(526), e529–517, https://doi.org/10.1016/j.amjmed.2005.10.049 (2006).
Li, X. et al. Cardiac-specific overexpression of tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation 102, 1690–1696 (2000).
Lanske, B. & Razzaque, M. S. Molecular interactions of FGF23 and PTH in phosphate regulation. Kidney international 86, 1072–1074, https://doi.org/10.1038/ki.2014.316 (2014).
Slatopolsky, E., Brown, A. & Dusso, A. Role of phosphorus in the pathogenesis of secondary hyperparathyroidism. American journal of kidney diseases: the official journal of the National Kidney Foundation 37, S54–57 (2001).
Dusso, A. S. et al. p21(WAF1) and transforming growth factor-alpha mediate dietary phosphate regulation of parathyroid cell growth. Kidney international 59, 855–865, https://doi.org/10.1046/j.1523-1755.2001.059003855.x (2001).
Yusni, A., Purba, A. & Tarigan, B. Essential Role of Serum Calcium for Muscle Strength in Football Athletes. IOP Conference Series: Materials Science and Engineering 180, 012186 (2017).
Fuller, T. J., Carter, N. W., Barcenas, C. & Knochel, J. P. Reversible changes of the muscle cell in experimental phosphorus deficiency. Journal of Clinical Investigation 57, 1019–1024 (1976).
Schubert, L. & DeLuca, H. F. Hypophosphatemia is responsible for skeletal muscle weakness of vitamin D deficiency. Arch Biochem Biophys 500, 157–161, https://doi.org/10.1016/j.abb.2010.05.029 (2010).
Bartali, B., Semba, R. D. & Araujo, A. B. Klotho, FGF21 and FGF23: Novel Pathways to Musculoskeletal Health? The Journal of frailty & aging 2, 179–183, https://doi.org/10.14283/jfa.2013.26 (2013).
Saito, T. & Fukumoto, S. Fibroblast Growth Factor 23 (FGF23) and Disorders of Phosphate Metabolism. International Journal of Pediatric Endocrinology 2009, 496514, https://doi.org/10.1155/2009/496514 (2009).
Takagi, Y., Shuman, H. & Goldman, Y. E. Coupling between phosphate release and force generation in muscle actomyosin. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 359, 1913–1920, https://doi.org/10.1098/rstb.2004.1561 (2004).
Fitts, R. H. The cross-bridge cycle and skeletal muscle fatigue. Journal of applied physiology (Bethesda, Md.: 1985) 104, 551–558, https://doi.org/10.1152/japplphysiol.01200.2007 (2008).
Boyas, S. & Guevel, A. Neuromuscular fatigue in healthy muscle: underlying factors and adaptation mechanisms. Annals of physical and rehabilitation medicine 54, 88–108, https://doi.org/10.1016/j.rehab.2011.01.001 (2011).
Millar, N. C. & Homsher, E. The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers. A steady-state and transient kinetic study. J Biol Chem 265, 20234–20240 (1990).
Fruen, B. R., Mickelson, J. R., Shomer, N. H., Roghair, T. J. & Louis, C. F. Regulation of the sarcoplasmic reticulum ryanodine receptor by inorganic phosphate. J Biol Chem 269, 192–198 (1994).
Duke, A. M. & Steele, D. S. Characteristics of phosphate-induced Ca(2+) efflux from the SR in mechanically skinned rat skeletal muscle fibers. Am J Physiol Cell Physiol 278, C126–135, https://doi.org/10.1152/ajpcell.2000.278.1.C126 (2000).
Lamb, G. D. Excitation-contraction coupling and fatigue mechanisms in skeletal muscle: studies with mechanically skinned fibres. J Muscle Res Cell Motil 23, 81–91 (2002).
Chang, A. R. & Grams, M. E. Serum phosphorus and mortality in the Third National Health and Nutrition Examination Survey (NHANES III): effect modification by fasting. American journal of kidney diseases: the official journal of the National Kidney Foundation 64, 567–573, https://doi.org/10.1053/j.ajkd.2014.04.028 (2014).
Wu, C. J. et al. Examining the association between anthropometric parameters and telomere length and mortality risk. Oncotarget 8, 34057–34069, https://doi.org/10.18632/oncotarget.15976 (2017).
Kramer, J. F. Reliability of knee extensor and flexor torques during continuous concentric-eccentric cycles. Archives of physical medicine and rehabilitation 71, 460–464 (1990).
Shaw, K. A. et al. Dual energy X-ray absorptiometry body composition and aging in a population-based older cohort. International journal of obesity (2005) 31, 279–284, https://doi.org/10.1038/sj.ijo.0803417 (2007).
Sanada, K. et al. Muscle mass and bone mineral indices: does the normalized bone mineral content differ with age? European journal of clinical nutrition 63, 465–472, https://doi.org/10.1038/sj.ejcn.1602977 (2009).
Bouchard, D. R. & Janssen, I. Dynapenic-obesity and physical function in older adults. The journals of gerontology. Series A, Biological sciences and medical sciences 65, 71–77, https://doi.org/10.1093/gerona/glp159 (2010).
Author information
Authors and Affiliations
Contributions
All authors contributed substantially in this work and approved the final version of the manuscript. Y.Y.C. contributed to the design of the study, was responsible for the management and retrieval of data, contributed to initial data analysis and interpretation, drafted the initial manuscript. Y.Y.C., T.W.K., C.W.C., C.J.W., H.F.Y., C.H.L., L.W.W., W.L.C. decided upon the data collection methods. Y.Y.C. and W.L.C. were also responsible for the data analysis decisions. W.L.C. conceptualized and designed the study, supervised all aspects of the study, critically reviewed and revised the manuscript, and approved the final manuscript as submitted. All authors meet the ICMJE criteria for authorship.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, YY., Kao, TW., Chou, CW. et al. Exploring the Link between Serum Phosphate Levels and Low Muscle Strength, Dynapenia, and Sarcopenia. Sci Rep 8, 3573 (2018). https://doi.org/10.1038/s41598-018-21784-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-21784-1
This article is cited by
-
Effect of early serum phosphate disorder on in-hospital and 28-day mortality in sepsis patients: a retrospective study based on MIMIC-IV database
BMC Medical Informatics and Decision Making (2024)
-
Association of circulating calciprotein particle levels with skeletal muscle mass and strength in middle-aged and older adults
Hypertension Research (2022)
-
Emerging cross-talks between chronic kidney disease–mineral and bone disorder (CKD–MBD) and malnutrition–inflammation complex syndrome (MICS) in patients receiving dialysis
Clinical and Experimental Nephrology (2022)
-
Patient-Reported Outcomes from a Randomized, Active-Controlled, Open-Label, Phase 3 Trial of Burosumab Versus Conventional Therapy in Children with X-Linked Hypophosphatemia
Calcified Tissue International (2021)
-
Effects of whole-body cryotherapy on the innate and adaptive immune response in cyclists and runners
Immunologic Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.