Abstract
Ageing and obesity are two major risk factors for the development of type 2 diabetes (T2D). A chronic, low-grade, sterile inflammation contributes to insulin resistance and β-cell failure. Toll-like receptor-4 (TLR4) is a major pro-inflammatory pathway; its ligands as well as downstream signals are increased systemically in patients with T2D and at-risk individuals. In the present study we investigated the combined effects of high fat/high sucrose diet (HFD) feeding, ageing and TLR4-deficiency on tissue inflammation, insulin resistance and β-cell failure. In young mice, a short-term HFD resulted in a mildly impaired glucose tolerance and reduced insulin secretion, together with a β-cell mass compensation. In older mice, HFD further deteriorated insulin secretion and induced a significantly impaired glucose tolerance and augmented tissue inflammation in adipose, liver and pancreatic islets, all of which was attenuated by TLR4 deficiency. Our results show that ageing exacerbates HFD-induced impairment of glucose homeostasis and pancreatic β-cell function and survival, and deteriorates HFD-induced induction of mRNA expression of inflammatory cytokines and pro-inflammatory macrophage markers. TLR4-deficiency protects against these combined deleterious effects of a high fat diet and ageing through a reduced expression of inflammatory products in both insulin sensitive tissues and pancreatic islets.
Similar content being viewed by others
Introduction
Type 2 Diabetes mellitus (T2D) is a chronic metabolic disorder characterized by insulin resistance, a progressive decline in pancreatic β-cell function and mass and subsequent hyperglycaemia; all of which is strongly associated with obesity. A chronic, low-grade, “sterile” inflammation is present in obesity, and pro-inflammatory mediators including cytokines and ROS/RNS cause insulin resistance in peripheral insulin sensitive tissues and lead to dysfunction and apoptosis of insulin-producing β-cells in pancreatic islets1,2.
The innate immunity and especially tissue macrophages contribute to such obesity-associated inflammation2,3. Pro-inflammatory macrophages (termed as M1 or classically activated macrophages) infiltrate insulin responsive tissues; the adipose tissue, liver as well as pancreatic islets, and outnumber homeostasis-maintaining and anti-inflammatory tissue-resident macrophages (termed as M2 or alternatively activated macrophages)4,5,6. This, over time, leads to a chronic low-grade tissue inflammation, subsequent insulin resistance and loss in compensatory adaptation of the pancreatic β-cells with progression to hyperglycemia and diabetes2,3.
Clinical as well as preclinical experimental studies show that Toll-like receptor 4 (TLR4) expression and activation is directly associated with obesity-induced tissue inflammation; abrogation of TLR4 is able to reverse insulin resistance and pancreatic β-cell dysfunction in experimental models7,8,9,10,11,12,13,14. Hyperlipidemia alone or in concert with hyperglycemia, termed as “lipoglucotoxicity” can induce a pro-inflammatory state, shown in fat, where elevated free fatty acids lead to impaired insulin sensitivity. In pancreatic islets, prolonged lipoglucotoxicity initiates a vicious cycle in β-cell destruction2,3. Both of them are shown to be mediated by TLR4 signaling4,6.
TLR4 is a member of the TLR family of pattern recognition receptors, and its signaling is one of the major pro-inflammatory pathways. Two tightly connected pathways in obesity activate TLR4 through specific ligands and result in the exacerbation of inflammation; elevated free fatty acids (FFA) as well as lipopolysaccharide (LPS)-linked to changes in gut microbiota. Three major ligands of TLR4; LPS, CXCL10 and FFA are systemically increased in patients with T2D as well as in at-risk individuals15,16,17,18. While LPS is the known classical ligand of TLR4, FFA stimulates TLR4 signalling19,20,21, but rather than directly bound to TLR4, it acts through the hepatokine fetuin-A22,23, which is also increased in obesity24 and independently associated with T2D25. Plasma LPS levels are increased in rodent models of obesity as well as in obese individuals, this metabolic endotoxemia could be due to increased intestinal permeability and enhanced LPS absorption by HFD26,27. Other described TLR4 ligands increased in T2D patients include HMGB1, hyaluronan, Hsp60/70 as well as S100A816,28.
Ageing is a major risk factor for the development of T2D, and is paralleled with developing glucose dys-homeostasis in both human and animal studies29,30. During ageing, a low-grade pro-inflammatory state has been observed with an elevation in pro-inflammatory cytokines, macrophages and superoxide products in fat, liver and pancreas, as well as in the circulation30,31,32,33,34,35. This may again be related to TLR4, as TLR4 mutant mice live longer, have stronger bones and muscles throughout their life36. In addition to diabetes, several other ageing-related diseases are mediated by increased inflammation through the pathological activation of TLR4, such as cardiovascular diseases, atherosclerosis, Alzheimer’s disease, arthritis and therefore, the term “inflamm-ageing” has been created to address such disease state with increased inflammation at an older age37.
Given this intersection of ageing, inflammation, TLR4 and diabetes, we hypothesized that ageing may have a potentiating effect upon obesity-induced tissue inflammation through TLR4, which would lead to an acceleration of the diabetes phenotype. Such possibility was addressed in the present study by short-term 8-week high fat/high sucrose diet-feeding of WT and TLR-4 knockout mice. We found that ageing could enhance diet-induced inflammatory cytokines in fat, liver and pancreatic islets, and aggravate impairment of glucose homeostasis and pancreatic β-cell dysfunction, which was prevented by TLR4-deficiency.
Results
Ageing further impairs high fat diet induced glucose intolerance and insulin resistance in old WT but not in TLR4 −/− mice
To evaluate whether ageing potentiates hyperglycemia in obesity, we fed WT C57BL/6 J mice a normal (ND) or a high fat/high sucrose diet (HFD, “Surwit”). After 8 weeks of HFD feeding, WT mice at an age of 14 weeks, when they are usually investigated, developed obesity and a slightly impaired glucose tolerance, which were severely potentiated in HFD fed older mice of 14 months (Fig. 1A,C). In contrast, glucose tolerance was almost uncompromised in Tlr4−/− mice of both ages (Fig. 1B,C).
Ageing further impairs high fat diet induced glucose intolerance and insulin resistance in old WT but not in Tlr4−/− mice (A–G) Young (6 weeks) and old (12 months) WT and Tlr4−/− mice were fed a normal (ND) or high fat/high sucrose diet (“Surwit”; HFD) for 8 weeks. (A–C) Intraperitoneal glucose tolerance test (ipGTT) with 1 g/kg body weight glucose of WT (A) and Tlr4−/− mice (B) and area-under-the-curve analysis by definite integrals of the same ipGTT results from the ND and HFD fed WT and Tlr4−/− mice after 8 weeks of diet (C). (D–G) Intraperitoneal insulin tolerance test (ipITT) of WT (D,F) and Tlr4−/− mice (E,G) with 0.75 IU/kg body weight insulin. Glucose levels were normalized to 100% before glucose injection (F,G). Data are means ±SE. *p < 0.05 ND vs. HFD; **p < 0.05 young vs. old mice; #p < 0.05 WT (F) vs. Tlr4−/− (G) mice. Due to the congested figure, we split the data from WT and Tlr4−/− mice used in the same experiments into two separate graphs (A/B, D/E, F/G). N = 12–15 mice per group; three independent experiments were performed.
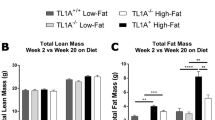
These data are in line with the impaired insulin tolerance in HFD fed aged mice. While young mice developed only slightly impaired insulin tolerance under the HFD feeding, compared to ND, insulin resistance worsened in the aged mice (Fig. 1D,F). In Tlr4−/− mice, insulin tolerance was unchanged -neither HFD nor ageing impaired insulin sensitivity during the 8-week feeding period (Fig. 1E,G). Body weight gain was significantly increased by the HFD, which was similar in both young and old WT and Tlr4−/− mice (Suppl. Fig. 1A,B). Also food intake was not affected by age or by genotype (Suppl. Fig. 1C,D).
HFD led to β-cell failure in aged mice, whereas TLR4-depletion could restore β-cell function and survival
Since HFD and ageing led to an impairment of both glucose and insulin tolerance, we tested whether this may also be a result of impaired β-cell function and survival. Fasted mice were injected with 2 g/kg glucose, and insulin secretion was measured before (0 min) and 30 min after glucose injection. In parallel to the HFD induced hyperglycemia (Fig. 1A), WT HFD fed young as well as old mice (but not Tlr4−/− mice) were hyperinsulinemic at the basal state (Fig. 2A), compared to ND mice.
HFD led to β-cell failure in aged mice, whereas TLR4-depletion could restore β-cell function and survival. (A–H) Young (6 weeks) and old (12 months) WT and Tlr4−/− mice were fed a normal (ND) or high fat/high sucrose diet (“Surwit”; HFD) for 8 weeks. (A,B) Insulin secretion during an ipGTT with 2 g/kg body weight glucose in week 8 measured before (0 min) and 30 min after glucose injection (A) and calculated as stimulatory index (B). (C–E) Mice were sacrificed at week 8 and islets isolated from all 8 treatment groups, cultured overnight and subjected to an in vitro GSIS assay. (C) Insulin secretion during 1h-incubation with 2.8 mM (basal) and 16.7 mM glucose (stimulated), normalized to (D) insulin content. (E) The insulin stimulatory index denotes the ratio of secreted/basal insulin during 1h-incubation with 16.7 mM and 2.8 mM glucose, respectively. (F) β-cell mass analysed from 10 sections/mouse spanning the whole pancreas. (G,H) Results and representative microscopic images from triple staining for TUNEL, insulin and DAPI expressed as percentage of TUNEL-positive β-cells ±SE. The mean number of β-cells scored was 10,252 for each treatment condition. (H) Arrows point to four TUNEL+ β-cells with remaining insulin from an old HFD fed WT mouse. Scale bar, 50 μm. *p < 0.05 ND vs. HFD; **p < 0.05 young vs. old mice; #p < 0.05 WT vs. Tlr4−/− mice. N = 5–9 mice per group; three independent experiments were performed.
The glucose stimulatory insulin secretion index was reduced in the young HFD mice and was fully abolished in the old HFD group, while it was fully maintained in Tlr4−/− mice (Fig. 2B). Similar data were obtained from an in vitro GSIS assay, in which islets from all 8 groups were isolated and plated on extracellular matrix coated dishes for one day of recovery. While basal insulin secretion was unaffected in all groups, glucose stimulatory index was reduced by the HFD in islets from young mice and completely deprived in islets from old HFD fed mice (Fig. 2C,E). Again, Tlr4−/− islets showed no impairment during the in vitro GSIS, and rescued high glucose induced insulin secretion in old mice fed a HFD compared to the wildtype littermates (Fig. 2C,E). Insulin content measured after the in vitro GSIS showed no significant changes among the 8 groups; except a significant increase in insulin content in the young Tlr4−/− mice in adaptation to the HFD (Fig. 2D).
Previously, we reported a compensatory increase in β-cell mass in mice during the first 8 weeks of HFD feeding38. This was again confirmed in this study; HFD feeding induced a compensatory increase in β-cell mass in young mice, while old mice were unable to increase β-cell mass in response to the HFD (Fig. 2F). In contrast, both young and old Tlr4−/− mice showed β-cell mass compensation (Fig. 2F). In line with the reduced β-cell mass, the number of apoptotic β-cells was increased in the old HFD fed mice (Fig. 2G), while apoptosis was significantly reduced in the old Tlr4−/− mice fed a HFD, compared to the WT mice of the same group (Fig. 2G,H).
HFD-induced inflammatory cytokine expression was enhanced in old mice and attenuated by TLR4-deficiency
Multiple studies have revealed that HFD feeding and obesity can induce chronic low-grade tissue inflammation in fat, liver and pancreatic islets. In this study, we attempted to investigate, whether ageing augments cytokine expression induced by HFD feeding. In young mice, 8 weeks of HFD feeding induced Il1b expression in adipose tissue (Fig. 3A) and Tnf expression in pancreatic islets (Fig. 3C). In old mice, HFD feeding induced expression of Il1b, Il6 and Ccl2 in fat (Fig. 3A), Il6, Tnf and Ccl2 in liver (Fig. 3B), Il1b in islets (Fig. 3C). While Il1b expression was already induced by HFD in fat of young mice, it was only induced in islets of older mice. Cytokine expression was also further accelerated in older mice on the HFD, especially in fat (e.g. Il6 and Ccl2), but also in the liver, which expressed more Tnf, compared to HFD fed young mice. In general in all investigated tissues, the expression of multiple inflammatory cytokines was elevated by a combination of HFD and ageing when compared with young mice fed the control normal chow diet (Fig. 3A–C). Ageing itself induced a pro-inflammatory phenotype under the ND; seen by the significantly increased Il6 expression in adipose tissue, but this was not observed in liver and islets (Fig. 3A–C).
HFD-induced inflammatory cytokine expression was enhanced in old mice and attenuated by TLR4-deficiency. (A–C) Young (6 weeks) and old (12 months) WT and Tlr4−/− mice were fed a normal (ND) or high fat/high sucrose diet (“Surwit”; HFD) for 8 weeks. RT-PCR analysis for inflammatory and anti-inflammatory genes with RNA extracted from fat (A), liver (B) and pancreatic islets (C) of mice treated under the indicated conditions. Y, young mice, O, old mice, ND, normal chow diet, HFD, high fat/high sucrose diet, WT, wildtype mice, Tlr4−/−, TLR4-knockout mice. All results are normalized to control young-ND, which is arbitrarily set as 1. Data are presented as means ± SE from n = 5–8 mice. *p < 0.05 ND vs. HFD; **p < 0.05 young vs. old mice; #p < 0.05 WT vs. Tlr4−/− mice, §p < 0.05 young ND vs. old HFD mice.
Analysis of the anti-inflammatory cytokines Il10, Tgfb and Il4 revealed that ageing together with HFD reduced Il10 expression in liver and islets (Fig. 3A–C, Suppl. Fig. 2), while Tgfb levels remained unchanged under all conditions in fat and liver (Fig. 3A,B) and Il4 was only detectable in liver but was unchanged by HFD and ageing (though TLR4 knockout showed a profound Il4 induction in old HFD fed mice compared to their WT counterparts (Suppl. Fig. 2)).
Next, we checked if TLR4-deficiency affected such ageing/HFD induced cytokine expression and thus may explain the protective effect of TLR4 inhibition on glycemia, β-cell function and survival. Overall, TLR4-depletion had a tendency to reduce ageing/HFD induced pro-inflammatory cytokine expression (Fig. 3A–C). One exception is Il6 and Tnf expression in liver, where was no change between Tlr4−/− mice and WT mice, neither at ageing nor at HFD condition or both (Fig. 3B). Similarly, Il10 expression was unchanged in fat and islets by TLR4-depletion, while it was increased together with Il4 by TLR4-deficiency in the liver of old HFD mice (Fig. 3A,B and Suppl. Fig. 2).
These results indicate a very complex regulation of different cytokines in the insulin responsive and insulin-producing tissues, and that TLR4 is an important but unlikely the only pathway to initiate such cytokine expression pattern.
Combined HFD-feeding and ageing shifted tissue macrophage polarization to a more pro-inflammatory phenotype
Since accumulation and classical activation of macrophages contributes to a pro-inflammatory phenotype observed in insulin sensitive and insulin-producing tissues under HFD feeding, we then analyzed macrophage markers in aged mice fed either ND or HFD. As general macrophage markers, we measured Cd68, Emr1 (F4/80) and the pan-myeloid marker Cd11b (Itgam). We measured Cd11c (Itgax) as M1 macrophage marker12,33,39,40 and Cd206 (mannose receptor, Mrc1) and Arg1 (arginase 1) as M2 macrophage markers9,12,40,41,42. Comparison of young and old mice fed a ND revealed that ageing alone did not significantly change macrophage accumulation and polarization (Fig. 4A–C). Paralleled with the induction of inflammatory cytokines, the expression of general macrophage markers significantly increased by the combination of HFD and ageing in all tested tissues (Fig. 4A–C). In contrast, M2 markers showed the opposite; reduced Mrc1 and Arg1 expression, coinciding with the anti-inflammatory cytokine Il10 (Fig. 3A–C), though detailed analysis revealed differences among tissues (Fig. 4A–C). Unlike in aged mice, the general macrophage markers were not uniformly induced by the 8-week HFD in young mice, only Cd68 was increased in the liver (Fig. 4A–C).
Combined HFD-feeding and ageing shifted tissue macrophage polarization to a more pro-inflammatory phenotype. (A–C) Young (6 weeks) and old (12 months) mice WT and Tlr4−/− mice were fed a normal (ND) or high fat/high sucrose diet (“Surwit”; HFD) for 8 weeks. RT-PCR analysis for general macrophage and macrophage polarization markers with RNA extracted from fat (A), liver (B) and pancreatic islets (C) of mice treated under the indicated conditions. Y, young mice, O, old mice, ND, normal chow diet, HFD, high fat/high sucrose diet, WT, wildtype mice, Tlr4−/−, TLR4-knockout mice. All results are normalized to control young-ND, which is arbitrarily set as 1. Data are presented as means ± SE from n = 5–8 mice. *p < 0.05 ND vs. HFD; **p < 0.05 young vs. old mice; #p < 0.05 WT vs. Tlr4−/− mice, §p < 0.05 young ND vs. old HFD mice.
Again, while an 8-week HFD regime triggers a minimal inflammatory profile in insulin responsive tissues and insulin-producing islets, ageing could potentiate HFD’s effect and augment tissue inflammation, macrophage accumulation and inflammatory activation.
Unlike the reduction in pro-inflammatory cytokine expression, TLR4 deficiency didn’t influence gene expression of macrophage accumulation and polarization in a universal manner. HFD induced Itgax expression was completely blocked in the liver and fat (Fig. 4A–C), and anti-inflammatory macrophage marker Arg1 expression in liver of HFD fed old mice was enhanced by TLR4-deficiency (Fig. 4A–C), which is in line with the increased Il4 and Il10 expression in the same setting (Fig. 3B and Suppl. Fig. 2). The results above demonstrate that TLR4-deficiency could attenuate inflammatory macrophages in fat and liver, in addition to restoring M2-like macrophage polarization in the liver of old mice, both of which could contribute to prevent HFD-induced inflammation in old mice.
Experimental Procedures
Animals
C57BL/6 J (wild type; WT) and C57BL/10ScCr (TLR4 knockout; Tlr4−/−)43 male mice comprising of young (6 weeks) and old (12 months) mice were obtained from Jackson Laboratories (Bar Harbor, ME) and separated into 4 groups. Half of the groups were fed a normal diet (ND) (Harlan Teklad Rodent Diet 8604 containing 12.2, 57.6, and 30.2% calories from fat, carbohydrate, and protein, respectively; Harlan Teklad, Madison, WI) or a high fat/high sucrose diet (HFD) (“Surwit,” containing 58, 26, and 16% calories from fat, carbohydrate, and protein, respectively44; Research Diets, Inc., New Brunswick, NJ) for 8 weeks. Body weight and food intake were measured weakly during the study. All mice were housed in a temperature-controlled room with a 12 hours light, 12 hours dark cycle, and were allowed free access to food and water according to the protocol approved by the “Bremen Senate of Health” (the Institutional Animal Care and Use Committee) in agreement with the §8 of the German animal protection law. All methods were carried out in accordance with the guidelines and regulations of the Institutional Animal Care and Use Committee.
Metabolic tests
For intraperitoneal glucose tolerance tests (ipGTT), mice were fasted 12 h overnight and injected i.p. with glucose (40%; B. Braun, Melsungen, Germany) at a dose of 1 g/kg body weight. Blood samples were obtained from the tail vein at time points 0, 15, 30, 60, 90 and 120 min for glucose measurements using a glucometer. Insulin secretion was measured before (0 min) and after (30 min) i.p. injection of glucose (2 g/kg body weight) with plasma taken from retro-orbital blood puncture using ultrasensitive mouse Elisa kit (ALPCO Diagnostics, Salem, NH) as described before45. For intraperitoneal insulin tolerance tests (ipITT), mice were fasted for 4 h and intraperitoneally injected with 0.75 IU/kg body weight of recombinant human insulin (InsHuman Rapid, Aventis, Germany) and blood glucose was measured 0, 15, 30, 60 and 90 minutes post injection.
Mouse tissue isolation
After 8 weeks of diet, mice were sacrificed and islets were isolated by pancreas perifusion with LiberaseTM (Roche, Mannheim, Germany) according to the manufacturer’s instructions and digested for 10 minutes at 37 °C as previously described45. Islets were purified by a density gradient of Histopaque (1:1; 1077 and 1119, Sigma-Aldrich, Steinheim, Germany) and subsequent hand-picking. High purity islets were cultured overnight in RPMI 1640 medium containing 11.1 mM glucose (Lonza, Basel, Switzerland), followed by pelleting islets and adding Trizol (PEQLAB GmbH, Erlangen, Germany) for RNA extraction. Liver and epididymal white adipose tissue (WAT) were cut into small pieces in RNAlater solution (Sigma-Aldrich, Steinheim, Germany) and then shaken at 4 °C overnight, followed by homogenization and addition of Trizol for RNA extraction.
Immunohistochemical analysis
Mouse pancreata were isolated and fixed with 4% PFA for 8 h at 4 °C and then paraffin embedded and cut into 4 μm sections. The slides were deparaffinized and immunostaining was carried out after heat antigen-retrieval45. Sections were incubated with anti-insulin (DAKO, Glostrup, Denmark; A0546, 1:100) for 12 h at 4 °C, and secondary antibody (biotin anti-guinea pig, 1:100; Jackson ImmunoResearch, PA) for 1 h at RT and thereafter with Vectastain ABC solution for 1 h at RT and DAB (HRP substrate kit, brown; both from Vector Laboratories, Burlingame, CA, USA) for 10 min. For morphometric analysis, ten sections (spanning the width of the pancreas) per mouse were analyzed as described before45. Pancreatic tissue area and insulin-positive area were determined by computer-assisted measurements using a Nikon MEA53200 (Nikon GmbH, Dusseldorf, Germany) microscope and images were acquired using NIS-Elements software (Nikon GmbH, Dusseldorf, Germany). Mean percent β-cell fraction per pancreas was calculated as the ratio of insulin-positive and whole pancreatic tissue area. β-cell mass was obtained by multiplying the β cell fraction by the weight of the pancreas. β-cell apoptosis was analyzed by the TUNEL technique according to the manufacturer’s instructions (In situ Cell Death Detection Kit, TMR red; Roche/now distributed by Sigma-Aldrich, Steinheim, Germany) and double stained for insulin, followed by FITC anti-guinea pig secondary antibody (Jackson).
Glucose-stimulated insulin secretion (GSIS) in vitro
For acute insulin release in response to glucose, primary mouse islets were washed and pre-incubated (30 min) in Krebs-Ringer bicarbonate buffer (KRB) containing 2.8 mM glucose and 0.5% BSA. KRB was then replaced by KRB 2.8 mM glucose for 1 h (basal), followed by an additional 1 h in KRB 16.7 mM glucose (stimulated). Thereafter, islets were washed with PBS and extracted with HCl (0.18 N) in 70% ethanol overnight at 4 °C. The acid-ethanol extracts were collected for determination of insulin content. Insulin was determined using mouse insulin ELISA (ALPCO Diagnostics, Salem, NH). Secreted insulin was normalized to total insulin content.
RNA extraction and quantitative RT-PCR analysis
Total RNA was isolated from mouse islets, liver and WAT with a Trizol extraction system (TriFast, PEQLAB GmbH, Erlangen, Germany). cDNA synthesis and quantitative RT-PCR was performed as previously described45. The Applied Biosystems StepOne Real-Time PCR system (Applied Biosystems, CA) with TaqMan® Fast Universal PCR Master Mix for TaqMan assays (Applied Biosystems, CA) was used for analysis. Cyclophilin (PPIA) and β-Actin (ACTB) were used as internal housekeeping controls and the quantitative analysis was performed with the ΔΔCT method. The following TaqMan® Gene Expression Assays were used: Ppia (Mm03024003_g1), Actb (Mm00607939_s1), Il1b (Mm00434228), Il6 (Mm00446190), Tnf (Mm00443258_m1), Ccl2 (Mm00441242_m1), Il4 (Mm00445259_m1), Il10 (Mm00439614_m1), Tgfb (Mm01178820_m1), Cd68 (Mm03047343_m1), Emr1 (F4/80) (Mm00802529_m1), Itgam (CD11b) (Mm00434455_m1), Itgax (CD11c) (Mm00498698_m1), Mrc1 (CD206) (Mm00485148_m1), Arg1 (Mm00475988_m1).
Statistical analysis
All values were expressed as means ± SE with the number of independent individual experiments (in vitro; biological replicates) or the number of mice (in vivo) presented in the figure legends. The different groups were compared by two-way ANOVA with Bonferroni post-tests. P value < 0.05 was considered statistically significant.
Discussion
The present study identifies a deleterious potentiation of impaired glucose homeostasis, β-cell dysfunction and chronic tissue inflammation by the combination of obesity and ageing. Data from our HFD/ageing mouse model support the concept that obesity with ageing leads to further deterioration in blood glucose regulation, which is likely due to a reduced capacity to balance inflammatory genes at an older age.
While our previous studies investigated the effect of chronic HFD feeding on glucose homeostasis and stages of β-cell adaptation, compensation and failure46, in this study we exposed the mice with the HFD for a relatively short term of 8 weeks, which only slightly impaired glucose and insulin tolerance in young mice of around 14 weeks, an age when they are usually investigated, but this was apparently exacerbated in older mice of 14 months of age.
Results from the in vivo GSIS displayed that β-cell function was impaired by the HFD regardless of age, but the definite amount of secreted insulin was only reduced in aged mice, while young mice could compensate for the increased insulin demand at a mildly insulin resistant stage. This suggests that β-cells from young mice keep a sufficient plasticity to maintain its insulin secretion function. This is also reflected by the changes in β-cell mass and in line with previous data showing that the ability of β-cells to proliferate is lost during ageing47,48. Young but not old mice respond to a HFD with β-cell mass expansion to meet the increased insulin demand. Such differences in the β-cell expansion capacity are not only observed with ageing but also with duration of diet. While high fat diet feeding for up to 8 weeks results in β-cell mass expansion, such adaptive increase in β-cell mass is not observed any more after 12 weeks38. This also correlates with β-cell apoptosis, which is only seen after longer periods of HFD feeding45, while after 8 weeks, HFD feeding does not result in changes of β-cell survival in young mice, but induces β-cell apoptosis in old mice. Such effects are certainly also dependent on the diet composition, as an even higher carbohydrate content in the diet (35% of calorie intake) can already severely impair glucose homeostasis in young mice49. While the diabetogenic “Surwit diet” of 58, 16 and 26% calories from fat, protein and carbohydrate, respectively44, disrupts insulin secretion later in life, β-cells can compensate for this in young mice. Their survival is not significantly affected yet, and there is only minor deterioration in glucose and insulin tolerance. This is reminiscent of our earlier study in human islets ex vivo, which shows that β-cell survival per se is not impaired in older individuals, but in response to diabetogenic stimulation, such as HFD or hyperglycemia, apoptosis is accelerated47. In aged HFD mice, there is a significantly elevated basal insulin secretion, and subsequently insulin secretion cannot be further induced in response to glucose during in vivo GSIS, which was just recently confirmed50. Such elevated basal insulin level is mainly due to insulin resistance and elevated FFA levels during an obese insulin resistant stage. The improvement in the β-cell stimulatory index by TLR4 deficiency is mainly attributed to the normalization in basal secretion. Interestingly, despite no obvious impairment in insulin sensitivity by the HFD in TLR4-KO mice, there is a compensatory increase in β-cell mass and insulin content, which suggests that such adaptation may be independent of insulin sensitivity.
In an in vitro GSIS assay, in which the effect of insulin resistance can be ruled out, basal insulin levels were similar and glucose stimulated insulin secretion reduced in young HFD mice, and fully abolished in old HFD mice, suggesting the secretory function is also compromised, while TLR4 deficiency protected the islets from such functional depletion. Such obvious loss in the secretory function was also observed in islets from senescent (21–22-month old) Fisher rats, compared to young rats (4–5-month old)51. Also in 7–8-month old Wistar rats, insulin production as well as secretion is impaired47, which is attributed at least in part to the reduction in PDX147,52, the factor for glucose mediated insulin production in mature β-cells. Other factors, which lead to an almost complete decline in β-cell regeneration in ageing are the increased expression of the cell cycle inhibitor P1648, which is initiated by decreased Bmi-1 binding to the Ink4a/Arf locus53 and by decreased Ezh254; both increase P16, and thus disable β-cell proliferation. As cell cycle and senescence markers have been identified in islets during ageing, we specifically focused here on markers of the inflammatory response; not only in islets but also in insulin responsive tissues. Overall, our study indicates that a mild ageing itself doesn’t induce β-cell functional impairment and survival, whereas it can potentiate the adverse effects of a short-term HFD.
“Sterile” chronic, low-grade inflammation without any obvious infection is a common feature of ageing, and people over the age of 65 have increased serum levels of IL-6, TNF, and IL-1855,56. Similarly, in rodent models of ageing, IL-1β, IL-6, MCP-1 (CCL2), TNF and IL-12b increase in fat and liver31,33,34. With respect to the pancreas, oxidative stress increases in aged mouse pancreases per se at the age of 14–16 months32 and TNF expression is elevated in pancreatic acinar cells in female mice aged 18–19 months35. In the present study, however, a pro-inflammatory cytokine expression by ageing alone was only seen in fat, but not in liver and islets, though differences in age, species and strains exist among this and other studies. We show that ageing alone neither induced metabolic deterioration nor an overall activated inflammatory state in metabolically active tissues.
Along with inflammatory cytokines, lipotoxicity contributes to insulin resistance and β-cell dysfunction through oxidative stress57. Free fatty acids activate TLR4, which further downstream leads to ROS/RNS production58. This is likely another mechanism, besides the inhibition of inflammation, by which TLR4 depletion ameliorated glucose homeostasis, β-cell function and survival in aged HFD fed mice in this study, even though we have not addressed such possible mechanism.
To the question whether the number of macrophages increase in tissues during ageing, several studies reported macrophage accumulation in fat, liver and/or pancreas of aged mice or rats30,31,34, although this was not confirmed by others32,33. In our current study, based on gene expression of accepted markers, neither macrophage accumulation nor their polarization status was changed in older mice in any of the three tissues, which is in line with the unchanged tissue inflammation. Mutually contradictive results were obtained from various studies, where down-regulation in both M1 and M2 polarization markers59, increased M2 macrophages60, or an overall macrophage polarization towards the M1 type during aging were observed33. The problem is that different age groups were used in these studies, with young animals ranging from 1–6 months and old animals ranging from 12–24 months age, thereby no corresponding correlation between an older age and worsening of the inflammatory phenotype could be drawn.
HFD feeding in the young mice for a short period of 8 weeks did not induce a full cytokine response at the mRNA level. The first elevated cytokines in response to the HFD were Il1b in fat and Tnf in islets. Since we didn’t observe compromised glucose tolerance, the very low-grade inflammation in young mice is consistent with the consensus that inflammation precedes hyperglycemia. In contrast, insulin secretion in young mice, tested by GSIS in vivo as well as in vitro, was already affected at this stage, together with the β-cell mass compensation response. Tnf was the only cytokine, which was induced in pancreatic islets by the short HFD feeding in the young mice, and this could act as mediator of β-cell dysfunction. This is in line with a previous study; while TNF does not affect β-cell apoptosis, it blunts GSIS from sorted β-cells61. Such results point to the possibility of early detrimental effects on β-cell function mediated by TNF. Especially, TNF is known to trigger insulin resistance and was also highly elevated at an insulin resistant stage in liver and fat in the old HFD mice. Thus, the results of this study also support the strategy to target TNF for the treatment of insulin resistance and β-cell failure62.
Neither ageing nor short term HFD itself induced severe hyperglycemia or massive changes in the cytokine pattern. But the combination of both synergistically induced inflammation in all insulin responsive and insulin secreting tissues- fat, liver and pancreatic islets. Along with hyperglycemia, insulin resistance, fully abolished insulin secretion and β-cell apoptosis, tissues were more inflamed in old HFD mice than in young, including a more and stronger pro-inflammatory and reduced anti-inflammatory cytokine expression. One limitation of this study is, that we only assessed mRNA levels of inflammatory products, which allowed quantitative analysis of cytokines at a very low expression levels. Moreover, cytokines are unstable and degrade rapidly, and thus often lay under the assay detection range, which makes their assessment on a protein level in tissues difficult.
Consistent with cytokine profiles, we found that only the combination of ageing and HFD feeding could increase the overall macrophage accumulation, again in line with the finding that ageing could potentiate HFD-induced gene expression of inflammatory cytokines, of markers of pro-inflammatory macrophages, along with a reduction in anti-inflammatory macrophage markers in fat and islets, metabolic dysfunction and β-cell failure.
A scenario emerges, how the HFD-ageing duo affects glucose metabolism: young mice are responsive to HFD with mildly increased macrophages in metabolism active tissues. This contributes to a mild inflammatory cytokine production and, in turn, results in an impairment of β-cell function. However, β-cells are still resilient to maintain compensation and glucose homeostasis. When mice get older, the same short-term diet stress not only increases M1-like macrophages but also attenuates M2-like macrophage activation, which further imbalances the macrophage phenotype and brings deterioration in the inflammatory status with more pro- and less anti-inflammatory cytokines. This then may lead to insulin resistance and compromised β-cell function, and in combination with reduced β-cell proliferation and increased β-cell apoptosis during ageing, it will finally result in definite insulin deficiency and concomitant hyperglycemia.
Being a crucial pattern recognition receptor and key player in inflammation, TLR4 is involved in many aspects of the pathogenesis of T2D, at the level of both β-cells and insulin responsive tissues7,8,10,13. As we aimed to identify the contribution of TLR4 on whole body glucose metabolism, we used whole-body TLR4-KO mice. This strategy, however, restrained the identification of the primary tissues affected by TLR4 signals. The generation of mouse models with tissue specific TLR4 re-expression in adipocytes/hepatocytes/β-cells/macrophages, respectively, on a TLR4-KO C57BL/10ScCr background would allow characterization of tissue specific effects, as well as a proof of a TLR4 specific effect upon its re-expression.
Tissue specific TLR4 effects have been studied in the past and confirmed observation from global deletions, e.g. myeloid-specific (as well as global) TLR4-deficiency improves insulin sensitivity and inhibits obesity-induced tissue inflammation in HFD and lipid infusion models7,11,22,63,64. However, in TLR4-deficienct mice, both reduced7,11,65 and unchanged ATM accumulation has been reported9,64. Notably, Orr et al. found that TLR4-depletion promoted M2 polarization in fat9. Similarly, Jia et al. observed that myeloid-specific Tlr4−/− had a trend to promote macrophage alternative activation in fat together with induced IL-10 production12.
Featuring the combinational effect of a mild HFD and ageing, our results in insulin-responsive and insulin-producing tissues from old HFD mice indicate an overall trend that TLR4-deficiency reduces mRNA expression of inflammatory cytokines and M1 macrophage markers, and additionally promotes alternative macrophage activation specifically in the liver. This in vivo study is also in line with previous ex vivo studies13,66; TLR4 activation in isolated islets induces cytokine expression, impairs glucose-stimulated insulin secretion and increases β-cell apoptosis. This is again supportive for the role of TLR4 activation in diabetes progression.
In summary, we found that ageing aggravated diet-induced impairment on glucose homeostasis, pancreatic β-cell function and survival and enhanced gene expression of inflammatory products in fat, liver and pancreatic islets in a HFD-fed mouse model. TLR4-deficiency exhibited protection against such deleterious effects through inhibiting pro-inflammatory cytokine expression and modulating tissue macrophage activation to a more anti-inflammatory phenotype. Ageing and obesity synergistically induce diabetes through TLR4, supporting the therapeutic potential of TLR4 inhibition to treat T2D.
References
Wellen, K. E. & Hotamisligil, G. S. Inflammation, stress, and diabetes. The Journal of clinical investigation 115, 1111–1119 (2005).
Donath, M. Y. & Shoelson, S. E. Type 2 diabetes as an inflammatory disease. Nature reviews. Immunology 11, 98–107 (2011).
Osborn, O. & Olefsky, J. M. The cellular and signaling networks linking the immune system and metabolism in disease. Nature medicine 18, 363–374 (2012).
Hill, A. A., Reid Bolus, W. & Hasty, A. H. A decade of progress in adipose tissue macrophage biology. Immunological reviews 262, 134–152 (2014).
Kraakman, M. J., Murphy, A. J., Jandeleit-Dahm, K. & Kammoun, H. L. Macrophage polarization in obesity and type 2 diabetes: weighing down our understanding of macrophage function. Frontiers in immunology 5, 470 (2014).
Morris, D. L. Minireview: Emerging Concepts in Islet Macrophage Biology in Type 2 Diabetes. Molecular endocrinology 29, 946–962 (2015).
Shi, H. et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. The Journal of clinical investigation 116, 3015–3025 (2006).
Mehta, N. N. et al. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes 59, 172–181 (2010).
Orr, J. S. et al. Toll-like receptor 4 deficiency promotes the alternative activation of adipose tissue macrophages. Diabetes 61, 2718–2727 (2012).
Amyot, J., Semache, M., Ferdaoussi, M., Fontes, G. & Poitout, V. Lipopolysaccharides impair insulin gene expression in isolated islets of Langerhans via Toll-Like Receptor-4 and NF-kappaB signalling. PLoS One 7, e36200 (2012).
Saberi, M. et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell metabolism 10, 419–429 (2009).
Jia, L. et al. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nature communications 5, 3878 (2014).
Nackiewicz, D. et al. TLR2/6 and TLR4-activated macrophages contribute to islet inflammation and impair beta cell insulin gene expression via IL-1 and IL-6. Diabetologia 57, 1645–1654 (2014).
Li, J. et al. TLR4 is required for the obesity-induced pancreatic beta cell dysfunction. Acta biochimica et biophysica Sinica 45, 1030–1038 (2013).
Creely, S. J. et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. American journal of physiology. Endocrinology and metabolism 292, E740–747 (2007).
Dasu, M. R., Devaraj, S., Park, S. & Jialal, I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes care 33, 861–868 (2010).
Herder, C. et al. Chemokines as risk factors for type 2 diabetes: results from the MONICA/KORA Augsburg study, 1984-2002. Diabetologia 49, 921–929 (2006).
Schulthess, F. T. et al. CXCL10 impairs beta cell function and viability in diabetes through TLR4 signaling. Cell metabolism 9, 125–139 (2009).
Hwang, D. Modulation of the expression of cyclooxygenase-2 by fatty acids mediated through toll-like receptor 4-derived signaling pathways. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 15, 2556–2564 (2001).
Lee, J. Y., Sohn, K. H., Rhee, S. H. & Hwang, D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. The Journal of biological chemistry 276, 16683–16689 (2001).
Nguyen, M. T. et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. The Journal of biological chemistry 282, 35279–35292 (2007).
Pal, D. et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nature medicine 18, 1279–1285 (2012).
Gerst, F. et al. Metabolic crosstalk between fatty pancreas and fatty liver: effects on local inflammation and insulin secretion. Diabetologia (2017).
Brix, J. M. et al. Elevated Fetuin-A concentrations in morbid obesity decrease after dramatic weight loss. The Journal of clinical endocrinology and metabolism 95, 4877–4881 (2010).
Stefan, N. et al. Impact of the adipokine adiponectin and the hepatokine fetuin-A on the development of type 2 diabetes: prospective cohort- and cross-sectional phenotyping studies. PloS one 9, e92238 (2014).
Cani, P. D. et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 (2008).
Cani, P. D., Delzenne, N. M., Amar, J. & Burcelin, R. Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathologie-biologie 56, 305–309 (2008).
Kuwabara, T. et al. Macrophage-mediated glucolipotoxicity via myeloid-related protein 8/toll-like receptor 4 signaling in diabetic nephropathy. Clinical and experimental nephrology 18, 584–592 (2014).
Meneilly, G. S. & Tessier, D. Diabetes in elderly adults. The journals of gerontology. Series A, Biological sciences and medical sciences 56, M5–13 (2001).
Almaca, J. et al. Young capillary vessels rejuvenate aged pancreatic islets. Proceedings of the National Academy of Sciences of the United States of America 111, 17612–17617 (2014).
Horrillo, D. et al. Age-associated development of inflammation in Wistar rats: Effects of caloric restriction. Archives of physiology and biochemistry 117, 140–150 (2011).
Yang, T. et al. Abrogation of adenosine A1 receptor signalling improves metabolic regulation in mice by modulating oxidative stress and inflammatory responses. Diabetologia 58, 1610–1620 (2015).
Lumeng, C. N. et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. Journal of immunology 187, 6208–6216 (2011).
Lin, L. et al. Ghrelin receptor regulates adipose tissue inflammation in aging. Aging 8, 178–191 (2016).
Xiong, Y. et al. Arginase-II Promotes Tumor Necrosis Factor-alpha Release from Pancreatic Acinar Cells Causing beta-Cell Apoptosis In Aging. Diabetes (2017).
Johnson, G. B., Riggs, B. L. & Platt, J. L. A genetic basis for the “Adonis” phenotype of low adiposity and strong bones. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 18, 1282–1284 (2004).
Salvioli, S. et al. Inflamm-aging, cytokines and aging: state of the art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Current pharmaceutical design 12, 3161–3171 (2006).
Meyer, A. et al. Manganese-mediated MRI signals correlate with functional beta-cell mass during diabetes progression. Diabetes 64, 2138–2147 (2015).
Fink, L. N. et al. Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans. Obesity 22, 747–757 (2014).
Han, M. S. et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 339, 218–222 (2013).
Odegaard, J. I. et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 (2007).
Odegaard, J. I. et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell metabolism 7, 496–507 (2008).
Coutinho, A., Forni, L., Melchers, F. & Watanabe, T. Genetic defect in responsiveness to the B cell mitogen lipopolysaccharide. European journal of immunology 7, 325–328 (1977).
Surwit, R. S. et al. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism: clinical and experimental 44, 645–651 (1995).
Ardestani, A. et al. MST1 is a key regulator of beta cell apoptosis and dysfunction in diabetes. Nature medicine 20, 385–397 (2014).
Sauter, N. S., Schulthess, F. T., Galasso, R., Castellani, L. W. & Maedler, K. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology 149, 2208–2218 (2008).
Maedler, K. et al. Aging correlates with decreased beta-cell proliferative capacity and enhanced sensitivity to apoptosis: a potential role for Fas and pancreatic duodenal homeobox-1. Diabetes 55, 2455–2462 (2006).
Tschen, S. I., Dhawan, S., Gurlo, T. & Bhushan, A. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes 58, 1312–1320 (2009).
Montgomery, M. K. et al. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 56, 1129–1139 (2013).
Aguayo-Mazzucato, C. et al. beta Cell Aging Markers Have Heterogeneous Distribution and Are Induced by Insulin Resistance. Cell metabolism 25, 898–910 e895 (2017).
Wang, S. Y., Halban, P. A. & Rowe, J. W. Effects of aging on insulin synthesis and secretion. Differential effects on preproinsulin messenger RNA levels, proinsulin biosynthesis, and secretion of newly made and preformed insulin in the rat. The Journal of clinical investigation 81, 176–184 (1988).
Reers, C. et al. Impaired islet turnover in human donor pancreata with aging. European journal of endocrinology 160, 185–191 (2009).
Dhawan, S., Tschen, S. I. & Bhushan, A. Bmi-1 regulates the Ink4a/Arf locus to control pancreatic beta-cell proliferation. Genes & development 23, 906–911 (2009).
Chen, H. et al. Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes & development 23, 975–985 (2009).
Ferrucci, L. et al. The origins of age-related proinflammatory state. Blood 105, 2294–2299 (2005).
Pedersen, M. et al. Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mechanisms of ageing and development 124, 495–502 (2003).
Keane, K. N., Cruzat, V. F., Carlessi, R., de Bittencourt, P. I. Jr. & Newsholme, P. Molecular Events Linking Oxidative Stress and Inflammation to Insulin Resistance and beta-Cell Dysfunction. Oxidative medicine and cellular longevity 2015, 181643 (2015).
Davis, J. E., Gabler, N. K., Walker-Daniels, J. & Spurlock, M. E. The c-Jun N-terminal kinase mediates the induction of oxidative stress and insulin resistance by palmitate and toll-like receptor 2 and 4 ligands in 3T3-L1 adipocytes. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 41, 523–530 (2009).
Mahbub, S., Deburghgraeve, C. R. & Kovacs, E. J. Advanced age impairs macrophage polarization. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research 32, 18–26 (2012).
Jackaman, C. et al. Targeting macrophages rescues age-related immune deficiencies in C57BL/6J geriatric mice. Aging cell 12, 345–357 (2013).
Bouzakri, K., Ribaux, P. & Halban, P. A. Silencing mitogen-activated protein 4 kinase 4 (MAP4K4) protects beta cells from tumor necrosis factor-alpha-induced decrease of IRS-2 and inhibition of glucose-stimulated insulin secretion. The Journal of biological chemistry 284, 27892–27898 (2009).
Donath, M. Y. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nature reviews. Drug discovery 13, 465–476 (2014).
Poggi, M. et al. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia 50, 1267–1276 (2007).
Suganami, T. et al. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochemical and biophysical research communications 354, 45–49 (2007).
Davis, J. E., Gabler, N. K., Walker-Daniels, J. & Spurlock, M. E. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity 16, 1248–1255 (2008).
Cucak, H. et al. Macrophage contact dependent and independent TLR4 mechanisms induce beta-cell dysfunction and apoptosis in a mouse model of type 2 diabetes. PloS one 9, e90685 (2014).
Acknowledgements
This work was supported by the German Research Foundation (DFG) and the European Research Council (ERC). We thank Katrischa Hennekens (University of Bremen) for excellent technical assistance.
Author information
Authors and Affiliations
Contributions
Conceived this project: K.M. Designed, performed and analyzed research: W.H. and K.M. Performed and analyzed this research: T.Y., D.C., H.H., K.A., B.L. Wrote the paper: W.H., K.M.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, W., Yuan, T., Choezom, D. et al. Ageing potentiates diet-induced glucose intolerance, β-cell failure and tissue inflammation through TLR4. Sci Rep 8, 2767 (2018). https://doi.org/10.1038/s41598-018-20909-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20909-w
This article is cited by
-
Toll-like receptor 4 (TLR4): new insight immune and aging
Immunity & Ageing (2023)
-
Aging mitigates the severity of obesity-associated metabolic sequelae in a gender independent manner
Nutrition & Diabetes (2021)
-
Comorbidity-associated glutamine deficiency is a predisposition to severe COVID-19
Cell Death & Differentiation (2021)
-
Lung Surfactant Accelerates Skin Wound Healing: A Translational Study with a Randomized Clinical Phase I Study
Scientific Reports (2020)
-
Age-Dependent Protection of Insulin Secretion in Diet Induced Obese Mice
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.