Abstract
The typical co-precipitation method was adopted to synthesized the Li-excess Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) series cathode materials. The influences of Zr4+ doping modification on the microstructure and micromorphology of Li1.20[Mn0.52Ni0.20Co0.08]O2 cathode materials were studied intensively by the combinations of XRD, SEM, LPS and XPS. Besides, after the doping modification with zirconium ions, Li1.20[Mn0.52Ni0.20Co0.08]O2 cathode demonstrated the lower cation mixing, superior cycling performance and higher rate capacities. Among the four cathode materials, the Li1.20[Mn0.50Zr0.02Ni0.20Co0.08]O2 exhibited the prime electrochemical properties with a capacity retention of 88.7% (201.0 mAh g−1) after 100 cycles at 45 °C and a discharge capacity of 114.7 mAh g−1 at 2 C rate. The EIS results showed that the Zr4+ doping modification can relieve the thickening of SEI films on the surface of cathode and accelerate the Li+ diffusion rate during the charge and discharge process.
Similar content being viewed by others
Introduction
Recently, the Li-excess Li1.20[Mn0.52Ni0.20Co0.08]O2 (0.6Li2MnO3·0.4LiNi0.50Co0.20Mn0.30O2) materials have attracted much study as cathodes for LIBs owing to the high specific discharge capacity (up to 250 mAh g−1) and less cost1,2,3. With further research, people have discovered that the Li2MnO3 phase (one of the components in the Li1.20[Mn0.52Ni0.20Co0.08]O2) will be activated and participate in the electrochemical reactions only when the cell voltage is charged to exceed 4.5 V4,5. However, the high working voltage will cause some drawbacks, such as severe capacity degradation and poor thermal stability, which restrict the practical applications, especially in EV and HEV6,7. Moreover, when the batteries have been used in the high temperature circumstance, the side reaction between the cathode and electrolyte will be more severe in comparison with the traditional cathode materials, such as LiCoO2 or LiNi0.50Co0.20Mn0.30O2 owing to the high working voltage for the Li-excess Li1.20[Mn0.52Ni0.20Co0.08]O2 materials8.
To maintain the stability of the cathode at high temperature, considerable effort has been made to resolve the intrinsic defects. For examples, the surface coating modification can effectively protect the cathode from reacting with the electrolyte and retard the thickening of SEI film during cycling. In addition, the suppression of the layered-to-spinel transformation for the Li-excess cathode materials can be obtained by the compact coating layer, leading to the improved electrochemical properties9,10,11,12. The ion doping modification can stabilize the cathode crystal structure and suppress the layer structural damage13,14. However, the surface coating modification technology has been complicated and the coating effect demonstrates to be difficult to control, while the ion doping modification shows the easy accessibility and obvious synthetic efficiency15. Therefore, the ion doping modification has been regarded as the competitive method to enhance the electrochemical properties of the Li-excess Li1.20[Mn0.52Ni0.20Co0.08]O2 materials.
Numerous studies have shown that the Zr4+ doping modification can effectively enhance the cyclical stability and rate capacity of cathodes. For example, when Zr4+ was doped into the LiCoO2 by using the ultrasonic spray pyrolysis method, the LiCo0.99Zr0.01O2 delivered the discharge capacity of 108 mAh g−1 at 1 C in the voltage range of 3.0–4.2 V after 50 cycles, while the un-doped sample rapidly dropped down to 23 mAh g−1 at the same condition16. When the LiNi0.5Co0.2Mn0.3O2 was doped modification with Zr4+ by solid-state method reaction, the Li(Ni0.5Co0.2Mn0.3)0.09Zr0.01O2 demonstrated the much more enhanced rate capability than that of the LiNi0.5Co0.2Mn0.3O2 by the suppression of electrode polarization17. While the radius of Zr4+ (0.072 nm) is larger than those of Mn4+ (0.053 nm), Ni2+ (0.069 nm), Co3+ (0.0685 nm) in the transition-metal layer, the Zr4+ adulteration will expand the diffusion path of Li+ insertion/extraction, leading to the improved electrochemical properties. On the other hand, the bond energy of Zr-O has found to be stronger than those of Ni-O Co-O and Mn-O, which will contribute to stabilizing the structure of cathode18. Based on the above evidence, Zr4+ will be an attractive doping element to dope into the Li-excess Li1.20[Mn0.52Ni0.20Co0.08]O2 and enhance the electrochemical properties.
In the work, the Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) series samples have been synthesized via using carbonate co-precipitation method. And then the combination of microstructural, particle morphology and electrochemical properties has been surveyed to evaluate the influence of different Zr4+ doping contents into Li1.20[Mn0.52Ni0.20Co0.08]O2 cathode.
Experimental
The Li-excess Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) series cathode materials were synthesized via using the carbonate co-precipitation method to synthesize the carbonate precursors, followed by sintering with LiOH·H2O powder at high temperature to obtain the cathode materials. The typical synthesis route has been shown as follows: (1) The stoichiometric amounts of MnSO4·H2O, NiSO4·6H2O, CoSO4·7H2O and Zr(NO3)4·5H2O were dissolved in distilled water to obtain a transparent solution; (2) Then the appropriate amount of NH3·H2O, as chelating agent and Na2CO3, as precipitant, were dropped into the above solution to make the metal ions deposit uniformly; (3) The acquired [Mn0.52−xZr x Ni0.20Co0.08](CO3)0.80 precursors were segregated, washed with deionized water until the impurities eliminate completely; (4) Then the stoichiometric amount of [Mn0.52−xZr x Ni0.20Co0.08](CO3)0.80 precursors and an excess 3 wt.% amount of LiOH·H2O powder were mixed uniformly, followed by pre-heated at 500 °C for 6 h and finally calcined at 950 °C for 12 h in tube furnace to acquire the Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) samples.
To investigate the influence of the Zr4+ doping on the crystal structure of Li1.20[Mn0.52Ni0.20Co0.08]O2, the XRD measurement were carried out by using Rigaku RINT2400 X-ray diffractometer with Cu Kα radiation in the 10° ≤ 2θ ≤ 80°, accompanied by a step size of 0.02° and a count time of 10.0s. Rietveld refinement of the cathode powder diffraction patterns were performed by using the GSAS/EXPGUI program. The morphologies of Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) were observed by using scanning electron microscopy (SEM, Ultra 55, Zeiss) and high-resolution transmission electron microscopy (TEM, FEI Titan G2 60–300) equipped with energy-dispersive X-ray spectroscopy (EDX, Oxford) to test the elemental distributions of cathode material (x = 0.02). The particle size was measured by using laser particle size Analysis (LPS, TOOLSO, 2005A). The chemical states of the doping element were determined by using X-ray photoelectron spectroscopy (XPS, Perkin Elmer PHI 1600). And the XPS spectra were fitted by using XPSPEAK software. The elemental composition, i.e. Ni, Co, Mn and Yb, was detected by ICP-OES (Inductively Coupled Plasma Optical Emission Spectrometer, iCAP 6000). Phase transformation studies of original and cycled Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.02) were carried out using a micro-Raman spectrometer (LabRAMHREvolution, HORIBA).
The electrochemical properties of Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) samples were measured by using galvanostatic charge and discharge with the coin cell of type CR2025. The coin cells were assembled as follows: (1) The 85 wt.% Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) samples, 10 wt.% carbon black and 5 wt.% polyvinylidene fluoride were evenly mixed to form the cathode slurry; (2) Then the slurry was casted onto Al foil by using a smudge stick and dried at 110 °C for 12 h in vacuum drying oven, followed by squeezed and punched into a circular disc with d = 12 mm; (3) The as-prepared cathode plate, the lithium metal plate as anode, the Celgard 2400 as the separator and 1 M LiPF6 dissolved in EC/DMC at mass ratio of 1:1 as the electrolyte were assembled in an argon-filled glove box to form the coin cells. The Galvanostatic charge-discharge tests were carried out by on a Land CT2001A (Wuhan, China) tester.
The cells were charged and discharged in the voltage range of 2.0 to 4.8 V at the different current densities (1C = 250 mA g−1). In addition, the CHI660D workstation was used to perform the electrochemical impedance spectroscopy (a frequency range from 0.01 Hz to 100 kHz and perturbation amplitude of 5 mV) and the cyclic voltammogram (a voltage range from 2.0 V to 4.8 V with a scanning rate of 0.1 mV s−1).
Results and Discussion
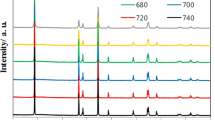
Figure 1 shows the X-ray diffraction patterns of the Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03)samples. The as-prepared samples have mainly demonstrated the typical XRD patterns of the hexagonal α-NaFeO2 structure with the space group R-3m (the LiMO2 features), except for the weak super lattice peaks between 20° and 25°, which are related to the Li2MnO3 phase, corresponding to the monocline unit cell C2/m19,20. In addition, the distinct splitting of (006)/(102) and (018)/(110) peaks have indicated that the as-prepared cathode materials have formed a well-developed hexagonal layered structure21. Besides, to further investigate the cation mixing between the Ni2+ and Li+ in the LiMO2 main phase, the Rietveld refinement of the diffraction patterns was performed based on the R-3m (used for LiNi0.50Co0.20Mn0.30O2 phase) and C2/m (used for Li2MnO3 phase) structure, as is shown in Fig. 1. And the structural parameters obtained from the refinement for the Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) samples are listed in Table 1. It can be seen that with the Zr4+ doping content increasing, the lattice parameters a and c of LiNi0.50Co0.20Mn0.30O2 phase have gradually risen owing to the larger radius of Zr4+. The larger lattice parameters a and c will contribute to enhancing the Li+ diffusion rate during the charge and discharge process22. Besides, the c/a ratio is related to the cation mixing and a high ratio represents the well cation ordering has been formed23. It can be observed the Zr4+-doped samples deliver the higher c/a ratio than that of the un-doped cathode, indicating the cation mixing of the as-prepared samples has been improved after the Zr4+ doping. Besides, according to the reports of J.R. Dahn24,25, the nominal formula of Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 can be assumed as [Li1−δNi δ ][Li δ Mn0.52−xZr x Ni0.20−δCo0.08]O2. The GSAS/EXPGUI program has been adopted to calculate the refined lattice structural data of as-prepared samples, as is shown in Table 1. It is clear that the amount of Ni in Li site for the Zr4+-doped samples is lower than that of the pristine Li1.20[Mn0.52Ni0.20Co0.08]O2 sample. And when the Zr4+ doping content aggrandizes, the amount of Ni in Li site first decreases from 0.059 to 0.041 and 0.032, then increases to 0.039, the Li1.20[Mn0.50Zr0.02Ni0.20Co0.08]O2 has demonstrated the optimal cation ordering. The lower cation mixing will not only suppress formation of spinel-like phase, but also improve the layered structure stability, finally contribute to enhancing the cyclic performance. Besides, the occupancy of Zr cations in 3b-site are respectively 0, 0.012, 0.019 and 0.031 with the Zr doping contents increasing, indicating the molar ratio for Zr doping can be designed experimentally. The Zr4+ doping can enlarge the lattice parameters, which facilitates Li-ion diffusion and subsequently enhances the high-rate capability.
Figure 2 shows the SEM images of the Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) samples. The as-prepared samples are composed of numerous crystallites with a diameter of 200~700 nm. And all particles present the similar morphology of rock-shaped grains without obvious aggregation. In addition, with the Zr4+ doping content increasing, the crystal particles surface become more smooth and the size of the particles become larger, which implies the crystallinity of the particles can be enhanced after the Zr4+ doping. To further analyze the influence of the Zr4+ doping on the cathode particles size, the size distribution of the Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) samples have been measured, as is shown in Fig. 3. It is obvious that the size of D50 gradually aggrandizes when the Zr4+ doping content increases, as is shown by the arrows, which is in good consistent with the observation of SEM images. A small amount of doped Zr ions may form continuous grain boundary phases in the Li1.20[Mn0.52Ni0.20Co0.08]O2 particles. These continuous grain boundary phases could enhance the mass diffusion transport at grain boundaries, finally promote the grain growth of Li1.20[Mn0.52Ni0.20Co0.08]O226,27. And the well crystallization will help to ameliorate the electrochemical properties of cathode. Besides, the STEM images of Li1.20[Mn0.50Zr0.02Ni0.20Co0.08]O2 and corresponding elemental maps of Ni, Mn, Co and Zr is shown in Fig. 4. The Fig. 4 demonstrates that not only the Ni, Co and Mn atoms have been distributed homogeneously, but also the doping element Zr atom have been evenly distributed in the cathode particles rather than segregated on the oxide surface, indicating the Zr4+ doping technology has obtained the obvious synthetic efficiency. Based on the above analysis, it has proved that the Zr4+ has been successfully doped into the Li1.20[Mn0.52Ni0.20Co0.08]O2 cathode material with uniform dispersion. The uniform dispersion of Zr dopant will make the function of Zr4+ doping modification more stability, which may be ready to provide a better cycling performance to some extent.
Figure 5 shows X-ray photoelectron spectroscopy (XPS) results of Zr, Mn, Ni and Co for the Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.02) samples. In Fig. 5(a), the obvious peaks at the binding energies of 184.9 eV and 182.6 eV are assigned to Zr 3d5/2 and Zr 3d3/2, respectively, which corresponds to the Zr-O bonds at the state of Zr4+ 28. In Fig. 5(c), the obvious peaks at the binding energies of 854.2 eV is assigned to Ni2p3/2, which corresponds to the oxidation state of Ni2+ and Ni3+ after fitting, respectively29,30. Besides, it can be calculated that the relative content of Ni2+ decreased after zirconium doping owing to the reduction of cation mixing degree. Compared with the pristine Li1.20[Mn0.52Ni0.20Co0.08]O2, the binding energies of Mn2p and Co2p peaks for Li1.20[Mn0.50Zr0.02Ni0.20Co0.08]O2 have no obvious changes, indicating the chemical properties of the Mn and Co elements have not been changed after the Zr4+ doping modification.
To acquire the elements composition of Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) samples, the ICP test was adopted and the results is demonstrated in Table 2. The measurement values of Ni, Co, Mn and Zr elements content are approximately equal to the theoretical analysis values, indicating that the molar ratios for Ni, Co, Mn and Zr elements have been synthesized in accordance with the experimental requirements.
Figure 6 shows the initial charge-discharge curves of the Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) samples in the voltage range of 2.0~4.8 V at 0.1 C rate. All samples have demonstrated the similar charge curve for the two typical charge steps. The first step of charging process exists in the potential region from 2.0 V to 4.5 V, corresponding to the Li+-extraction from layer LiNi0.50Co0.20Mn0.30O2 component and the oxidation of Ni2+ to Ni4+ and Co3+ to Co4+ 31,32. For the second step, all samples exhibit a long voltage plateau at about 4.5 V, where the irreversible Li+ extract and oxygen release from the Li2MnO3 phase33,34. Table 3 shows the initial cycle electrochemical data of Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) cathodes at 0.1 C rate in the voltage range of 2.0~4.8 V. With the Zr4+ doping content increasing, the initial charge capacities of as-prepared samples gradually decline owing to the electrochemical inactive of doped Zr4+, While the discharge capacities first enhance and then decrease and the Li1.20[Mn0.50Zr0.02Ni0.20Co0.08]O2 sample delivers the highest discharge capacity of 272.4 mAh g−1. In addition, the lowest irreversible capacity loss for the Li1.20[Mn0.50Zr0.02Ni0.20Co0.08]O2 sample has promoted the highest initial coulombic efficiency, which indicates that the Zr4+ doping can restrain the release of oxygen from the Li2MnO3 and decrease the irreversible capacity loss. Compared to the bonds break energy values for theΔHƒ298(Ni-O) = 391.6 kJ∙mol−1, ΔHƒ298(Co-O) = 368 kJ∙mol−1 and ΔHƒ298(Mn-O) = 402 kJ∙mol−1, the Zr-O delivers the higher bonds break energy value ofΔHƒ298(Zr-O) = 760 kJ mol−1, therefore with the Zr4+ doping, the oxygen release of the Zr4+-doped samples will face more resistance than the un-doped sample, subsequently the irreversible capacity loss has been suppressed17.
Figure 7 shows the rate capabilities of the Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) samples with various current densities in the voltage range of 2.0~4.8 V. Obviously, the Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0.01, 0.02, 0.03) samples have all demonstrated the higher discharge capacities than those of the pristine Li1.20[Mn0.52Ni0.20Co0.08]O2 at the rate of 0.1 C, 0.2 C, 0.5 C, 1 C, 2 C and 5 C, thereinto the Li1.20[Mn0.50Zr0.02Ni0.20Co0.08]O2 sample delivers the optimum rate capacity. In addition, with current density increasing, the superiority has become particularly evident, indicating the advantage of Zr4+ doping on the rate capacity of Li1.20[Mn0.52Ni0.20Co0.08]O2 is much more significant at high rate. As is seen in Table 4, the discharge capacity of the Li1.20[Mn0.50Zr0.02Ni0.20Co0.08]O2 is only 8.8 mAh g−1 higher than that of the bare Li1.20[Mn0.52Ni0.20Co0.08]O2. However when the current density enhances to 5 C rate, the bare sample shows a discharge capacity of 86.6 mAh g−1 and this value is increased to 105.3, 114.7 and 108.6 mAh g−1 for the Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0.01, 0.02, 0.03) samples, respectively. The superior rate capacity of the Zr4+-doped Li1.20[Mn0.52Ni0.20Co0.08]O2 samples have mainly been attributed to the fast Li+ migration speed during the charge and discharge process. One reason is that with the Zr4+ doping, the larger lattice parameters of the Zr4+-doped samples have contributed to enhancing the Li+ diffusion speed. Besides, the better crystallization property of the Zr4+-doped samples will also help to strengthen the conductivity ability of ions and electrons during the charge-discharge process.
Figure 8 shows the cycling performance of the Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) samples at 0.5 C rate in the voltage range of 2.0~4.8 V at room temperature (25 °C). It can be observed that the Zr4+-doped samples have delivered the higher discharge capacity than that of the bare Li1.20[Mn0.52Ni0.20Co0.08]O2. And with the cycles going on, the cycling performance of the bare Li1.20[Mn0.52Ni0.20Co0.08]O2 is similar to those of the Zr4+-doped samples, the discharge capacities have all gradually attenuated followed the same trend. Table 5 shows the discharge capacity of Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) at 0.5 C rate in the voltage range of 2.0~4.8 V at 25 °C. With the Zr4+ doping content increasing, the initial discharge capacities are198.0, 202.0, 208.3 and 203.0 mAh g−1, respectively. And after 100 cycles, the corresponding capacity retentions still maintain 86.9%, 88.5%, 90.4% and 88.7%, respectively. It has proved that the Zr4+ doping modification can enhance the specific capacity and cycling performance of the Li1.20[Mn0.52Ni0.20Co0.08]O2 cathode, owing to the lower cation mixing and faster Li+ migration speed for the Zr4+-doped samples. Besides, the discharge voltage plateau will gradually decrease during the cyclic process, owing to the enlargement of polarization and the formation of spinel-like phase for cathode materials35. It can be observed that the discharge voltage drops to lower plateau for the all cathodes after different cycles, as the arrows pointed in Fig. 9. Table 5 shows the declining value of voltage plateau between 1st and 100th (ΔV) for the Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) samples and with the Zr4+ doping contents increasing, the ΔV values are 0.298, 0.259, 0.211 and 0.236 V, respectively. The smaller ΔV values of the Zr4+-doped cathodes have indicated that the Zr4+ doping modification can improve the layered structural stability by restraining the cation mixing between the Ni2+ and Li+ and the formation of spinel-like phase. While the smaller ΔV values of Zr4+-doped cathodes will contribute to maintaining the high power output of cells.
The poor cycling performance at high temperature for the Li-excess Li1.20[Mn0.52Ni0.20Co0.08]O2 has become one of the main drawbacks for the commercial application owing to the enhancement of the side reaction between cathode and electrolyte. Figure 10 shows the cycling performance of the Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) at 0.5 C rate in the voltage range of 2.0~4.8 V at 45 °C. In comparison with the cycling performance at room temperature, the bare Li1.20[Mn0.52Ni0.20Co0.08]O2 demonstrates the more severe capacity fading. During the early cycle period, the fast capacity attenuation can be observed owing to the bare cathode particles surface. After several cycles, the side reaction between the cathode and electrolyte can generate some by-product, which will deposit at the electrode/electrolyte interface to form the Solid Electrolyte Interface (SEI) film. And the SEI film will protect the cathode materials from erosion by the electrolyte, making the capacity attenuation slightly slow36,37. The initial discharge capacities are 221.8, 226.6 and 218.0 mAh g−1 for Zr4+-doped Li1.20[Mn0.52Ni0.20Co0.08]O2 electrodes with the doping contents of 0.01, 0.02 and 0.03, respectively, larger than that (208.3 mAh g−1) of the un-doped Li1.20[Mn0.52Ni0.20Co0.08]O2, as is seen in Table 6. After 100 cycles, with the Zr4+ doping contents increasing, the Zr4+-doped Li1.20[Mn0.52Ni0.20Co0.08]O2 samples exhibit the discharge capacity of 180.2, 190.7 and 176.9 mAh g−1 respectively, corresponding that the capacity retentions first enhance from 86.3% to 88.7% and then decline to 86.5%. As for the bare Li1.20[Mn0.52Ni0.20Co0.08]O2, the discharge capacity decreases acutely to 172.5 mAh g−1 with the capacity retention of only 82.8%. During the charge-discharge process at high temperature, the cathodes have suffered from the attack of HF, dissolution of the Mn ions, structural change and decomposition of electrolyte on the cathode surface38. While the stronger total metal–oxygen bonding for the Zr4+-doped samples can contribute to stabilizing the structure of cathode during cycling, leading to the improved cycling performance. However, when the Zr4+ doping content reaches to 0.03, the cycling performance of Li1.20[Mn0.49Zr0.03Ni0.20Co0.08]O2 is not as good as that of the Li1.20[Mn0.50Zr0.02Ni0.20Co0.08]O2 for that the inhomogeneity phase of the ZrO2 existed in the compound can hinder the Li+ intercalation/deintercalation from the cathode17.
To further understand the influence of Zr4+ doping on the electrochemical properties of Zr4+-doped Li1.20[Mn0.52Ni0.20Co0.08]O2, the electrochemical impedance spectroscopy (EIS) for the four samples have been carried out after charging to 4.5 V in the 1st, 30th cycles. Figure 11 shows the Nyquist curves of the four cathodes and all the Nyquist curves demonstrate the similar characteristics, containing a small semicircle in the high frequency, a large semicircle in the high to medium frequency and a quasi-straight line in the low frequency, which respectively correspond to the impedance of Li+ migration across the SEI film (R sf and CPE sf ), the impedance of charge transfer (R ct and CPE dl ) and the impedance of Li-ion migration in the cathode (Z W )39,40. The corresponding equivalent circuit in Fig. 10(e) is used to simulate the Nyquist curves and the corresponding Rs, Rsf and Rct values can be acquired, as is shown in Table 7. In the 1st cycle, the R sf values of Zr4+-doped Li1.20[Mn0.52Ni0.20Co0.08]O2 are lower than that of the bare one, therefore the superior initial discharge capacity can be obtained for the Zr4+-doped Li1.20[Mn0.52Ni0.20Co0.08]O2, which is in consistency with the results of Table 3. With the cycles going on, the SEI film will thicken, causing the increase of the R sf value. After 30 cycles, with the Zr4+ doping contents increasing, the Zr4+-doped Li1.20[Mn0.52Ni0.20Co0.08]O2 samples deliver the R sf values of 445.8, 363.1 and 428.8 Ω respectively, corresponding that the ΔR sf values first enhance from 287.1 to 210.8 and then drop to 284.9 Ω. As for the bare Li1.20[Mn0.52Ni0.20Co0.08]O2, the R sf value rise promptly to 544.5 Ω, with the ΔR sf value of 371.4 Ω. It indicates the samples after the Zr4+ doping can relieve the thickening of SEI films on the surface of cathode, which contributes to decreasing the Li+ migration resistance across the SEI films and enhancing the electrochemical properties. Besides, the Li+ diffusion rate (D Li +) in the cathode can be calculated using the following equations41:
where, F, n, A, C R is gas constant, T is the absolute temperature, F represents the Faraday constant, n is the number of electrons per molecule during oxidation, A corresponds to the area of the electrode-electrolyte interface, i.e. 1.13 cm2 and C is the concentration of lithium ion, respectively. Besides, τ W is the Warburg coefficient of the bulk cathode, which is can be calculated by the Eqs (2). Thereinto, the Zre is the real part of impedance, ω is the angular frequency42 and Fig. 12 shows the plots comparison of Zre vs. ω−1/2 for the Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.01, 0.02, 0.03) samples after 30 cycles. Thus τ W can be obtained from the linear fitting of Zre vs. ω−1/2.
According to Eqs (1) and (2), after 30 cycles, with the Zr4+ doping contents increasing, the Zr4+-doped Li1.20[Mn0.52Ni0.20Co0.08]O2 samples exhibit the DLi+ values of 3.61 × 10−14 cm2 s−1, 8.32 × 10−14 cm2 s−1 and 5.46 × 10−14 cm2 s−1 respectively, higher than that (7.63 × 10−15 cm2 s−1) of the pristine electrode. Therefore, the Zr4+-doped Li1.20[Mn0.52Ni0.20Co0.08]O2 samples have demonstrated the superior rate capacity.
Figure 13 shows the Raman spectra of original and cycled Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.02) (100 cycles at 45 °C). The Raman band at 427 cm−1 corresponds to the monoclinic Li2MnO3 phase, which can be observed in the spectrum of original Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.02) in Fig. 13(a) and disappear in the cycled electrode in Fig. 13(b) owing to the disappearance of the monoclinic Li2MnO3 component after cycling43. Besides, the other two significant Raman bands at 483 and 595 cm−1 for the Li1.20[Mn0.52Ni0.20Co0.08]O2 (481 and 593 cm−1 for the Li1.20[Mn0.50Zr0.02Ni0.20Co0.08]O2 owing to the Zr4+ doping) belong to the bending Eg and stretching A1g modes, respectively44 in Fig. 13(a). After 100 cycles, the Raman bands at 595 cm−1 for the Li1.20[Mn0.52−xZr x Ni0.20Co0.08]O2 (x = 0, 0.02) have both shifted to higher values, indicating the cathode structure transformation from the layered to defect spinel structure45. The Raman band for the Li1.20[Mn0.50Zr0.02Ni0.20Co0.08]O2 have shifted from 593 to 602 cm−1 after 100 cycles, much lower than that of the pristine Li1.20[Mn0.52Ni0.20Co0.08]O2 (from 595 to 615 cm−1). Therefore, the Zr4+ doping have restrained the layered-to-spinel phase change of Li1.20[Mn0.52Ni0.20Co0.08]O2 during cycling, forming the superior electrochemical properties by Zr doping.
Conclusions
In order to enhance the electrochemical properties of Li-excess Li1.20[Mn0.52Ni0.20Co0.08]O2, the different contents of Zr4+ have been doped into the pristine Li1.20[Mn0.52Ni0.20Co0.08]O2. After the Zr4+ doping, the cation mixing between Li+ and Ni2+ has been lowered and the cathode particles have been aggrandized. In comparison with the pristine cathode, the Zr4+-doped Li1.20[Mn0.52Ni0.20Co0.08]O2 samples have demonstrated the more stable cycling performance and higher rete capacities. Especially at high temperature (45 °C), the Zr4+ doping modification has delivered the more obvious superiority. After 100 cycles, with the Zr4+ doping contents increasing, the Zr4+-doped Li1.20[Mn0.52Ni0.20Co0.08]O2 samples exhibit the capacity retentions of 86.3%, 88.7% and 86.5% respectively, larger than that (82.8%) of the bare Li1.20[Mn0.52Ni0.20Co0.08]O2. The stronger total metal–oxygen bonding for the Zr4+-doped samples has mainly contributed to stabilizing the structure of cathode and improving the cycling performance. The Zr4+ doping modification has provided a potential approach to enhance the electrochemical properties of the Li-excess cathodes for Li-ion battery.
References
Chen, D. et al. Synthesis and performances of Li-Rich@AlF3@Graphene as cathode of lithium ion battery. Electrochim. Acta 193, 45–53 (2016).
Li, B. et al. Manipulating the electronic structure of Li-rich manganese-based oxide using polyanions: towards better electrochemical performance. Adv. Funct. Mater. 24(32), 5112–5118 (2014).
Chen, Z. et al. Surface-oriented and nano flake-stacked LiNi0.5Mn1.5O4 spinel for high-rate and long-cycle-life lithium ion batteries. J. Mater. Chem. 22, 17768–17772 (2012).
Chen, Z. et al. Building honeycomb-like hollow microsphere architecture in a bubble template reaction for high-performance lithium-rich layered oxide cathode materials. ACS Appl. Mater. Interfaces 9, 30617–30625 (2017).
He, Z. et al. Electrochemical performance of zirconium doped lithium rich layered Li1.2Mn0.54Ni0.13Co0.13O2 oxide with porous hollow structure. J. Power Sources 299, 334–341 (2015).
Yu, H. et al. High-energy cathode materials (Li2MnO3–LiMO2) for lithium-ion batteries. J. Phys. Chem. Lett. 4(8), 1268–1280 (2013).
He, Z. et al. A novel architecture designed for lithium rich layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 oxides for lithium-ion batteries. J. Mater. Chem. A 3(32), 16817–16823 (2015).
Mohanty, D. et al. Structural transformation of a lithium-rich Li1.2Co0.1Mn0.55Ni0.15O2 cathode during high voltage cycling resolved by in situ X-ray diffraction. J. Power Sources 229, 239–248 (2013).
Zhao, T. et al. Design of surface protective layer of LiF/FeF3 nanoparticles in Li-rich cathode for high-capacity Li-ion batteries. Nano Energy 15, 164–176 (2015).
Candace, S. et al. Aluminum borate coating on high-voltage cathodes for Li-ion batteries. J. Electrochem. Soc. 162, A2259–A2265 (2015).
Xu, M. et al. Highly crystalline alumina surface coating from hydrolysis of aluminum isopropoxide on lithium-rich layered oxide. J. Power Sources 281, 444–454 (2015).
Xu, M. et al. Mitigating capacity fade by constructing highly ordered mesoporous Al2O3/polyacene double-shelled architecture in Li-rich cathode materials. J. Mater. Chem. A 3, 13933–13945 (2015).
Zhao, J. et al. Synthesis and electrochemical characterization of Zn-doped Li-rich layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material. Ceram. Int. 41, 1–6 (2015).
Jin, X. et al. Excellent rate capability of Mg doped Li[Li0.2Ni0.13Co0.13Mn0.54]O2 cathode material for lithium-ion battery. Electrochim. Acta 136, 19–26 (2014).
Feng, X. et al. Enhanced electrochemical performance of Ti-doped Li1.2Mn0.54Co0.13Ni0.13O2 for lithium-ion batteries. J. Power Sources 317, 74–80 (2016).
Kim, S. H. et al. Improving the rate performance of LiCoO2 by Zr doping. J. Electroceram. 23, 254–257 (2009).
Wang, D. et al. Role of zirconium dopant on the structure and high voltage electrochemical performances of LiNi0.5Co0.2Mn0.3O2 cathode materials for lithium ion batteries. Electrochim. Acta 188, 48–56 (2016).
Luo, W. et al. The impact of Zr substitution on the structure, electrochemical performance and thermal stability of Li[Ni1/3Mn1/3−zCo1/3Zr z ]O2. J. Electrochem. Soc. 158, A428–A433 (2011).
Park, K. et al. Re-construction layer effect of LiNi0.8Co0.15Mn0.05O2 with solvent evaporation process. Sci. Rep. 7, 44557, https://doi.org/10.1038/srep44557 (2017).
Wang, D. et al. Polyaniline modification and performance enhancement of lithium-rich cathode material based on layered- spinel hybrid structure. J. Power Sources 293, 89–94 (2015).
Chen, Z. et al. Hierarchical Porous LiNi1/3Co1/3Mn1/3O2 Nano-/Micro Spherical Cathode Material: Minimized Cation Mixing and Improved Li+ Mobility for Enhanced Electrochemical Performance. Sci. Rep. 6, 25771, https://doi.org/10.1038/srep25771 (2016).
Kang, S. et al. Preparation and electrochemical performance of Yttrium-doped Li[Li0.20Mn0.534Ni0.133Co0.133]O2 as cathode material for Lithium-Ion batteries. Electrochim. Acta 144, 22–30 (2014).
Jafta, C. J. et al. Synthesis, characterisation and electrochemical intercalation kinetics of nanostructured aluminium-doped Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for lithium ion battery. Electrochim. Acta 85, 411–422 (2012).
Luo, W. et al. Synthesis, characterization and thermal stability of LiNi1/3Mn1/3Co1/3−zMg z O2, LiNi1/3−zMn1/3Co1/3Mg z O2 and LiNi1/3Mn1/3−zCo1/3Mg z O2. Chem. Mater. 22, 1164–1172 (2010).
Luo, W. et al. The impact of Zr substitution on the structure, electrochemical performance and thermal stability of Li[Ni1/3Mn1/3−zCo1/3Zr z ]O2. J. The Electrochem. Soc. 158(4), A428–A433 (2011).
Han, J. et al. Grain growth in Mn-doped ZnO. J. Eur. Ceram. Soc. 20, 2753–2758 (2000).
Gong, M. M. et al. Modeling the grain growth kinetics of doped nearly fully dense nanocrystalline ceramics. Ceram. Int. 43, 6677–6683 (2017).
Zhu, W. et al. The adhesion performance of epoxy coating on AA6063 treated inTi/Zr/V based solution. Appl. Surf. Sci. 384, 333–340 (2016).
Noh, M. et al. Optimized synthetic conditions of LiNi0.5Co0.2Mn0.3O2 cathode materials for high rate Lithium batteries via Co-precipitation method. J. Electrochem. Soc. 160, A105–A111 (2012).
Wang, D. et al. Improved high voltage electrochemical performance of Li2ZrO3-coated LiNi0.5Co0.2Mn0.3O2 cathode material. J. Alloy. Compd. 647, 612–619 (2015).
Lim, J.-M. et al. Intrinsic Origins of Crack Generation in Ni-rich LiNi0.8Co0.1Mn0.1O2 layered oxide cathode material. Sci. Rep. 7, 39669, https://doi.org/10.1038/srep39669 (2017).
Yu, R. B. et al. Investigation on the enhanced electrochemical performances of Li1.2Ni0.13Co0.13Mn0.54O2 by surface modification with ZnO. Electrochim. Acta 173, 515–522 (2015).
Liu, X. Y. et al. CaF2-coated Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials for Li-ion batteries. Electrochim. Acta 109, 52–58 (2013).
Xu, G. et al. Enhanced oxygen reducibility of 0.5Li2MnO3·0.5LiNi1/3Co1/3Mn1/3O2 cathode material with mild acid treatment. J. Power Sources 248, 894–899 (2014).
Wang, C. et al. Electrochemical properties of αMoO3-coated Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for Li-ion batteries. Electrochim. Acta 176, 1171–1181 (2015).
Zuo, X. et al. Effect of diphenyl disulfide as an additive on the electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2/graphite batteries at elevated temperature. Electrochim. Acta 245, 705–714 (2017).
Lee, J. N. et al. N-(triphenylphosphoranylidene) aniline as a novel electrolyte additive for high voltage LiCoO2 operations in lithium ion batteries. Electrochim. Acta 56, 5195–5200 (2011).
Liu, X. et al. Surface phase transformation and CaF2 coating for enhanced electrochemical performance of Li-rich Mn-based cathodes. Electrochim. Acta 163, 82–92 (2015).
Wang, Y. et al. High capacity spherical Li[Li0.24Mn0.55Co0.14Ni0.07]O2 cathode material for lithium ion batteries. Solid State Ionics 233, 12–19 (2013).
Xu, G. et al. Understanding the electrochemical superiority of 0.6Li[Li1/3Mn2/3]O2−0.4Li[Ni1/3Co1/3Mn1/3]O2 nano-fibers as cathode material for lithium ion batteries. Electrochim. Acta 173, 672–679 (2015).
Li, N. et al. Incorporation of rubidium cations into Li1.2Mn0.54Co0.13Ni0.13O2 layered oxide cathodes for improved cycling stability. Electrochim. Acta 231, 363–370 (2017).
He, Z. et al. Structural and electrochemical characterization of layered 0.3Li2MnO3·0.7LiMn0.35−x/3Ni0.5−x/3Co0.15−x/3Cr x O2 cathode synthesized by spray drying. Adv. Powder Technol. 25(2), 647–653 (2014).
Nayak, P. K. et al. Electrochemical and structural characterization of carbon coated Li1.2Mn0.56Ni0.16Co0.08O2 and Li1.2Mn0.6Ni0.2O2 as cathode materials for Li-ion batteries. Electrochim. Acta 137, 546–556 (2014).
Amalraj, F. et al. Synthesis of integrated cathode materials xLi2MnO3·(1−x) LiMn1/3Ni1/3Co1/3O2 (x = 0.3, 0.5, 0.7) and studies of their electrochemical behavior. J. Electrochem. Soc. 157, A1121–A1130 (2010).
Amalraj, F. et al. Study of the lithium-rich integrated compound xLi2MnO31(1−x)LiMO2 (x around 0.5; M = Mn, Ni, Co; 2:2:1) and its electrochemical activity as positive electrode in lithium cells. J. Electrochem. Soc. 160, A324–A337 (2013).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51604110, 51774135, U1361118, 51504093 and 51374003), Provincial Natural Science of Hunan (2017JJ3074), China Postdoctoral Science Foundation (2017M612558) and Research project of Hunan Provincial Education Department (17C0641).
Author information
Authors and Affiliations
Contributions
Y. Lu conceived and designed this work, M. Pang and S. Shi assisted the experiments. Q. Ye did the XRD refinement, Z. Tian did the SEM, LPS and XPS test and T. Wang performed the electrochemical properties measurement. Y. Lu wrote the manuscript. All authors discussed the results on the manuscript and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, Y., Pang, M., Shi, S. et al. Enhanced Electrochemical Properties of Zr4+-doped Li1.20[Mn0.52Ni0.20Co0.08]O2 Cathode Material for Lithium-ion Battery at Elevated Temperature. Sci Rep 8, 2981 (2018). https://doi.org/10.1038/s41598-018-21345-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-21345-6
This article is cited by
-
Effect of co-precipitation and solid-state reaction synthesis methods on lithium-rich cathodes Li1.2Ni0.2Mn0.6O2
Journal of Solid State Electrochemistry (2022)
-
Effect of Different Calcination Temperatures on the Structure and Properties of Zirconium-Based Coating Layer Modified Cathode Material Li1.2Mn0.54Ni0.13Co0.13O2
Acta Metallurgica Sinica (English Letters) (2022)
-
Superior electrochemical properties of Zirconium and Fluorine co‐doped Li1.20[Mn0.54Ni0.13Co0.13]O2 as cathode material for lithium ion batteries
Journal of Materials Science: Materials in Electronics (2021)
-
Al-doped Li1.21[Mn0.54Ni0.125Co0.125]O2 cathode material with enhanced electrochemical properties for lithium-ion battery
Applied Physics A (2020)
-
Improved electrochemical properties and thermal stability of Li1.20Mn0.54Ni0.13Co0.13O2 cathode material by Li2ZrO3 coating for Lithium-ion batteries
Journal of Materials Science: Materials in Electronics (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.