Abstract
We compared progression-free survival (PFS) and overall survival (OS) among 292 metastatic renal cell carcinoma (mRCC) patients either undergoing nephrectomy (Nx, 61.6%) or not (non-Nx, 38.4%), stratified according to the MSKCC and Heng risk models, treated with either immunotherapy (IT, 45.2%) or targeted therapy (TT, 54.8%) between 2000 and 2015. During the follow-up duration of 16.6 months, PFS/OS of the Nx (6.0/30 months) and non-Nx (3.0/6.0 months) groups were significantly different despite differences among baseline parameters (p < 0.05). The intermediate- and poor-risk patients defined using either model showed significantly longer PFS and OS in the Nx group than in the non-Nx group (p < 0.05). After stratifying groups by systemic therapy and risk models, both the Nx and non-Nx groups showed no significant differences in intermediate and poor-risk models (p > 0.05). In both synchronous and metachronous mRCC patients, both PFS and OS showed similar survivals; the Nx group had significantly longer PFS and OS than the non-Nx group, even after considering each systemic therapy and prognostic model. Nx showed a significant positive benefit in PFS and OS compared to no Nx upon patient stratification according to the MSKCC and Heng risk models. The metastatic type did not significantly affect survival between the two groups.
Similar content being viewed by others
Introduction
The standard therapy for metastatic renal cell carcinoma (mRCC) has been systemic therapy including cytokine immunotherapy (IT) in the past decade and systemic targeted therapy (TT) in recent decades. However, the outcomes of systemic therapy are unsatisfactory with a dismal 5-year overall survival (OS) rate of <20%, because RCC is resistant to radiotherapy, chemotherapy, and immunotherapy1. Complete surgical resection of the tumor (radical or partial) is the only known curative therapy for RCC localized within the kidney2.
Regarding mRCC, cytoreductive nephrectomy (CNx) is often indicated as part of an integrated therapeutic management strategy for mRCC to reduce the potential tumor burden as well as control cancer-related symptoms, including hemorrhage, pain, and paraneoplastic syndrome3. CNx has also been proven to improve prognosis of mRCC treated either with IT or TT; however, randomized clinical trials have not yet clarified the definite survival benefit of CNx with TT. Several large-scaled retrospective studies and meta-analyses have shown the favorable effects of CNx on survival with spontaneous regression of metastasis in up to 4% of cases4,5,6,7, a decreased risk of death and a 5.8-month survival advantage before IT8,9,10.
In this retrospective study, we aimed to evaluate the prognostic significance of Nx on progression-free survival (PFS) and OS in patients with mRCC through stratification by types of systemic therapy, metastatic types, and the widely used prognostic risk models (the Memorial Sloan-Kettering Cancer Center [MSKCC] and the International Metastatic Renal Cell Carcinoma Database Consortium [IDMC, also known as Heng]11 risk models).
Materials and Methods
Ethics statement
The Institutional Review Board of the National Cancer Center (IRB No. NCC 2015-0212) approved this retrospective study and waived the need for written consent. All patient data were anonymized and de-identified prior to analysis. All study protocols were performed in accordance with the tenets of the ethical guidelines and regulations of the ‘World Medical Association Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects.’
Criteria for patients
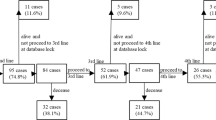
We analyzed the data of 292 mRCC patients treated with first-line systemic therapy using either TT (N = 160, 54.8%) or IT (N = 132, 45.2%) including sunitinib, sorafenib, pazopanib, temsirolimus as first-line TT and interferon-alpha and interleukin-2 as IT between 2000 and 2015, as cited in previously published reports12. A total of 183 (61.6%) patients who underwent Nx and 109 (38.9%) patients who did not (non-Nx) were enrolled for the evaluation PFS and OS, and were stratified by their MSKCC and Heng (favorable, intermediate, and poor) risk criteria and metastatic types (synchronous and metachronous type), as well as types of systemic therapies used (IT or TT). Furthermore, patient records were retrospectively extracted from the data prospectively recorded by our institutional RCC database to obtain baseline information regarding age, body mass index, ECOG–performance status (ECOG-PS), TNM stage, cell histology, Fuhrman nuclear grade, and survival outcomes. The radical nephrectomy (RNx) or CNx procedure was performed by open access or laparoscopically by a single surgeon (J.C.). If CNx was not performed, histological confirmation of RCC was performed by biopsies from primary or metastatic lesions or both. The RECIST criteria 1.113 were used for the evaluation of response to systemic therapies after each therapeutic regimen and follow-up protocol cited in previous reports11.
Statistical analysis
The baseline characteristics are presented as frequency (percentage) for categorical variables and median (range) for continuous variables. Differences in distributions were compared between SM and MM using the Student’s t-test, Pearson’s Chi-square test, Fisher’s exact test, and Log-rank test as appropriate. Survival curves and their differences are presented using the Kaplan-Meier curve and log-rank test to analyze times to progression (PFS) for first-line systemic therapy and death (OS) after systemic therapy according to the number of MSKCC and Heng risk factors. All results were considered statistically significant when two-sided p-values were <0.05 using SAS 9.2 software (SAS Institute Inc., Cary, NC, USA).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Results
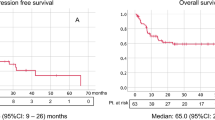
During a median 10.0- months of treatment and 16.6 months of follow-up, the median PFS/OS was 11.0 (8.0-14.0)/4.0 (2.8–5.2) months with an objective response rate of 17.5% and disease control rate of 63.4% after first-line therapy (Table 1). The median number of metastatic organs was 2.0 (range, 1–5) with the lung being the most frequently metastasized organ (75.7%), followed by bones (45.2%), lymph nodes (24.3%), brain (18.8%), and the liver (17.1%). A total of 106 patients had only one metastatic site (36.2%) and 98 (33.3%) patients had at least two or more metastatic organs. The overall patients’ Charlson comorbidity index was a median of 7.0 (range, 6–13) with hypertension (43.8%) and diabetes (22.3%) being the most frequent underlying diseases among enrolled patients.
The median PFS/OS for IT and TT were 2.0 (1.1–2.9) months/10.0 (6.8–13.2) months and 5.0 (3.5–6.5) months/13.0 (8.4–17.6) months, respectively (OS for IT-TT, 24.0 [11.2–36.8] months). The Nx group had significantly longer PFS/OS (6.0/30.0 months) than the non-Nx group (3.0/8.0 months, p < 0.001, Supplementary Figure 1). The comparison of PFS/OS between IT and TT according to the prognostic model showed significant differences. TT had better prognostic effects on both PFS (9.0 and 4.0 months) and OS (32.0 and 9.0 months) than IT (PFS, 4.0 and 2.0 months; OS, 26.0 and 6.0 months) among intermediate and poor risks, and the IT-TT group had the best PFS/OS among all MSKCC and Heng risk groups (p < 0.05, Table 2 and Supplementary Figure 2). Despite the significant differences in baseline characteristics between the Nx and non-Nx groups, including those in metastatic type, ECOG-performance status, number of MSKCC and Heng risk factors, treatment duration, clinical and pathologic T stage, first line systemic therapy, rate of treatment-free interval <1 year, and survival rate (p < 0.05, Table 3), patients in the Nx group had significantly prolonged PFS/OS compared to the non-Nx group (p < 0.05, Table 4).
After stratification of prognostic risks and systemic therapies, patients in the Nx and non-Nx groups had insignificantly different PFS and OS, especially among the poor-risk patients with no benefits in PFS and OS (p > 0.05, Table 5). Regarding the comparison between Nx and non-Nx for SM and MM, the Nx group had significantly better PFS (5.0 vs. 3.0 months) and OS (22.0 vs. 7.0 months) than the non-Nx group regardless of the types of systemic therapy and prognostic risk models (p < 0.05, Supplementary Tables 1 and 2).
Discussion
The therapeutic concepts of Nx in metachronous and synchronous mRCC are different, although radical (or partial) Nx for localized RCC and CNx for mRCC both have a significant survival gain6,14,15. Radical (or partial) Nx removes all cancer cells en-bloc within the primary kidney rendering the patient completely free of cancer until metastasis or recurrence occurs. Recurrent or metastasized cancers in metachronous mRCC are composed of dormant cancer cells that have re-appeared or new cancer cells with different metabolic activity in metastatic lesions, and create a tumor microenvironment that is different from lesions in localized RCC. Meanwhile, CNx for synchronous mRCC removes the primary loco-regional tumor as much as possible to decrease the tumor burden in order to achieve better responses to systemic therapy and focuses more on the metastatic lesions16,17. Although CNx does not guarantee a surgically curable status, a few selected patients achieved complete remission after CNx in combination with targeted therapy18.
The effects of Nx for mRCC with regard to the prognostic risks, metastatic types, and type of systemic therapy were compared to those of no Nx. Nx had significant benefits for OS compared to no Nx in intermediate risk patients with mRCC (Table 4) despite the more favorable baseline characteristics of patients in the Nx group (p < 0.05, Tables 1 and 2). The Nx group was mostly consisted of patients with favorable risk group and metachronous type of metastasis. However, the Nx and no Nx groups failed to show any significant differences in PFS among any risk groups and in OS within the poor-risk group (Table 5).
Additional findings on the metastatic types showed that they did not significantly affect the prognostic survival differences between patients who underwent Nx and those who did not (Supplementary Tables 1 and 2). The synchronous and metachronous sets showed almost no difference in the survival of patients who underwent Nx and those who did not. Both sets of patients with mRCC had a significantly better PFS and OS after Nx than without Nx upon stratification by types of systemic therapy and in the prognostic risk models. This implied that Nx was beneficial for both synchronous and metachronous mRCCs when the patient’s surgical condition and disease status allowed it, and thus, was a significant factor for better survival in mRCC. Regarding the type of systemic therapy, in both metastatic types, Nx with IT or with TT resulted in similar median OS of 20 months and 22–25 months, respectively, and no Nx with IT or TT resulted in similar OS (6 months vs. 9 months) within the MSKCC and Heng risk models (Supplementary Tables 1 and 2).
The recent development of targeted agents using a genetic analysis technique permitted prolonged survival of mRCC patients compared to that with IT, especially in the intermediate Heng risk and Nx groups with more than two-fold increase in OS, (p < 0.001, Table 2), which is similar to the results of this study. Moreover, Nx with adequate and proper selection of the targeted therapeutic agents as well as of patients with a prognostic model would provide the best opportunities for survival compared to no Nx6,15. Bamias et al.19 showed that patients with primary mRCC treated with sunitinib and CN had a significantly longer OS than patients who did not undergo CNx (23.9 vs. 9 months), and found the Heng and MSKCC risk models to be associated with prognostic significance. The present study also demonstrated that in favorable and intermediate risk patients with general conditions susceptible to Nx, TT had much greater chances of controlling tumors in mRCC patients than non-Nx. Other retrospective studies20,21,22 including a Surveillance, Epidemiology, and End Results (SEER) database study21 have suggested CNx as an independent factor of OS with 19 months of improvement after CNx in the TT era even after adjusting for established prognostic risk factors (vs. 13 months in the IT era)20. Finally, two famous randomized phase 3 trials namely, CARMENA with Nx followed by sunitinib vs. sunitinib alone and SURTIME, with immediate vs. deferred (after 3 complete cycles of sunitinib) CNx23 are currently ongoing and would provide definite answers to questions on the benefit and timing of CNx for mRCC treated with TT24.
Discussions on CNx have included the indications for CNx by properly selecting patients. Regarding the better prognosis of CNx, the current indications for mRCC were patients with good performance status, large primary tumors accounting for most of the disease volume, and low metastatic volume, but not patients with poor PS or Heng or MSKCC poor-risk disease, with relatively small primary tumors and high metastatic volume, and/or with sarcomatoid tumors4,23. There is little doubt that patients with solitary tumors or oligometastasis could either potentially be cured by CNx and complete resection of metastatic sites, or benefit from a substantial delay and reduction in associated side-effects of systemic therapy before further progression requires medication6,16,17,18.
However, because of the clinical and biological heterogeneity of synchronous mRCCs and the lack of biomarkers, not all patients with mRCC benefited from CNx, especially among the poor-risk group25. CNx in patients who have a poor performance (ECOG 2/3 patients) might serve a palliative function, but it should be performed with caution because of the poor outcomes in such patients26. This study did not show a significant beneficial effect on survival compared to that in the non-CNx poor-risk group, similar to the studies by Choueiri and Heng11,21. Heng et al. also found that most patients with 4 or more IMDC risk factors did not benefit from tumor removal22.
Despite the CNx effect on survival, the use of CNx declined to 34–38% in 2005–2010 since the introduction of TT, suggesting that more patients would eventually be treated with the primary tumor in situ11,27. The reason for this decline was that, unlike IT, TT alone could control the disease, particularly at the primary tumor site; thus, Nx might not be necessary. This might avoid the morbidity and mortality associated with surgery followed by a postoperative delay in starting systemic treatment allowing further disease progression.
Another issue regarding CNx was its effect on mRCC with the combination of TT in a neoadjuvant (in the meaning of “presurgical”) setting with delayed CNx. The theoretical advantage of neoadjuvant TT with delayed CNx was downsizing the primary renal tumor and rapid initiation of effective systemic therapy without any delay associated with planning, performing, and recovering from Nx after supportive care for improving general conditions143. Moreover, patients with primary refractory disease would be accurately identified, and therefore, might have an opportunity to promptly switch to another targeted agent. However, it should be noted that approximately 30% of patients failed to go on the planned Nx in neoadjuvant trials due to changes in the performance state of individuals and downsizing of the tumor with TT seemed to be quite modest (2–6% by the RECIST criteria), which was not enough to facilitate CNx14. The frequent occurrence of disease progression (approximately 33–37% by the RECIST criteria)28 during a surgery-related break in treatment was another important issue that occurred approximately 3–4 weeks postoperatively29. However, this neoadjuvant TT and delayed CNx might be one option for poor-risk patients with an ECOG score of 2 to 3 in the poor-risk group. TT might be proposed as the first-line treatment to these patients, especially with MSKCC intermediate-risk disease in a phase 2 trial with pazopanib; CNx should be considered only after an objective response to the systemic treatment30.
In addition, the rapid progression after cessation of TT with CNx remains unclear, as to whether it was due to the withdrawal of anti-angiogenic therapy before surgery, the release of growth factors after surgery, a combination of both, or if it was progression due to TT resistance. Translational research on primary tumor tissue has shown that withdrawal of sunitinib led to rapid endothelial cell proliferation31. This seems to support the hypothesis that a treatment break might trigger progression in some patients, although that patient population cannot be identified4. A tumor flare phenomenon suggested by Escudier et al.32 showed acceleration of tumor growth rate and induction of tumor flares after cessation of systemic treatment, which could negatively affect mRCC prognosis.
The limitations of this study were its retrospective design with a small number of poor-risk patients. Additionally, the intraoperative measures and further analyses of systemic agents, including pathological and metastatic information have not been accounted in the survival rate of Nx group. However, this is an evidence-based retrospective study providing the positive aspects of CNx in the TT era. A carefully selected group of patients with synchronous mRCC can obtain a clinical benefit with survival gain after combinational local surgery such as metastatectomy or radiation therapy with systemic TT.
Conclusion
The prognostic significance of Nx for PFS and OS according to the MSKCC and Heng criteria showed a positive effect in the intermediate- and poor-risk groups among mRCC patients treated with first-line systemic therapies. A stratified comparison of PFS and OS by systemic therapies and metastatic types between the Nx and non-Nx groups showed no significant differences among those with poor risk; however, significant differences among those with intermediate risk were observed.
References
Ljungberg, B. et al. The epidemiology of renal cell carcinoma. Eur Urol. 60, 615–21 (2011).
Basso, M., Cassano, A. & Barone, C. A survey of therapy for advanced renal cell carcinoma. Urol Oncol. 28, 121–33 (2010).
Hong, X. et al. Prognostic value of cytoreductive nephrectomy combined with targeted therapy for metastatic renal cell carcinoma: a meta-analysis. Int Urol Nephrol. 48, 967–75 (2016).
Bex, A., Ljungberg, B., van Poppel, H. & Powles, T. The Role of Cytoreductive Nephrectomy: European Association of Urology Recommendations in 2016. Eur Urol. 70, 901–905 (2016).
Culp, S. H. Cytoreductive nephrectomy and its role in the present-day period of targeted therapy. Ther Adv Urol. 7, 275–85 (2015).
Noe, A., Stewart, G. D. & Bex, A. The role of cytoreductive surgery in the era of targeted agents. Curr Opin Urol. 25, 374–80 (2015).
Aslam, M. Z. & Matthews, P. N. Cytoreductive nephrectomy for metastatic renal cell carcinoma: a review of the historical literature and its role in the era of targeted molecular therapy. ISRN Urol. 2014, 717295 (2014).
Mickisch, G. H., Garin, A., van Poppel, H., de Prijck, L. & Sylvester, R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 358, 966–70 (2001).
Flanigan, R. C. et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 345, 1655–9 (2001).
Flanigan, R. C. et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 171, 1071–6 (2004).
Heng, D. Y. et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 27(34), 5794–9 (2009).
Kim, S. H. et al. Systemic Treatments for Metastatic Renal Cell Carcinoma: 10-Year Experience of Immunotherapy and Targeted Therapy. Cancer Res Treat. 48, 1092–101 (2016).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 45, 228–47 (2009).
Mathieu, R. et al. Nephrectomy improves overall survival in patients with metastatic renal cell carcinoma in cases of favorable MSKCC or ECOG prognostic features. Urol Oncol. 33(339), e9–15 (2015).
Vaishampayan, U.N. The Role of Nephrectomy for Kidney Cancer in the Era of Targeted and Immune Therapies. Am Soc Clin Oncol Educ Book. 35, e16–20 (2016).
Barbastefano, J. et al. Association of percentage of tumour burden removed with debulking nephrectomy and progression-free survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. BJU Int. 106, 1266–9 (2010).
Dabestani, S. et al. Local treatments for metastases of renal cell carcinoma: a systematic review. Lancet Oncol. 15, e549–61 (2014).
Albiges, L. et al. Complete remission with tyrosine kinase inhibitors in renal cell carcinoma. J Clin Oncol. 30, 482–7 (2012).
Bamias, A. et al. Prognostic significance of cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma treated with first-line sunitinib: a European multiinstitutional study. Clin Genitourin Cancer. 12, 373–83 (2014).
Conti, S. L. et al. Utilization of cytoreductive nephrectomy and patient survival in the targeted therapy era. Int J Cancer. 134, 2245–52 (2014).
Choueiri, T. K. et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol. 185, 60–6 (2011).
Heng, D. Y. et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol. 66, 704–10 (2014).
Patard, J. J. et al. ICUD-EAU International Consultation on Kidney Cancer 2010: treatment of metastatic disease. Eur Urol. 60, 684–90 (2011).
Stewart, G. D. et al. Cytoreductive Nephrectomy in the Tyrosine Kinase Inhibitor Era: A Question That May Never Be Answered. Eur Urol. pii: S0302-2838(16)30743-6 https://doi.org/10.1016/j.eururo.2016.10.029 (2016).
Bex, A. & Powles, T. Selecting patients for cytoreductive nephrectomy in advanced renal cell carcinoma: who and when. Expert Rev Anticancer Ther. 12, 787–97 (2012).
Shuch, B. et al. Performance status and cytoreductive nephrectomy: redefining management in patients with poor performance. Cancer. 113, 1324–31 (2008).
Tsao, C. K. et al. Cytoreductive nephrectomy for metastatic renal cell carcinoma in the era of targeted therapy in the United States: a SEER analysis. World J Urol. 31, 1535–9 (2013).
Powles, T. et al. A prospective evaluation of VEGF-targeted treatment cessation in metastatic clear cell renal cancer. Ann Oncol. 24, 2098–103 (2013).
Culp, S. H. et al. Can we better select patients with metastatic renal cell carcinoma for cytoreductive nephrectomy? Cancer. 116, 3378–88 (2010).
Powles, T. et al. Safety and Efficacy of Pazopanib Therapy Prior to Planned Nephrectomy in Metastatic Clear Cell Renal Cancer. JAMA Oncol. 2, 1303–9 (2016).
Griffioen, A. W. et al. Rapid angiogenesis onset after discontinuation of sunitinib treatment of renal cell carcinoma patients. Clin Cancer Res. 18, 3961–3971 (2012).
Iacovelli, R. et al. Evidence and Clinical Relevance of Tumor Flare in Patients Who Discontinue Tyrosine Kinase Inhibitors for Treatment of Metastatic Renal Cell Carcinoma. Eur Urol. 68, 154–60 (2015).
Author information
Authors and Affiliations
Contributions
Manuscript conception and preparation: J.C., H.K.S., K.H.L., J.Y.J., S.H.K. Data collection and analysis: J.C., S.H.K. Statistical analysis: S.H.K. Manuscript writing: S.H.K., J.C. Manuscript approval: All authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, S.H., Jeong, KC., Joung, J.Y. et al. Prognostic significance of nephrectomy in metastatic renal cell carcinoma treated with systemic cytokine or targeted therapy: A 16-year retrospective analysis. Sci Rep 8, 2974 (2018). https://doi.org/10.1038/s41598-018-20822-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20822-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.