Abstract
The scaffold protein DLGAP1 is localized at the post-synaptic density (PSD) of glutamatergic neurons and is a component of supramolecular protein complexes organized by PSD95. Gain-of-function variants of DLGAP1 have been associated with obsessive-compulsive disorder (OCD), while haploinsufficient variants have been linked to autism spectrum disorder (ASD) and schizophrenia in human genetic studies. We tested male and female Dlgap1 wild type (WT), heterozygous (HT), and knockout (KO) mice in a battery of behavioral tests: open field, dig, splash, prepulse inhibition, forced swim, nest building, social approach, and sucrose preference. We also used biochemical approaches to examine the role of DLGAP1 in the organization of PSD protein complexes. Dlgap1 KO mice were most notable for disruption of protein interactions in the PSD, and deficits in sociability. Other behavioral measures were largely unaffected. Our data suggest that Dlgap1 knockout leads to PSD disruption and reduced sociability, consistent with reports of DLGAP1 haploinsufficient variants in schizophrenia and ASD.
Similar content being viewed by others
Introduction
The Disks Large Associated Protein 1 gene encodes the protein DLGAP1 (also known as GKAP or SAPAP1), which localizes at the postsynaptic density (PSD)1,2,3,4,5,6,7. Genetic variants of DLGAP1 and abnormalities of the PSD have been associated with neuropsychiatric disorders including schizophrenia8, autism spectrum disorder (ASD)9, and obsessive-compulsive disorder (OCD)10. For example, the case-control portion of the first Genome Wide Association Study (GWAS) for OCD found that the two single nucleotide polymorphisms (SNPs) with the lowest P-values were both located within an intron of DLGAP111. Furthermore, a 63 kb duplication of four exomes of DLGAP1 was observed in a sib-pair with pediatric OCD, and other comorbid disorders12. Conversely, a de novo deletion CNV of DLGAP113, and a number of rare protein-altering variants, including missense and stop variants, have also been identified in individuals with schizophrenia14,15. Furthermore, ASD patients show an increased incidence of carrying non-synonymous variants of DLGAP19. Rodent studies have shown that DLGAP1 protein complexes isolated from the hippocampal CA1 region are enriched in proteins considered risk factors for schizophrenia and ASD16,17. Thus, emerging data suggests that genetic variants increasing DLGAP1 function may predispose to OCD, while variants decreasing DLGAP1 function might predispose to schizophrenia or ASD.
The postsynaptic terminal of excitatory synapses is characterized by an electron-dense accumulation of protein, called the Post Synaptic Density (PSD). The proteome of the PSD has been extensively characterized in vertebrates (vPSD), which has a conserved set of ~1000 proteins2. All PSD proteins are physically organized into a hierarchy of complexes and supercomplexes (complexes of complexes)17,18,19. DLGAP1 is a scaffold component of 1.5 MDa supercomplexes, which also includes a number of different scaffolds such as Dlg1-4, Shank1-3 and also includes N-methyl-D-aspartate (NMDA) receptors, ion channels and signaling proteins5,19,20. Mutations in PSD95 (Dlg4) and other proteins in these supercomplexes alter the capacity of synapses to detect and respond to patterns of neural activity, control of short- and long-term synaptic strength, and modulate innate and learned behaviors17,21,22,23,24,25,26,27,28.
Scaffold proteins in the Dlg family (PSD95/Dlg4, PSD93/Dlg2, SAP102/Dlg3, SAP97/Dlg1) have been shown to be essential for assembly of postsynaptic complexes18,29, however, the requirement of DLGAP1 for the organization of protein interactions at the PSD has not been directly examined. DLGAP1 links Dlg family scaffolds to SHANK (SH3 and multiple ankyrin repeat domains proteins) family proteins (SHANK1-3), and protein complexes from these three families of scaffold proteins have been shown to be enriched in a variety of psychiatric and other complex brain disorders30. Therefore, Dlgap1 deletion could disrupt the organization of the PSD by uncoupling these families of scaffold proteins. Furthermore, DLGAP1 is most highly expressed within neocortex, hippocampus, olfactory bulb, and cerebellum31,32, brain regions which have relevance to symptoms observed in neuropsychiatric disorders.
We evaluated Dlgap1 wild type (WT), heterozygous (HT), and knockout (KO) mice for protein interactions within the PSD, and behavioral phenotypes relevant to OCD, ASD, and/or schizophrenia. OCD is characterized by obsessions, compulsions, or both33; schizophrenia is characterized by positive symptoms, including hallucinations and delusions, and negative symptoms, including anhedonia and social deficits34; core features of ASD include repetitive behaviors, and social deficits35. We assessed mice for sociability, grooming behaviors, sensorimotor gating, exploratory behavior, anxiety, locomotion, nest building, anhedonia, and depression-like behavior.
Methods
Animals
Dlgap1 KO mice on a mixed 129/C57BL/6 J background were obtained from Dr. Seth Grant at Edinburgh University. Mice were backcrossed to C57BL/6 J mice for one generation and then HT × HT breeding produced experimental cohorts. Adult male and female Dlgap1 WT, HT, and KO mice were subjects. Mice were maintained on a 12-hour light/dark schedule with food and water available ad libitum, and all tests occurred during the animals’ light cycle. All methods were performed in accordance with the local Institutional Animal Care and Use Committee (IACUC) guidelines and regulations.
Several cohorts were used for studies. Cohort 1 consisted of mice (n = 16/sex/genotype) aged 8–11 weeks at the start of experiments. Mice were assessed in the following tests: open field, dig test, splash test (week 1); social approach, nest building (week 2); prepulse inhibition (PPI) (week 4); forced swim test (week 6). Cohort 2 consisted of mice (males: 14 WT, 14 HT, 12 KO; females: 14 WT, 15 HT, 13 KO) aged 8–11 weeks at the beginning of testing. Mice were tested for sociability and sucrose preference. Cohort 3 consisted of 6 KO and 6 WT male mice (2–4 months), and were used for biochemical studies of the PSD. Sample sizes were selected based on estimates from our previous work36,37,38.
Biochemistry
Immunoprecipitation of the DLG layer of the PSD scaffold was performed using a pan-Membrane Associated Guanylate kinase family (MAGUKs) antibody and determining the levels of SHANK proteins by HPLC-MS/MS. PSD-enriched fractions were prepared using a 3-step protocol as described previously16. Briefly, adult mouse cortex was homogenized, homogenates were spun for 4 min at 500 g, the supernatant was collected and centrifuged at 10,000 g, and the membrane fraction was solubilized. Solubilized membranes were centrifuged at 30,0000 RPM in a Beckman Optima Max rotor MLA-130 for 40 minutes, pellet was collected and solubilized. Enrichment and quality of PSD fractions was monitored against a number of protein controls including glutamate receptor subunits, presynaptic markers, cytoplasmic proteins, and core PSD scaffolds as previously described16. Samples were separated by NuPAGE® Novex® 4–12% Bis-Tris Gels and subsequently fixed and then stained with Instant blue®. Lanes were cut and placed into 96-well plates for de-staining, and digested by tripsin at 37 °C for 1 hour. Peptides were then extracted with acetonitrile. Peptide desalting and reverse phase separation of peptides was performed using the Nano/Capillary Liquid Chromatography (LC) System Ultimate 3000 (Thermo/Dionex) and samples then processed in a hybrid linear ion trap–Fourier transform ion cyclotron resonance (FTICR) mass spectrometer (MS). Antibodies for immunoisolation were screened for specificity using Dlgap1 KO and Shank3c-terminal deleted KO mice, and anti-GST as negative controls17. Antibody concentrations were standardized for optimum protein recovery within ranges: 0.4–0.8 ug/ul. Immunoprecipitations were performed as described previously16,30, with minor modifications. Protein interactions were considered positive if at least two unique peptides were present in duplicate assays and absent in controls. MS data were processed using Proteome Discoverer 1.4 (PD, Thermo Scientific) and searched by both Sequest and Mascot V2.4 (Matrix science) against a modified mouse database that was downloaded from Uniprot and was combined with its decoy database. The mass tolerance used for searching was set as 10 ppm for precursor ions and 0.8 Da for fragment ions. No more than two missed cleavage sites were allowed. Static modification was set as cysteine carboxyamidation and dynamic modification was set as methionine oxidation. False discovery rates (FDR) were automatically calculated by the Percolator node of PD based on decoy database hits. A peptide FDR of 0.01 were used for cut-offs. Peptides with high confidence were considered as true hits and proteins with at least two different peptides were accepted.
Open Field Test
Mice were placed into the corner of automated activity chambers, which were 41 cm × 41 cm with a 16 × 16 photobeam grid, and 2.54 cm between each photobeam39 (Accusan, Columbus, OH). Activity was recorded for 60 minutes. Total distance traveled was quantified to assess locomotor activity. Vertical activity was used to assess rearing, an exploratory behavior. Percent center distance was calculated as [(center distance/total distance) × 100] and was used as a measure of anxiety, with increases in center distance interpreted as a decrease in anxiety. Spatial d was calculated using BMDP, Python, and Night Owl to assess the degree to which consecutive movements were along a straight line (d ≈ 1), meandering (d ≈ 1.5), or contained directional changes (d ≈ 2)40.
Dig Test
Immediately following the open field test, mice were placed into a novel cage with 1″ of standard, clean bedding and behavior was videotaped for 3 minutes. Videos were then scored by an experimenter blind to genotype and sex. Measures were: latency to dig, total time digging, number of bouts digging, and average bout duration. A digging bout was defined as significant displacement of bedding due to limb or nose movement lasting at least one second, and not separated by more than one second of rest. Increases in digging behavior indicate increased exploration41.
Splash Test
Following the dig test, mice were allowed to habituate to the test cage for an hour. Mice were then removed from the test cage, sprayed twice on their dorsal surface from approximately 5″ away with a 10% sucrose solution, and returned to the test cage42. Behavior was videotaped for 5 minutes, and then scored by a blind experimenter for latency to groom, total time grooming, number of grooming bouts, and average bout duration. A grooming bout was defined as any number of leg strokes along the body, a minimum of 2 arm strokes over the face/head, or any amount of time spent licking/biting the fur, with a break not separated by more than 2 seconds. Decreases in grooming in response to sucrose spray was interpreted as increased anhedonia43.
Nest Building
Nest building was used to assess general home cage behavior. Mice were placed into a novel cage with an unused nestlet (a 2″ × 2″ square of cotton fiber; Ancare). Nests were photographed at 0, 2, 4, and 6 hrs. Nests were scored by an experimenter blinded to genotype and sex, as described previously44. Briefly, nests were given a score of 0–5 according to the following criteria: 0 = untouched; 1 = minimal pieces ripped off; 2 = major pieces of nestlet square left intact; 3 = all/most ripped up but not organized; 4 = all ripped up, organized into clear nest but not perfect, no tight walls; 5 = all ripped up, organized into clear nest, tight walls45. Additionally, nests were weighed at time 0 and 6 hours, and the amount of nestlet used was calculated by subtracting the intact portion of the nestlet remaining at 6 hours from the original nestlet weight at time 0.
Prepulse Inhibition
Prepulse inhibition was used as an index of sensorimotor gating, which is deficient in patients with schizophrenia46,47, ASD48,49, and OCD50,51. Mice were placed into startle chambers that consisted of a Plexiglas cylinder resting on a Plexiglas platform in a sound-attenuating, ventilated chamber (San Diego Instruments, CA). Sessions were 20 minutes long. The first 5 minutes consisted of acclimation to the background (65 dB) noise. The testing consisted of a pseudo random representation of five different types of trials: a 40-millisecond broadband 120 dB burst (pulse alone trial); three different prepulse pulse trials in which 20-millisecond long 3 dB, 6 dB, or 12 dB above background stimuli preceded the 120 dB pulse by 100 milliseconds (onset to onset); and a no stimulus trial, in which only background noise (65 dB) was presented52,53. PPI was calculated as [(startle amplitudepulse − startle amplitudeprepulse + pulse)/startle amplitudepulse) × 100].
Forced Swim Test
The forced swim test was used as an index of a depression-like behavior. Mice were placed into a bucket (24 cm high and 19 cm in diameter filled 19 cm high) filled with 23 °C to 25 °C tap water. Behavior was videotaped from above for 6 minutes, with the last four minutes scored by a blinded experimenter for time climbing, swimming, and immobile. Mice were also exposed to a 6-minute pretest 24 hours earlier. An increase in immobility or decrease in active behavior reflects a depression-like phenotype54.
Social Approach Experiment 1
Cohort 1 mice were habituated to open field chambers (106 × 106 × 76 cm; Accuscan, Columbus, OH) for 5 minutes. The test mouse was then removed, and a novel mouse (C57BL/6 J mouse of same sex, but lower body weight) was placed in a clear plastic cage (10 L × 8 W × 12 H cm) in one corner of the chamber. A novel object [a clear plastic cage (10 L × 8 W × 12 H cm)] was placed in the opposite corner of the chamber. The test mouse was then placed back in the chamber for an additional 5 minutes and allowed to move freely throughout the chamber. Data was collected automatically within the open field and specifically within the interaction area, defined as a 5 cm radius around the novel object or novel mouse. Dependent measures were entries, vertical time, total distance traveled, and time spent within the interaction area.
Social Approach Experiment 2
Cohort 2 mice were run in another social approach paradigm to assess number of sniffs made towards the novel mouse versus novel object. Mice were allowed to habituate to open field chambers for 10 minutes. During habituation, two wire cups (H: 10.5 cm, D: 9.5 cm) were present, centered along the left and right wall of the chamber. Following the 10-min habituation period, the test mouse was removed. A novel object (lego) was placed under one cup and a novel mouse was placed under the other cup. The novel mouse was a C57BL/6 J mouse of the same sex, but lower weight than the test mouse. The position of the mouse and object were counterbalanced among test mice. The test mouse was then returned to the open field chamber for 10 minutes. Behavior was videotaped and scored blindly for latency to first sniff, and number of sniffs. The chamber, cups, and novel object were cleaned between each mouse with 70% isopropyl alcohol. Since latency data was not normally distributed, latency data were log10 transformed.
Sucrose Preference
Mice were individually housed into assigned test cages, where they had access to water and sucrose in separate bottles for 4 hours. The concentrations of sucrose (0.5, 1, and 2%) were tested on sequential days, in ascending order, with the left/right sucrose bottle side switched each session. Volumes were recorded before and after each session, and sucrose preference was calculated as [(ml sucrose/ml sucrose + water) * 100].
Statistical Analyses
Two-way ANOVAs were performed for all measures with sex and genotype as factors. Main effects of genotype were resolved using Student Newman-Keuls post hoc tests. Interactions were resolved using one-way ANOVAs to compare the sexes, and Student Newman-Keuls post hoc tests to compare genotypes. For all measures, outliers were removed if their value was greater than two standard deviations above or below the mean. Alpha was set at 0.05. Variance was comparable between all compared groups.
Results
Biochemistry
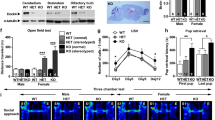
Within the PSD of excitatory glutamatergic synapses, a simplified scheme can describe a core scaffold structure (Fig. 1D), with three main groups of scaffold proteins: (1) an “upper layer” of scaffold proteins associated with glutamate receptor ion channels, including DLGs. (2) a “bottom layer” of scaffold components, including the SHANKs 1–3, and (3) an intermediate layer of scaffold molecules, functioning as a link between the “upper and lower layers”. This “middle layer” includes the disk large associated protein family (DLGAP1-4)17.
(A) The number of unique peptides identified in pan-MAGUK immunoprecipitation (IP) assays detected by HPLC-MS/MS from adult mouse cortex are shown. Columns show results from triplicate experiments, protein ID (Gene name), number of unique peptides and number of peptide spectrum matches (PSMs) (average of triplicate assays) for WT and Dlgap1 KO mice. (B) Representative blots showing two major isoforms of DLGAP1 in WT and their absence in Dlgap1 KO adult mouse cortex. (C) Representative blot of triplicate assays showing decreased (p = 0.0080, two-tailed non-parametric t-test) levels of DLG4 (left), and no changes in Homer1 in Shank3 IP in Dlgap1 KO adult mouse cortex (right). (D) Cartoon shows scheme of three layers of PSD scaffold proteins. Top: DLGs (Dlg1-4), Middle: DLGAPs (DLGAPs1-4) and Bottom: SHANKs (Shank1-3). Cartoons indicate decreased association of top and bottom layers in Dlgap1 KO (right) relative to WT (left). (E) Box plots and representative western blots of total protein levels of Homer1, Dlg4, Grin2A, Grin2B and Syngap1 in WT and Dlgap1 KO adult mouse cortex. No changes in total protein levels were observed.
As a first approach, we asked if the deletion of Dlgap1 impairs the interaction between the three scaffold-layers of the PSD. We immunoprecipitated DLG scaffolds with a pan-MAGUK antibody30 and identified their protein interactions by HPLC-MS/MS in WT and Dlgap1 KO mouse cortex. Figure 1A shows the number of unique peptides, run in triplicate, identified in pan-MAGUK immunoprecipitation (IP) as well as number of peptide spectrum matches (PSMs) (average of triplicate assays). Figure 1B shows that a pan-MAGUK antibody was able to coimmunoprecipitate DLGAP1 and SHANKs (SHANK1,2,3) together with the DLG family of scaffolds (DLG1,2,3,4) in WT cortex. This finding is consistent with previous results indicating that DLG proteins directly bind DLGAP155, which in turn binds to SHANK proteins56. Moreover, our previous studies show that DLG4, DLGAP1 and SHANK3 form large protein interaction complexes with each other30. Here, we also found that immunoisolation of DLG scaffolds from Dlgap1 KO mice recovered few SHANK1-3 peptides, which were below the level of detection (Fig. 1A).
To confirm that DLGAP1 was required for connecting DLG and SHANK proteins in PSD supercomplexes, we immunoprecipitated SHANK3 from WT and Dlgap1 KO mice cortex and performed Western Blots for Dlg4 (Psd95). Figure 1B,C shows that Dlgap1 deletion impairs 59.6% (p = 0.0080) of the association of Dlg4 to Shank3 supercomplexes. Since Shank3 complexes also contain other members of the DLGAP family in adult mouse cortex such as DLGAP2, DLGAP3, and DLGAP430, a fraction of Shank3 protein can still associate to DLG supercomplexes through interaction with DLG2, 3 and 4 (Fig. 1D). Moreover, while SHANK3 connection to DLG upper scaffolds was impaired in Dlgap1 KO, the interaction of SHANK3 with the downstream component Homer1 was unaffected (p = 0.823) (Fig. 1E), indicating that DLGAP1 selectively associates DLGs to SHANKs supercomplexes.
Finally, we asked if changes in protein interactions in Dlgap1 KO mice were a consequence of general changes in total protein levels. Figure 1E shows that NMDAR receptors subunits, Grin2A and Grin2B and PSD scaffolds and adaptors DLG4, SynGAP1, and Homer1 were unchanged in Dlgap1 KO mice versus WT mice.
Open Field Test
The measures of total distance traveled (Fig. 2A), rearing (Fig. 2B), percent of total distance traveled in the center (Fig. 2C), and spatial d (Fig. 2D) were not altered by genotype or sex. No interactions were observed.
Dig Test
A genotype by sex interaction [F(2,88) = 3.485] (p < 0.05) (Fig. 3A) and post hoc tests revealed an increased latency to dig in male Dlgap1 KO mice compared to male WT mice [F(2,45) = 3.31] (p < 0.05). Furthermore, male Dlgap1 KO mice had longer latencies to dig than female KO mice (p < 0.05). A main effect of genotype on number of digging bouts was also found [F(2,90) = 3.12] (p < 0.05) (Fig. 3B), with Dlgap1 KO mice exhibiting fewer digging bouts than WT mice (p < 0.05). Neither total time spent digging (Fig. 3C) nor average bout duration (Fig. 3D) were affected by genotype or sex.
Splash Test
Analysis revealed a genotype by sex interaction for average bout duration [F(2,88) = 5.70](p < 0.01) (Fig. 4A). Post hoc tests showed that within males, Dlgap1 KOs had fewer grooming bouts than HTs [F(2,43) = 4.02] (p < 0.05). Additionally, within HTs, males had longer grooming bouts than females (p < 0.05). Furthermore, Dlgap1 KO mice showed a longer latency to groom compared to either WT or HT mice [F(2,88) = 6.25] (p < 0.01) (Fig. 4B). No main effects or interaction of sex and genotype were observed for the number of grooming bouts (Fig. 4C). A genotype by sex interaction and post hoc tests revealed that within Dlgap1 HTs, males spent more time grooming than females [F(2,88) = 3.60] (p < 0.05) (Fig. 4D).
Nest Building
Across groups, nest quality increased over time (p < 0.001) (Supp. Figure 1A). The average nest quality score was rated at 2.6 at the 6-hour time-point. Therefore, either increases or decreases in nest quality should have been detectable in Dlgap1 KO mice compared to WT mice on the 5 point scale used, with 2.6 representing an intermediate score. However, no main effects or interaction of sex and genotype were found for nest quality. Similarly, the amount of nestlet used by the 6-hour time-point did not differ between groups (Supp. Figure 1B).
PPI
Neither genotype nor sex affected baseline startle (data not shown) or PPI (Supp. Figure 2). There were no interactions including genotype, sex, or prepulse intensity.
FST
No interactions or main effects of genotype or sex were found for time immobile (Supp. Figure 3A), climbing (Supp. Figure 3B), or swimming (Supp. Figure 3C). There was a main effect of sex on climbing, in which males climbed more than the females [F(2,90) = 7.17] (p < 0.01) (Supp. Figure 3B).
Social Approach
Experiment 1: For the number of entries, a three-way interaction of sex by genotype by condition (mouse vs. object) [F(2,90) = 4.83] (p < 0.01) (Fig. 5A) and post hoc tests showed that within males, both Dlgap1 HTs and KOs made fewer entries into the zone containing the mouse than WTs, indicating less sociability (p < 0.05). In addition, male WTs made more entries into either zone (containing the mouse or object) than female WTs [F(2,90) = 4.81] (p < 0.01), indicating increased general exploration. The greater number of entries made into the zone containing the mouse versus the object by male WT mice did not achieve statistical significance [F(1,15) = 3.56] (p = 0.08). For distance traveled, a three-way interaction of sex by genotype by condition [F(2,90) = 3.45] (p < 0.05) (Fig. 5B) and post hoc tests indicated that male Dlgap1 WTs traveled more distance near the mouse than female WTs [F(2,45) = 3.68] (p < 0.05), indicating more social approach. Furthermore, male HT and KO mice showed reduced distance traveled in the zone containing the mouse than male WT mice, indicating reduced social approach. The increased distance traveled around the mouse versus the object by male WT mice did not achieve statistical significance [F(1,15) = 4.33] (p = 0.05). For time spent rearing, an interaction of genotype by condition and post hoc analysis showed that WT mice spent more time rearing near the mouse than near the object, while Dlgap1 HT and KO mice did not [F(2,90) = 6.12] (p < 0.01) (Fig. 5C, Supp. Figure 4). Furthermore, WT mice spent more time rearing around the mouse than HT or KO mice. Finally, there was an interaction of sex by condition [F(1,90) = 4.22] (p < 0.05). Post hoc tests showed that female, but not male, mice spent more time rearing around the mouse than the object [F(1,47) = 6.93] (p < 0.01), and females spent less time rearing around the object than males (Supp. Figure 5) (P < 0.05). For horizontal time in the interaction zone, a trend for interaction of genotype by sex by condition was found [F(2,88) = 2.81] (p = 0.07). Post hoc tests showed that across genotypes, females mice spent more time in the zone surrounding the mouse versus the object [F(1,88) = 10.15] (p < 0.01) (Fig. 5D). Within males, WTs spent more time in the zone around the mouse than the object [F(2,88) = 4.59] (p < 0.05). Lastly, Newman Keuls post hoc tests showed that male WT mice spent more time around the mouse than male HT or KO mice. For the measure of duration, one male WT and one female WT were outliers, and were excluded from analysis.
Effect of Dlgap1 genotype on social approach in the first social interaction test for (A) number of entries, (B) total distance traveled, and (C) total time spent rearing, and (D) total horizontal time, all with respect to the zone containing the novel mouse or the object. Effect of Dlgap1 genotype in the second social interaction test for (E) log latency to the first sniff and (F) number of sniffs with respect to the mouse or the object. *Indicates a significant difference between conditions (mouse vs. object) (p < 0.05); +p indicates a difference between conditions at the trend level (0.05 < p < 0.10); #indicates a significant difference between genotype or sex (p < 0.05).
Experiment 2: For the log latency to the first sniff, a genotype by condition interaction [F(2,73) = 4.83] (p < 0.01) (Fig. 5D; Supp. Figure 6) and post hoc tests revealed that each genotype showed a shorter latency to sniff the mouse than the object (all p’s < 0.0001); however, Dlgap1 KO mice showed a shorter latency to sniff the object than WT and HT mice (p < 0.05). For the number of sniffs, no main effects or interactions were found. However, planned comparisons revealed that within each genotype and sex, all groups sniffed the mouse more than the object (all p’s < 0.008), with the exception of female Dlgap1 KO mice (Fig. 5E). Additionally, Dlgap1 KO females sniffed the mouse less than Dlgap1 KO males (p < 0.05).
Sucrose Preference
Sucrose preference was not altered by genotype or sex, and no interactions were found (Supp. Figure 7).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Discussion
Here we show that Dlgap1 KO mice exhibit impairments in the physical organization of the PSD and selective deficits in sociability. Alterations of the PSD in Dlgap1 KO mice included loss of association between core scaffold proteins that are components of postsynaptic signaling complexes. We also observed deficits in sociability in Dlgap1 KO mice using two distinct behavioral paradigms that rely on different dependent measures. One paradigm used automated data collection and measured horizontal time, number of entries, distance traveled, and vertical time within a zone proximal to the target mouse or object; the other paradigm assessed sniffing of the target mouse or object rather than mere proximity. Dlgap1 KO male mice also showed reductions in exploratory behavior in the dig test, and increased anhedonia in the splash test. However, these findings were not corroborated by other tests assessing similar constructs. For example, no differences in exploration were found in the open field test, and no differences in anhedonia were found in the sucrose preference test. Furthermore, no effect of genotype was found for locomotor behavior, nest building, startle reactivity, forced swim test behavior, or prepulse inhibition. Therefore, disorganization of specific protein complexes within the PSD and social approach deficits constitute selective deficits observed in Dlgap1 KO mice. Our findings are consistent with reports suggesting that genetic variation decreasing the function of DLGAP1 might contribute to risk for both schizophrenia13 and ASD9, disorders characterized by social deficits57,58.
Our biochemical results reveal that DLGAP1 is involved in connecting the top and bottom layers of the PSD scaffold machinery (Fig. 1). Immunoprecipitation of SHANK3 showed a decrease in the ternary association to DLG4 through DLGAP1. Moreover, immunoisolation of DLG scaffolds recovered few peptides for SHANK1-3 which could not be detected and quantitated by HPLC-MS/MS in all the replicates of Dlgap1 KO mice (Fig. 1A). These findings suggest a general impairment in the association of SHANKs and DLGs supercomplexes in Dlgap1 KO mice. Furthermore, we observed similar levels of the NMDAR receptors subunits Grin2A and Grin2B, PSD scaffolds, and adaptors DLG4, SynGAP1, and Homer1 between Dlgap1 WT and KO mice, suggesting that the alteration in protein interactions between the top and bottom layers of the PSD scaffold in Dlgap1 KO mice is not due to alterations in the levels of these proteins at the PSD. These protein interactions are influenced by several factors, including proteins’ binding affinities and the local concentration of proteins. One possibility is that lack of DLGAP1 frees available binding sites for other members of the DLGAP family (DLGAP2-4) to interact with DLG and SHANK family members, producing “novel” and perhaps abnormal PSD interactions. Thus, changes in the copy number of scaffolds such as DLGAP1 might increase or decrease the total number of “slots” available for interactions, and strongly influence PSD signaling machinery59. Furthermore, the interaction between SHANK3 and Homer1 was found to be unaltered in Dlgap1 KO mice. Homer1 has recently been implicated in depression-related behaviors in rodents60,61 and humans62. Our results indicating normal interactions between SHANK3 and Homer1 in Dlgap1 KO mice is consistent with the absence of any depression-related phenotype in these mice, including lack of effects in the FST and sucrose preference test.
Animal models have previously implicated NMDA receptor hypofunction in deficits in sociability. For example, mouse models of NMDAR hypofunction, including knockdown of the NR1 subunit63 or tissue specific and/or inducible knockout of NR163,64,65,66, have all reported reduced sociability. Specifically, knockout of NR1 in the forebrain during adulthood reduced social motivation64. Other genetic manipulations impairing NMDA receptor function, such as reduced NMDA glycine site affinity, also produce social deficits in mice67. Thus, the reduction in social approach observed in Dlgap1 KO mice might result from impaired NMDA receptor signaling due to disrupted organization of the supercomplexes containing NMDARs, DLG, DLGAP1 and SHANKs. In addition to the components of the supercomplexes, downstream effector proteins including AMPA receptors also play a role in social interactions. Indeed, AMPA receptors also regulate sociability68,69. Constitutive GluR1 receptor knockout mice show reductions in sociability70; however, deletion of GluR1 during late adolescence using a tamoxifen-inducible system leads to cognitive impairments, PPI deficits, and hyperlocomotion, but not social approach deficits71. Furthermore, haploinsufficiency of the ASD/SCZ associated gene Shank3 leads to reduced sociability in mice72. Our results extend this finding by showing that the reduced social behavior Dlgap1 KO mice also correlates with a deficit in SHANK3 protein interactions at the PSD (Fig. 1A). Thus, disruption of core scaffold proteins can contribute in a reciprocal manner to the disorganization of the core signaling machinery of the PSD and result in endophenotypes for psychiatric disorders.
We observed sex differences in the effect of Dlgap1 genotype on sociability. For example, male, but not female, Dlgap1 HT and KO mice showed reductions in horizontal time, vertical time, number of entries, and distance traveled in the region surrounding the target mouse (Fig. 5A,B). However, this finding is due in part to the greater horizontal time, number of entries, and distance traveled by WT males than WT females; thus, the lack of effect in females might be due to a floor effect (Fig. 5). However, female Dlgap1 HT and KO mice showed reductions in vertical time spent in the region surrounding the target mouse compared to WT mice (Fig. 5C; Supp. Figure 4), indicating reduced social approach. Interestingly, in the second social interaction test, all Dlgap1 genotypes showed a similar latency to sniff the mouse, but Dlgap1 KO mice had a shorter latency to sniff the object than Dlgap1 WT or HT mice, suggesting increased exploration of the object (Fig. 5E). Finally, female Dlgap1 KO mice were the only group that did not sniff the mouse more times than the object (Fig. 5E). Thus, decreased Dlgap1 expression reduced social approach in both sexes, although different measures were affected in males versus females. These findings demonstrate the importance of assessing multiple measures of sociability, since assessing only one could lead to an erroneous conclusion regarding sex differences in sociability. Future studies should further investigate the deficits in sociability in Dlgap1 KO mice; for example, assessing ultrasonic vocalizations could determine whether this phenotype has an early onset73.
Dlgap1 KO mice also showed alterations in several other behavioral measures. They showed decreased exploration in the dig test (Fig. 3); however, we did not find alterations in other exploratory behaviors, such as rearing in the open field test. We also assessed sensorimotor gating (PPI), which is reduced in patients with OCD, ASD, or schizophrenia, but did not observe any differences due to genotype in mice (Supp. Figure 2). Even though we found consistent reductions in the social approach of Dlgap1 KO mice, we found few genotypic differences in other tests of anhedonia, such as the sucrose preference test. In the splash test, Dlgap1 KO male mice showed evidence of anhedonia in only one measure, the latency to groom (Fig. 3F). Finally, Dlgap1 KO mice show normal levels of locomotor activity (Fig. 2A), unlike the mice harboring null alleles of other PSD proteins such as SHANK274 or SHANK375. Since the consequences of gene knockout can vary on different genetic backgrounds76, more studies will be required to determine whether knockout of Dlgap1 primarily affects social approach behavior in different mouse strains. Although Dlgap1 HT and KO mice showed reductions in sociability, but not other behavioral abnormalities observed in schizophrenia or autism patients such as PPI deficits46,47,48,49, reduced DLGAP1 function may still contribute to risk for these disorders. Recent human genetic studies indicate that the risk for neuropsychiatric disorders is sometimes conferred by single gene variants, but is often conferred by variation in many genetic loci, such that one gene contributes only to a portion of the behavioral syndrome77,78. Here, we suggest that reduction of DLGAP1 function may contribute to the reduced sociability observed in disorders linked to this gene, including autism9 and schizophrenia8,13,14,15.
The Dlgap family has distinct regional expression patterns in the brain31. While Dlgap1 is expressed at a high density in the cortex, a brain region implicated in social cognition79, Dlgap3 is enriched in striatum80. Interestingly, Dlgap3 has also been associated with OCD-related phenotypes80. Thus, behavioral phenotypes associated with mutations in Dlgap family members might better correlate to their relative expression levels in different brain regions, rather than to specific protein interactions within the PSD. Furthermore, other PSD elements, including the SHANK proteins81, are also differentially expressed across brain regions. Thus, although mutations in Dlgaps or Shanks might disrupt the scaffold structure of the PSD, the correlation between mutations and behavioral phenotypes might depend on the overlapping patterns of expression of each family member. For example, Shank1 or Dlgap1 mutations might correlate with behaviors dependent on cortical function, while Shank3 or Dlgap3 mutations may have a better correlation with behaviors modulated by striatal activity.
Our present findings show that lack of Dlgap1 in mice disrupts protein interactions within the PSD of the cortex, and induces selective deficits in sociability. Our results suggest that one function of DLGAP1 protein is to interlink and connect scaffold proteins in the PSD. The reductions in sociability were highly specific, with no changes in locomotor activity, anxiety, sensorimotor gating, depression-like behaviors, or nest-building observed in Dlgap1 KO mice. Yet, several reports suggest that alterations to PSD proteins can produce age-dependent changes in anxiety or depression-related behaviors82,83. Thus, our present results cannot rule out that DLGAP1 KO mice might have alterations in these phenotypes during the juvenile period, or following aging. Our findings are consistent with observations that individuals affected by schizophrenia or ASD carry rare genetic variants that are predicted to reduce the function of DLGAP1. In sum, the present findings extend upon previous reports that PSD dysfunction can lead to deficits in sociability. Future studies should further establish the precise mechanisms by which Dlgap1 KO contributes to social deficits.
References
Bayes, A. et al. Comparative study of human and mouse postsynaptic proteomes finds high compositional conservation and abundance differences for key synaptic proteins. PLoS One 7, e46683, https://doi.org/10.1371/journal.pone.0046683 (2012).
Bayes, A. et al. Evolution of complexity in the zebrafish synapse proteome. Nat Commun 8, 14613, https://doi.org/10.1038/ncomms14613 (2017).
Bayes, A. et al. Characterization of the proteome, diseases and evolution of the human postsynaptic density. Nat Neurosci 14, 19–21, https://doi.org/10.1038/nn.2719 (2011).
Collins, M. O. et al. Proteomic analysis of in vivo phosphorylated synaptic proteins. J Biol Chem 280, 5972–5982, https://doi.org/10.1074/jbc.M411220200 (2005).
Fernandez, E. et al. Targeted tandem affinity purification of PSD-95 recovers core postsynaptic complexes and schizophrenia susceptibility proteins. Mol Syst Biol 5, 269, https://doi.org/10.1038/msb.2009.27 (2009).
Husi, H., Ward, M. A., Choudhary, J. S., Blackstock, W. P. & Grant, S. G. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci 3, 661–669, https://doi.org/10.1038/76615 (2000).
Distler, U. et al. In-depth protein profiling of the postsynaptic density from mouse hippocampus using data-independent acquisition proteomics. Proteomics 14, 2607–2613, https://doi.org/10.1002/pmic.201300520 (2014).
Focking, M. et al. Proteomic and genomic evidence implicates the postsynaptic density in schizophrenia. Mol Psychiatry 20, 424–432, https://doi.org/10.1038/mp.2014.63 (2015).
Li, J. et al. Integrated systems analysis reveals a molecular network underlying autism spectrum disorders. Mol Syst Biol 10, 774, https://doi.org/10.15252/msb.20145487 (2014).
Ting, J. T., Peca, J. & Feng, G. Functional consequences of mutations in postsynaptic scaffolding proteins and relevance to psychiatric disorders. Annu Rev Neurosci 35, 49–71, https://doi.org/10.1146/annurev-neuro-062111-150442 (2012).
Stewart, S. E. et al. Genome-wide association study of obsessive-compulsive disorder. Mol Psychiatry 18, 788–798, https://doi.org/10.1038/mp.2012.85 (2013).
Gazzellone, M. J. et al. Uncovering obsessive-compulsive disorder risk genes in a pediatric cohort by high-resolution analysis of copy number variation. J Neurodev Disord 8, 36, https://doi.org/10.1186/s11689-016-9170-9 (2016).
Kirov, G. et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry 17, 142–153, https://doi.org/10.1038/mp.2011.154 (2012).
Genovese, G. et al. Increased burden of ultra-rare protein-altering variants among 4,877 individuals with schizophrenia. Nat Neurosci 19, 1433–1441, https://doi.org/10.1038/nn.4402 (2016).
Xing, J. et al. Resequencing and Association Analysis of Six PSD-95-Related Genes as Possible Susceptibility Genes for Schizophrenia and Autism Spectrum Disorders. Sci Rep 6, 27491, https://doi.org/10.1038/srep27491 (2016).
Li, J. et al. Long-term potentiation modulates synaptic phosphorylation networks and reshapes the structure of the postsynaptic interactome. Sci Signal 9, rs8, https://doi.org/10.1126/scisignal.aaf6716 (2016).
Yao, W. D. et al. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron 41, 625–638 (2004).
Frank, R. A. et al. NMDA receptors are selectively partitioned into complexes and supercomplexes during synapse maturation. Nature communications 7, 11264, https://doi.org/10.1038/ncomms11264 (2016).
Frank, R. A. W., Zhu, F., Komiyama, N. H. & Grant, S. G. N. Hierarchical organization and genetically separable subfamilies of PSD95 postsynaptic supercomplexes. J Neurochem, https://doi.org/10.1111/jnc.14056 (2017).
Husi, H. & Grant, S. G. Isolation of 2000-kDa complexes of N-methyl-D-aspartate receptor and postsynaptic density 95 from mouse brain. J Neurochem 77, 281–291 (2001).
Carlisle, H. J., Fink, A. E., Grant, S. G. & O’Dell, T. J. Opposing effects of PSD-93 and PSD-95 on long-term potentiation and spike timing-dependent plasticity. J Physiol 586, 5885–5900, https://doi.org/10.1113/jphysiol.2008.163469 (2008).
Charlesworth, P., Morton, A., Eglen, S. J., Komiyama, N. H. & Grant, S. G. Canalization of genetic and pharmacological perturbations in developing primary neuronal activity patterns. Neuropharmacology 100, 47–55, https://doi.org/10.1016/j.neuropharm.2015.07.027 (2016).
Elias, G. M. et al. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron 52, 307–320, https://doi.org/10.1016/j.neuron.2006.09.012 (2006).
Grant, S. G., Marshall, M. C., Page, K. L., Cumiskey, M. A. & Armstrong, J. D. Synapse proteomics of multiprotein complexes: en route from genes to nervous system diseases. Hum Mol Genet 14(Spec No. 2), R225–234, https://doi.org/10.1093/hmg/ddi330 (2005).
Komiyama, N. H. et al. SynGAP regulates ERK/MAPK signaling, synaptic plasticity, and learning in the complex with postsynaptic density 95 and NMDA receptor. J Neurosci 22, 9721–9732 (2002).
MacLaren, E. J., Charlesworth, P., Coba, M. P. & Grant, S. G. Knockdown of mental disorder susceptibility genes disrupts neuronal network physiology in vitro. Mol Cell Neurosci 47, 93–99, https://doi.org/10.1016/j.mcn.2010.12.014 (2011).
Migaud, M. et al. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 396, 433–439, https://doi.org/10.1038/24790 (1998).
Nithianantharajah, J. et al. Synaptic scaffold evolution generated components of vertebrate cognitive complexity. Nat Neurosci 16, 16–24, https://doi.org/10.1038/nn.3276 (2013).
Frank, R. A., Zhu, F., Komiyama, N. H. & Grant, S. G. Hierarchical organisation and genetically separable subfamilies of PSD95 postsynaptic supercomplexes. Journal of Neurochemistry (submitted) (2017).
Li, J. et al. Spatiotemporal profile of postsynaptic interactomes integrates components of complex brain disorders. Nat Neurosci 20, 1150–1161, https://doi.org/10.1038/nn.4594 (2017).
Welch, J. M., Wang, D. & Feng, G. Differential mRNA expression and protein localization of the SAP90/PSD-95-associated proteins (SAPAPs) in the nervous system of the mouse. J Comp Neurol 472, 24–39, https://doi.org/10.1002/cne.20060 (2004).
Kindler, S., Rehbein, M., Classen, B., Richter, D. & Bockers, T. M. Distinct spatiotemporal expression of SAPAP transcripts in the developing rat brain: a novel dendritically localized mRNA. Brain Res Mol Brain Res 126, 14–21, https://doi.org/10.1016/j.molbrainres.2004.03.014 (2004).
Ruscio, A. M., Stein, D. J., Chiu, W. T. & Kessler, R. C. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 15, 53–63, https://doi.org/10.1038/mp.2008.94 (2010).
Wu, E. Q., Shi, L., Birnbaum, H., Hudson, T. & Kessler, R. Annual prevalence of diagnosed schizophrenia in the USA: a claims data analysis approach. Psychol Med 36, 1535–1540, https://doi.org/10.1017/S0033291706008191 (2006).
Samson, A. C. et al. Emotion dysregulation and the core features of autism spectrum disorder. J Autism Dev Disord 44, 1766–1772, https://doi.org/10.1007/s10803-013-2022-5 (2014).
Ho, E. V. et al. Clinically effective OCD treatment prevents 5-HT1B receptor-induced repetitive behavior and striatal activation. Psychopharmacology (Berl) 233, 57–70, https://doi.org/10.1007/s00213-015-4086-8 (2016).
Jiao, J., Opal, M. D. & Dulawa, S. C. Gestational environment programs adult depression-like behavior through methylation of the calcitonin gene-related peptide gene. Mol Psychiatry 18, 1273–1280, https://doi.org/10.1038/mp.2012.136 (2013).
Dulawa, S. C., Scearce-Levie, K. A., Hen, R. & Geyer, M. A. Serotonin releasers increase prepulse inhibition in serotonin 1B knockout mice. Psychopharmacology (Berl) 149, 306–312 (2000).
Zhu, H. et al. Abnormal response to stress and impaired NPS-induced hyperlocomotion, anxiolytic effect and corticosterone increase in mice lacking NPSR1. Psychoneuroendocrinology 35, 1119–1132, https://doi.org/10.1016/j.psyneuen.2010.01.012 (2010).
Paulus, M. P., Dulawa, S. C., Ralph, R. J. & Mark, A. G. Behavioral organization is independent of locomotor activity in 129 and C57 mouse strains. Brain Res 835, 27–36 (1999).
Deacon, R. M. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc 1, 122–124, https://doi.org/10.1038/nprot.2006.20 (2006).
Ducottet, C. & Belzung, C. Behaviour in the elevated plus-maze predicts coping after subchronic mild stress in mice. Physiol Behav 81, 417–426, https://doi.org/10.1016/j.physbeh.2004.01.013 (2004).
Yalcin, I., Belzung, C. & Surget, A. Mouse strain differences in the unpredictable chronic mild stress: a four-antidepressant survey. Behav Brain Res 193, 140–143, https://doi.org/10.1016/j.bbr.2008.04.021 (2008).
Deacon, R. M. Assessing nest building in mice. Nat Protoc 1, 1117–1119, https://doi.org/10.1038/nprot.2006.170 (2006).
Hess, S. E. et al. Home improvement: C57BL/6J mice given more naturalistic nesting materials build better nests. J Am Assoc Lab Anim Sci 47, 25–31 (2008).
Braff, D. L. Prepulse inhibition of the startle reflex: a window on the brain in schizophrenia. Curr Top Behav Neurosci 4, 349–371 (2010).
Ludewig, K., Geyer, M. A. & Vollenweider, F. X. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biol Psychiatry 54, 121–128 (2003).
Perry, W., Minassian, A., Lopez, B., Maron, L. & Lincoln, A. Sensorimotor gating deficits in adults with autism. Biol Psychiatry 61, 482–486, https://doi.org/10.1016/j.biopsych.2005.09.025 (2007).
McAlonan, G. M. et al. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain 125, 1594–1606 (2002).
Ahmari, S. E., Risbrough, V. B., Geyer, M. A. & Simpson, H. B. Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacology 37, 1216–1223, https://doi.org/10.1038/npp.2011.308 (2012).
Hoenig, K., Hochrein, A., Quednow, B. B., Maier, W. & Wagner, M. Impaired prepulse inhibition of acoustic startle in obsessive-compulsive disorder. Biol Psychiatry 57, 1153–1158, https://doi.org/10.1016/j.biopsych.2005.01.040 (2005).
Shanahan, N. A. et al. Chronic reductions in serotonin transporter function prevent 5-HT1B-induced behavioral effects in mice. Biol Psychiatry 65, 401–408, https://doi.org/10.1016/j.biopsych.2008.09.026 (2009).
Shanahan, N. A., Velez, L. P., Masten, V. L. & Dulawa, S. C. Essential role for orbitofrontal serotonin 1B receptors in obsessive-compulsive disorder-like behavior and serotonin reuptake inhibitor response in mice. Biol Psychiatry 70, 1039–1048, https://doi.org/10.1016/j.biopsych.2011.07.032 (2011).
Detke, M. J., Johnson, J. & Lucki, I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol 5, 107–112 (1997).
Kim, E. et al. GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules. J Cell Biol 136, 669–678 (1997).
Naisbitt, S. et al. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron 23, 569–582 (1999).
Okruszek, L. et al. Social cognition in neuropsychiatric populations: a comparison of theory of mind in schizophrenia and mesial temporal lobe epilepsy. Sci Rep 7, 484, https://doi.org/10.1038/s41598-017-00565-2 (2017).
Schenkel, L. S., Spaulding, W. D. & Silverstein, S. M. Poor premorbid social functioning and theory of mind deficit in schizophrenia: evidence of reduced context processing? J Psychiatr Res 39, 499–508, https://doi.org/10.1016/j.jpsychires.2005.01.001 (2005).
Walkup, W. G. et al. A model for regulation by SynGAP-alpha1 of binding of synaptic proteins to PDZ-domain ‘Slots’ in the postsynaptic density. Elife 5, https://doi.org/10.7554/eLife.16813 (2016).
Serchov, T., Heumann, R., van Calker, D. & Biber, K. Signaling pathways regulating Homer1a expression: implications for antidepressant therapy. Biol Chem 397, 207–214, https://doi.org/10.1515/hsz-2015-0267 (2016).
Wagner, K. V. et al. Homer1/mGluR5 activity moderates vulnerability to chronic social stress. Neuropsychopharmacology 40, 1222–1233, https://doi.org/10.1038/npp.2014.308 (2015).
Leber, S. L. et al. Homer1a protein expression in schizophrenia, bipolar disorder, and major depression. J Neural Transm (Vienna) 124, 1261–1273, https://doi.org/10.1007/s00702-017-1776-x (2017).
Milenkovic, M., Mielnik, C. A. & Ramsey, A. J. NMDA receptor-deficient mice display sexual dimorphism in the onset and severity of behavioural abnormalities. Genes Brain Behav 13, 850–862, https://doi.org/10.1111/gbb.12183 (2014).
Jacobs, S. & Tsien, J. Z. Adult forebrain NMDA receptors gate social motivation and social memory. Neurobiol Learn Mem 138, 164–172, https://doi.org/10.1016/j.nlm.2016.08.019 (2017).
Finlay, J. M. et al. Effects of prefrontal cortex and hippocampal NMDA NR1-subunit deletion on complex cognitive and social behaviors. Brain Res 1600, 70–83, https://doi.org/10.1016/j.brainres.2014.10.037 (2015).
Saunders, J. A. et al. Knockout of NMDA receptors in parvalbumin interneurons recreates autism-like phenotypes. Autism Res 6, 69–77, https://doi.org/10.1002/aur.1264 (2013).
Labrie, V., Lipina, T. & Roder, J. C. Mice with reduced NMDA receptor glycine affinity model some of the negative and cognitive symptoms of schizophrenia. Psychopharmacology (Berl) 200, 217–230, https://doi.org/10.1007/s00213-008-1196-6 (2008).
Gascon, E. et al. Alterations in microRNA-124 and AMPA receptors contribute to social behavioral deficits in frontotemporal dementia. Nat Med 20, 1444–1451, https://doi.org/10.1038/nm.3717 (2014).
O’Connor, E. C., Bariselli, S. & Bellone, C. Synaptic basis of social dysfunction: a focus on postsynaptic proteins linking group-I mGluRs with AMPARs and NMDARs. Eur J Neurosci 39, 1114–1129, https://doi.org/10.1111/ejn.12510 (2014).
Wiedholz, L. M. et al. Mice lacking the AMPA GluR1 receptor exhibit striatal hyperdopaminergia and ‘schizophrenia-related’ behaviors. Mol Psychiatry 13, 631–640, https://doi.org/10.1038/sj.mp.4002056 (2008).
Inta, D. et al. Phenotype of mice with inducible ablation of GluA1 AMPA receptors during late adolescence: relevance for mental disorders. Hippocampus 24, 424–435, https://doi.org/10.1002/hipo.22236 (2014).
Bozdagi, O. et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism 1, 15, https://doi.org/10.1186/2040-2392-1-15 (2010).
Gandal, M. J. et al. Mice with reduced NMDA receptor expression: more consistent with autism than schizophrenia? Genes Brain Behav 11, 740–750, https://doi.org/10.1111/j.1601-183X.2012.00816.x (2012).
Schmeisser, M. J. et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 486, 256–260, https://doi.org/10.1038/nature11015 (2012).
Wang, X. et al. Altered mGluR5-Homer scaffolds and corticostriatal connectivity in a Shank3 complete knockout model of autism. Nat Commun 7, 11459, https://doi.org/10.1038/ncomms11459 (2016).
Sittig, L. J. et al. Genetic Background Limits Generalizability of Genotype-Phenotype Relationships. Neuron 91, 1253–1259, https://doi.org/10.1016/j.neuron.2016.08.013 (2016).
Geschwind, D. H. & Flint, J. Genetics and genomics of psychiatric disease. Science 349, 1489–1494, https://doi.org/10.1126/science.aaa8954 (2015).
Kim, Y. S. & State, M. W. Recent challenges to the psychiatric diagnostic nosology: a focus on the genetics and genomics of neurodevelopmental disorders. Int J Epidemiol 43, 465–475, https://doi.org/10.1093/ije/dyu037 (2014).
Frith, C. D. The social brain? Philos Trans R Soc Lond B Biol Sci 362, 671–678, https://doi.org/10.1098/rstb.2006.2003 (2007).
Bienvenu, O. J. et al. Sapap3 and pathological grooming in humans: Results from the OCD collaborative genetics study. Am J Med Genet B Neuropsychiatr Genet 150B, 710–720, https://doi.org/10.1002/ajmg.b.30897 (2009).
Monteiro, P. & Feng, G. SHANK proteins: roles at the synapse and in autism spectrum disorder. Nat Rev Neurosci 18, 147–157, https://doi.org/10.1038/nrn.2016.183 (2017).
Nosyreva, E., Autry, A. E., Kavalali, E. T. & Monteggia, L. M. Age dependence of the rapid antidepressant and synaptic effects of acute NMDA receptor blockade. Front Mol Neurosci 7, 94, https://doi.org/10.3389/fnmol.2014.00094 (2014).
Inta, D. et al. Significant increase in anxiety during aging in mGlu5 receptor knockout mice. Behav Brain Res 241, 27–31, https://doi.org/10.1016/j.bbr.2012.11.042 (2013).
Acknowledgements
Brain Research Foundation (SCD), NARSAD Independent Investigator Award (SCD), IMHRO Rising Star Depression Research Award in Memory of George Largay (SCD), and a gift from the Norton Gerali Foundation (SCD); T32MH020065 and NARSAD Young Investigator Award (MJR); Welcome Trust (NHK, SGNG); National Institute of Child Health and Human Development (MH104603-01A1; to M.P.C.); Della Martin Foundation (JAK).
Author information
Authors and Affiliations
Contributions
M.P.C., R.M.J., and S.C.D. wrote the manuscript. M.P.C. performed biochemical experiments. R.M.J., E.V.H., and S.L.T. performed behavioral experiments. K.N.H. and G.G.N. provided DLGAP1 knockout mice. K.J.A. made intellectual contributions. All authors edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coba, M.P., Ramaker, M.J., Ho, E.V. et al. Dlgap1 knockout mice exhibit alterations of the postsynaptic density and selective reductions in sociability. Sci Rep 8, 2281 (2018). https://doi.org/10.1038/s41598-018-20610-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20610-y
This article is cited by
-
The dark side of synaptic proteins in tumours
British Journal of Cancer (2022)
-
Dissecting indirect genetic effects from peers in laboratory mice
Genome Biology (2021)
-
Gut microbiome partially mediates and coordinates the effects of genetics on anxiety-like behavior in Collaborative Cross mice
Scientific Reports (2021)
-
Intrauterine hyperglycemia impairs memory across two generations
Translational Psychiatry (2021)
-
Cognitive impairment and autistic-like behaviour in SAPAP4-deficient mice
Translational Psychiatry (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.