Abstract
The dispersion of pathogenic microorganisms consists of the transport of pathogens from their source to inoculate a new host. Agricultural and economic importance of the Soybean root rot justifies studying this disease, especially the role of insects as dispersers. The aim of this study was to evaluate the role of the ladybird beetle, Cycloneda sanguinea Linnaeus (Coleoptera: Coccinellidae) in the dispersion of pathogens that cause Soybean root rot. Three pathogen species, Macrophomina phaseolina (Tassi) (Sphaeropsidales: Botryosphaeriaceae), Fusarium incarnatum-equiseti species complex (FIESC), and F. commune (Skovgaard) O’Donnell & Nirenberg were isolated from the midgut of ladybird beetles and cultured. Macrophomina phaseolina was identified by morphology while for the other two species, DNA was sequenced. The DNA extracted was amplified in the Internal Transcriber Spacer (ITS) region, sequenced and compared to voucher sequences deposited in the GenBank. Sequences of nucleotide ITS1-5.8 S were identified in the regions of rDNA-ITS4 ribosomal DNA. This is the first report of Macrophomina phaseolina (Tassi) (Sphaeropsidales: Botryosphaeriaceae), Fusarium incarnatum-equiseti species complex (FIESC), and F. commune (Skovgaard) O’Donnell & Nirenberg, being dispersed by C. sanguinea in Brazilian soybean fields.
Similar content being viewed by others
Introduction
Infectious disease has a major influence on the demography of human, plant, and animal populations. Plant pathogen dispersion is a process of movement of propagules from one host to a new host. In this context, plant pathogens produce a large number of propagules with most of them lost before reaching the host plant. Survival strategies of microorganisms such as hyphae and spores in an insect-vector enable the primary cycle initiation of a disease. Insects transmit some plant diseases and can retain the pathogens in the absence of host plants, contributing to the pathogen’s survival1. Some microorganisms can multiply, reproduce, and undergo cyclic changes inside an insect vector1,2,3,4,5.
Fusarium (Hypocreales: Nectriaceae) species cause rot diseases in different crops in South America. Fusarium incarnatum (Roberge) Saccardo was isolated from soil and aerial plant parts in banana and palm trees. This fungus has been implicated in plant diseases, including walnut canker, kangaroo paw blight of ornamental plants, bean pod and seed rot, reducing sorghum seed germination and seedling growth, melon corky dry rot and storage rot problems in mushrooms, besides being the dominant fungus in pearl millet grain6. Fusarium equiseti (Corda) Saccardo is a cosmopolitan soil inhabitant worldwide, mainly in dry areas. Damage caused by this fungus occurs in senescent plant tissue and produces diseases such as the sour cherry tree cankers, rot in pumpkin and cucurbit fruits in contact with soil, and diseases in the date palm6. Fusarium commune Skovgaard, O’Donnell & Nirenberg was isolated from soil and pea in Denmark and it is widespread in the Northern hemisphere in different crops such as carnation, corn, carrot, barley, and western white pine7. In tropical areas, F. commune was isolated from Dajiao bananas in Guangdong, China and Cavendish bananas in Brazil8. Fusarium commune caused damping-off and seedling root rot on soybean in the United States9.
Many different insect species are known to vector Fusarium species such as Xylosandrus compactus (Eichhoff) (Coleoptera: Curculionidae)10, Dendroctonus sp. (Coleoptera: Curculionidae)11, Reticulitermes flavipes (Kollar) (Blattodea: Rhinotermitidae)12, Diabrotica speciosa (Germar) (Coleoptera: Chrysomelidae)13, Spodoptera littorallis (Boisduval) (Lepidoptera: Noctuidae)14, Xyleborus fornicatus (Eichhorn) (Coleoptera: Curculionidae)15, Triatoma sp. (Hemiptera: Reduviidae)16,17, Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae)16,18, Cyclocephala modesta Burm, Dyscinetus gagates Burm, and Diloboderus abderus Sturm (Coleoptera: Scarabaeidae)5.
The ladybird beetle, Cycloneda sanguinea Linnaeus (Coleoptera: Coccinellidae) occurs in several countries in the Americas19. Larvae of C. sanguinea feed voraciously on aphids, ingesting fluid from their body, while adults devour the aphids completely19,20. This insect is a predator of Aphis gossypii Glover, Aphis papaveris Fabricius, Hyadaphis foeniculi, Hyalopterus pruni Geoffroy, Macrosiphum euphorbiae Thomas, Macrosiphum rosae Linnaeus, Macrosiphum persicae Sulzer, Ropalosiphum maidis Fitch, Toxoptera aurantii (Boyer de Fonscolombe) and Schizaphis graminum Rondani (Aphididae)3,18,19,20,21,22.
The increasing incidence of the soybean root rot in Southern Brazil justifies researches on insects dispersing and spreading inoculum of this disease. The aim of this study was to evaluate the dispersal of fungal root pathogens, by isolating the fungi of C. sanguinea collected in soybean fields, followed by a pathogenicity test in healthy soybean plants.
Results
Molecular fungi identification
Three pathogen species were isolated from the midgut of C. sanguinea: Macrophomina phaseolina, Fusarium incarnatum-equiseti and F. commune. Fungi were identified based on morphological characteristics of Macrophomina phaseolina (Tassi) Goidanich. Specific identification of the other two species was possible by extracting and sequencing DNA from fungi isolated from the digestive tract of larvae and adults. Sequenced ITS1-5.8S-ITS4 regions of the ribosomal DNA were identified as F. incarnatum-equiseti complex species and F. commune (Fig. 1). GenBank accession numbers are KR082312 and KR082314.
Association of detected pathogens with environmental parameters
The frequency of isolation of the fungal pathogens from the C. sanguinea was correlated with soil parameters and weather data. Macrophomina phaseolina had a positive relationship with Ca, Mg, Al, S, clay, P, K, Cu, Zn, pressure, temperature; and had a negative one with organic matter, pH (water), humidity and dew point. Fusarium incarnatum-equiseti and F. commune were negatively correlated with clay, P, Zn, humidity and rain; and a positive correlation with temperature and solar radiation per year (Table 1).
Pathogenicy tests
The healthy soybean plants inoculated with F. incarnatum-equiseti and F. commune isolates originating from the gastric cecum (digestive tract) of C. sanguinea larvae and adults showed the following symptoms of root rot: internerval leaf chlorosis, internerval necrosis and seedling damping-off and necrosis in shoots and branches. The F. incarnatum-equiseti and F. commune isolation frequency in soybean roots (12.50 ± 3.30% SD) also reduced the seed germination (average 87.50 ± 33.07% SD), lap diameter (average 0.51 ± 0.20% SD) during plant reproduction phase and, increased leaf (2.00 ± 0.89% SD) and root (1.75 ± 0.68% SD) symptoms. Macrophomina phaseolina was not tested for pathogenicity as it is an established pathogen of soybeans.
Discussion
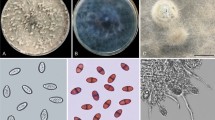
Fusarium species isolated from the C. sanguinea digestive tract were morpho-physiologically identified and confirmed by molecular analysis of the ITS region of group 99, the F. incarnatum-equiseti species complex23. Fusarium incarnatum has a history of taxonomic and nomenclatural disagreements because of its isolate morphological variability23. Fusarium equiseti have two forms, macroconidia and microconidia. Macroconidia are strongly septate, sickle-shaped, and thick with a different curvature in the mycelium that is oval6. The sexual stage of F. equiseti is given by the specific epithet Gibberella intricans Wollenweber. Fusarium incarnatum also have macroconidia and microconidia, slender, with dorsal curved, and a straighter ventral surface (Fig. 2C). The apical cell is curved and tapers to a point; the basal cell is foot-shaped24. Microconidia are pyriform and uniseptate. The sexual or teleomorph stage of this fungus has not been described6,24.
Fusarium species. (A) Fusarium incarnatum-equiseti. (B) Fusarium commune mass spores on soybean stem. (C) Fusarium incarnatum-equiseti macroconidia 3- to 5-septate curved spores (120 µm). (D) Fusarium commune microconidia aseptate oval or elliptical spores (4.2 µm). Figures (A–D) were obtained by Salgado-Neto.
Fusarium commune have macroconidia and microconidia. Differing from other Fusarium species, the macroconidia of F. commune are short to medium long (3 to 5 µm), straight to slightly curved, slender, and thin-walled. The apical cell is tapered and curved, and sometimes with a sling hook25. Microconidia are oval, elliptical, or kidney shaped, usually aseptate (Fig. 3D). Conidia are produced on short monophialides in false heads, singly or in pairs on the aerial mycelium. The sexual or teleomorph stage of F. commune is unknown7.
Macrophomina phaseolina infect roots, stems, leaves, and fruits of various plant species in Brazil. It was first reported infecting beans and soybeans, causing charcoal rot disease with numerous tiny, black, and irregularly shaped microsclerotia (Fig. 3). For this fungus, these microsclerotia are survival structures allowing it to persist for a long time in the soil. Transport of contaminated soil during soil tillage and preparation is the one of the main dispersal methods of M. phaseolina within and between fields, and consequently, this fungus has become a globally important crop pathogen26,27. However, M. phaseolina lost its ability to reproduce sexually, or can do it only under special environmental conditions26,27,28.
The presence of F. incarnatum-equiseti and F. commune spores and hyphae was confirmed into midgut and mouthparts of C. sanguinea on soybean roots and stems. Similar studies have indicated a symbiotic relationship between F. solani and H. hampei16. Larvae of Scarabaiedae such as C. modesta, D. gagates, and D. abderus may spread F. oxysporum inoculum5. Fusarium spores are abundant in nature and can be transmitted and dispersed when passing ungerminated through insect alimentary tracts. Passage of Fusarium spores through the midgut of Musca domestica L. (Muscidae: Diptera) did not alter the spores in their physical appearance or ability to germinate29. One potential biochemical capability detected in Fusarium isolates that may benefit insect hosts include sterol synthesis, detoxification of (plant or venomous preys) derived allelochemicals and secondary metabolites, and nitrogen scavenging from unconventional substrates, including cyanide and formamide30. The presence of Fusarium species in the midgut suggests that C. sanguinea may be harboring this fungus in order to benefit from its metabolic capabilities.
Coccinellids are mycophagous, foraging in patches of asexual spores, may acquire conidial inoculum acting as mechanical vectors of pathogens31,32. The fungal spores are attractive as food for coccinellids, and therefore should not survive digestion. However, spores remained viable in the mycophagy Halyziini-powdery mildew system31,32. Another system involves a diet of aphids present on apple for the coccinellid Hippodamia convergens Guérin-Méneville carrying the fungal pathogen Discula destructiva Redlin (dogwood anthracnose) excreting viable spores31,32,33. In this context, spores Fusarium are found in plant tissues where phytophagous insects can acquire them via the cuticle or midgut, and, consequently, these spores are acquired by C. sanguinea through predation.
The presence of spores and hyphae of F. incarnatum-equiseti and F. commune in C. sanguinea indicates a close interaction between C. sanguinea larvae and the Fusarium complex. The teleomorph stage of F. incarnatum and F. commune is unknown but possibly occurs inside the midgut of larvae of C. sanguinea. The dispersion of M. phaseolina, F. incarnatum-equiseti and F. commune by C. sanguinea possibly starts with larval feeding (mycophagy, phytophagy, and zoophagy), absorbing spores and hyphae, near the soil in soybean stem as a protein and sugar source for this insect. The midgut of C. sanguinea can shelter pathogenic fungi so they may complete the reproductive cycle and disperse intact spores by excretion34.
High deficiencies or concentrations of N, P, K and micronutrients, and organic matter occurs in sandy and compacted soils, also linked to low pH reduces plant growth, favoring root rot development35,36. Mineral nutrients such as K, Ca, Mg, S, B, Mn and Fe favour healthy plants and avoid the entrance of external pathogens37. However, the effect of a nutrient on a particular combination (pathogen-host plant), cannot be generalized because some of them may increase disease severity, while others reduce it, as found here.
The weather factors affect the release and dispersal of Fusarium spores into the environment. A passively-released spore is a particle in a given area and its release from a pustule or conidiophore depends on mechanical force (caused by wind or rain) overcoming cohesive forces. Mechanical force from wind or rain is the main cause for particles adhering to plant surfaces to be removed. The chemicals exuded by splash-dispersed fungi, help spores to adhere to plant surfaces when they dry and also help the spores to be splashed when wet by reducing the surface tension of the water film. Changes in humidity, solar radiation and the atmospheric electric charge have been correlated with Fusarium spore mass release38. In this study, the mass release of Fusarium spores was observed after fog in the soybean crops (Fig. 2A,B).
This is the first study to report the presence F. incarnatum-equiseti species complex and F. commune with Fusarium disease symptoms dispersed by C. sanguinea in soybean crops. It is also the first report of M. phaseolina, which causes charcoal rot disease, and F. incarnatum-equiseti species complex, and F. commune simultaneously spread by C. sanguinea. This information contributes to our understanding of the geographical distribution of root phytopathogens in Brazil. Moreover, it brings new findings to the insect dispersing phytopathogens and to improve crop protection measures.
Methods
Local data and biological material
Experiments were carried out in the municipality of Santa Maria, Rio Grande do Sul state, Brazil (29.4056351°S, 53.4421171°W). Insect sampling was done four times a year on the seeds and roots of G. max between 2012 and 2013. Larvae and adults of C. sanguinea were collected in two soybean cultivars (BMX Power RR variety) with trenches (50 cm L × 25 cm W × 30 cm H) with 20 samples and 25 m distance.
Molecular fungi identification
Larvae and adults of C. sanguinea were disinfected in ethanol and rinsed in sterile distilled water. A tube in which the beetle was immersed was covered by the barrier film, inverted three times, and sonicated for 2 min at 40 kHz (model 8851-34, Cole Parmer, Vernon Hills, IL); the rinse was repeated with sterile distilled water. The mouthparts, prothorax, cuticle, and midgut were dissected and placed in the Eppendorf tubes with 100 mL of 0.85% saline solution18 in the laboratory of phytopathology at the Universidade Federal de Santa Maria (UFSM). Then, samples of C. sanguinea in saline solution were added to Petri dishes with 3% PDA (Potato Dextrose Agar) media and incubated in a growth chamber (25 ± 6 °C, 75 ± 5% and a photoperiod 12:12 h [L:D]) for seven days5.
DNA was extracted in cetyltrimethylammonium bromide (CTAB)39. The mycelium was triturated in micro centrifuge tubes (1.5 mL, 10.8 × 40.6 mm) with the aid of a plastic pestle. The extracted genomic DNA was subjected to polymerase chain reaction (PCR) to amplify the ITS (Internal Transcribed Spacer) region located between the genes encoding the 18 S and 28 S ribosomal RNAs. The primers used were ITS1 (5′-TCCGTAGG TGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′).
Amplification and direct sequencing of fungi ribosomal RNA genes for phylogenetic analysis were conducted40. The products amplified were purified by precipitation with polyethylene glycol41, sequenced by the chain termination reaction method employing the reagent Big Dye 3.1 (Applied Biosystems), and analyzed in an automated capillary sequencer 3500 L (Applied Biosystems).
Species identification was based on the similarity of GenBank sequence databases. Similar sequences were searched in the GenBank using GenBank’s BLAST tool and aligned on the BioEdit program with the ClustalW algorithm. An outgroup was also chosen in the GenBank and aligned together to prepare a phylogenetic dendrogram using the MEGA5 software42.
A phylogenetic dendrogram was built for the Fusarium spp. isolates obtained from the midgut of C. sanguinea larvae and adults. Evolutionary distances were calculated and evolutionary history inferred with the Maximum Likelihood Method43. The branches show the percentage of trees with the associated taxa clustered together. Initial tree(s) for the heuristic search were obtained automatically. The maximum parsimony method was used when the number of common sites was <100 or lower than one-fourth of the total number of sites. The Bioinformatics Neighbor Joining (BIONJ) method with Maximum Composite Likelihood (MCL) distance matrix was used showing branches with the highest probability (log −1416.3605).
Soil and weather analysis
In the soybean crops, soil was removed and sieved in a 2 mm metal wire mesh (0.8 × 0.4 m). The percentage of clay, organic matter, pH (water), and quantities of P, K, Ca, Mg, S, Al, Zn, B, and Cu from soil samples were analyzed at the “Centro de Ciências Rurais” of the “Universidade Federal de Santa Maria” (Santa Maria, Rio Grande do Sul, Brasil). Temperature (°C), humidity (%), dew point (%), pressure (hPa), wind speed (m/s), radiation (kJ/m²), and rainfall (mm) were obtained from the “Instituto Nacional de Meteorologia” (INMET) (Brazil) for the days that samples were taken.
Pathogenicy test
Pathogenicity test was performed in plastic pots, (21 cm W × 17 cm H), filled with HA PLANTMAX substrate with two soybean seeds per pot. Pots were placed in a greenhouse under a natural photoperiod and temperature ranged from 25 to 30 °C during March, April, and May 2013. The three treatments, Fusarium oxysporum Schlechtendahl emend. Snyder and Hansen isolated from the alimentary the gut of C. sanguinea larvae (T1), F. incarnatum-equiseti and F. commune isolated from the gut of C. sanguinea larvae/adults (T2) and uninfected control (T3) were arranged in a completely randomized design with eight replications.
Statistics
Data were analyzed by non-parametric Kruskal-Wallis test for stochaistic dominance. A Dunn’s test of multiple comparisons was also used for comparisons of the means at 5% significance level. Presence of Fusarium root and seed germination was analyzed using the exact binomial test (5% probability level) and compared with 2 × 2 treatments. The tests were performed with the R software44.
References
Douglas, A. E. & Beard, C. B. Microbial symbioses in the midgut of insects. In: Biology of the insect midgut (eds Lehane, M. J. and Billingsley, P. F.) 419–429 (Chapmam & Hall, 1996).
Ma, L. J. et al. Fusarium Pathogenomics. Annu. Rev. Microbiol. 67, 399–416, https://doi.org/10.1146/annurev-micro-092412-155650 (2013).
Ploetz, R. C. Fusarium induced diseases of tropical, perennial crops. Phytopathology 96, 648–652, https://doi.org/10.1094/PHYTO-96-0648 (2006).
Raman, A., Wheatley, W. & Popay, A. Endophytic fungus-vascular plant-insect interactions. Environ. Entomol. 41, 433–447, https://doi.org/10.1603/EN11317 (2012).
Salgado-Neto, G., Vaz, M. A. B., Guedes, J. V. C., Muniz, M. F. B. & Blume, E. Fusarium oxysporum dispersion by larvae of Cyclocephala modesta, Dyscinetus gagates and Diloboderus abderus in Brazil. Cienc. Rural 46, 943–949, https://doi.org/10.1590/0103-8478cr20150471 (2016).
Leslie, J. F. & Summerell, B. A. The Fusarium laboratory manual (Blackwell Publishing, 2006).
Skovgaard, K., Rosendahl, S., O’Donnell, K. & Nirenberg, H. I. Fusarium commune is a new species identified by morphological and molecular phylogenetic data. Mycologia 95, 630–636 (2003).
Li, C. Y., Mostert, G., Zuo, C. W., Beukes, I. & Yang, Q. S. Diversity and distribution of the banana wilt pathogen Fusarium oxysporum f. sp. cubense in China. Fungal Genom. Biol. 3, 1 (2013).
Ellis, M. L., Díaz Arias, M. M., Cruz Jimenez, D. R., Munkvold, G. P. & Leandro, L. F. First report of Fusarium commune causing damping-off, seed rot, and seedling root rot on soybean (Glycine max) in the United States. Plant Dis. 97, 284, https://doi.org/10.1094/PDIS-07-12-0644-PDN (2013).
Le Pelley, R. H. Pests of coffee (Longman, 1968).
Whitney, H. S. Relationships between bark beetles and symbiotic organisms. In: Bark beetles in North American conifers. A system for study of evolutionary biology (eds. Milton, J. B. & Stureeon, R. B.) 183–211 (University of Texas Press, 1982).
Zoberi, M. & Grace, K. Fungi associated with subterranean termite Reticulitermes flavipes (Kollar) in Ontario. Mycology 82, 289–294, https://doi.org/10.2307/3759899 (1990).
Silva-Werneck, J. O., De Faria, M. R., Abreu Neto, J. R. M. V., Magalhães, B. P. & Schimidt, F. G. V. Técnica de criação de Diabrotica speciosa (Germ.) (Coleoptera; Chrysomelidae) para bioensaios com bacilos e fungos entomopatogênicos. An. Soc. Entomol. Bras. 24, 45–52 (1995).
Ismail, M. A. & Abdel-Sater, M. A. Fungi associated with the Egyptian cotton leaf worm Spodoptera littoralis Boisduval. Mycopathologia 124, 79–86, https://doi.org/10.1007/BF01103106 (1993).
Kumar, N. S., Hewavitharanage, P. & Adikaram, N. K. B. Histology and fungal flora of shot-hole borer beetles (Xyleborus fornicatus) galleries in tea (Camellia sinensis). J. Natl. Sci. Found. 26, 195–207 (1998).
Morales-Ramos, J. A., Rojas, M. G., Sittertz-Bhatkar, H. & Saldana, G. Symbiotic relationship between Hypothenemus hampei (Coleoptera: Scolytidae) and Fusarium solani (Moniliales, Tuberculariaceae). Ann. Entomol. Soc. Am. 93, 541–547, https://doi.org/10.1603/0013-8746(2000)093[0541:SRBHHC]2.0.CO;2 (2000).
Rocha, L. L. V., Andrade, N. C., Zanuncio, J. C. & Serrão, J. E. Endocrine and regenerative cells in the midgut of Chagas disease vector Triatoma vitticeps during different starvation periods. Folia Biol-Krakow 62, 259–267, https://doi.org/10.3409/fb62_3.259 (2014).
Gama, F. C., Teixeira, C. A., Garcia, A., Costa, J. N. M. & Lima, D. K. S. Diversity of filamentous fungi associated with Hypothenemus hampei (Ferrari) (Coleoptera: Scolytidae) and its galleries in berries of Coffea canephora (Pierre). Neotrop. Entomol. 35, 573–578, https://doi.org/10.1590/S1519-566X2006000500002 (2006).
Santos, G. P. & Pinto, A. C. Q. Biologia de Cycloneda sanquinea e sua associação com pulgão em mudas de mangueira. Pesqui. Agropecu. Bras. 16, 473–476 (1981).
Fernandes, F. S. et al. Within-plant distribution of cotton aphids, Aphis gossypii Glover (Hemiptera: Aphididae), in Bt and non-Bt cotton fields. B. Entomol. Res. 102, 78–87, https://doi.org/10.1017/S0007485311000381 (2012).
Fernandes, F. S. et al. Within plant distribution and dynamics of Hyadaphis foeniculi (Hemiptera: Aphididae) in field fennel intercropped with naturally colored cotton. Fla. Entomol. 96, 92–103, https://doi.org/10.1653/024.096.0112 (2013).
Malaquias, J. B. et al. Estimating development of the fennel aphid, Hyadaphis foeniculi (Passerini) (Hemiptera: Aphididae) using nonlinear models. Pest Manag. Sci. 71, 744–751, https://doi.org/10.1002/ps.3845 (2015).
Nirenberg, H. I. Recent advances in the taxonomy of Fusarium. Stud. Mycol 32, 91–101 (1990).
Geiser, D. M. et al. One fungus, one name: defining the genus Fusarium in a scientifically robust way that preserves longstanding use. Phytopathology 103, 400–408, https://doi.org/10.1094/PHYTO-07-12-0150-LE (2013).
Stewart, J. E., Mee-Sook, K., Robert, L. J., Dumroese, R. K. & Klopfenstein, N. B. Molecular characterization of Fusarium oxysporum and Fusarium commune isolates from a conifer nursery. Phytopathology 96, 1124–1133, https://doi.org/10.1094/PHYTO-96-1124 (2006).
Agrios, G. N. Plant Pathology (Elsevier, 2005).
Desmukh, S. K., Misra, J. K., Tewari, J. P. & Papp, T. Fungi: Applications and Management Strategies (CRC Press, 2016).
Webster, J. & Weber, R. W. S. Introduction to Fungi (Cambridge University Press, 2007).
Bolton, H. T. & Hansens, E. J. Ability of the house fly, Musca domestica, to ingest and transmit viable spores of selected fungi. Ann. Ent. Soc. Am. 63, 98–100, https://doi.org/10.1093/aesa/63.1.98 (1970).
Teetor-Barsch, G. H. & Roberts, D. W. Entomogenous Fusarium species. Mycopathologia 84, 3–16, https://doi.org/10.1007/BF00436991 (1983).
Hodek, I. & Evans, E. W. 2012. Food relationships in Ecology and Behaviour of the Ladybird Beetles (Coccinellidae) (eds. Hodek, I., Van Emden, H.F. & Honek, A.)141–274 (Wiley-Blackwell, 2012).
Sutherland, A. M. & Parrella, M. P. Mycophagy in Coccinellidae: review and synthesis. Biol. Control 51, 284–293, https://doi.org/10.1016/j.biocontrol.2009.05.012 (2009).
Hed, B. E., Windham, M. T. & Grant, J. F. Survival of conidia of Discula destructiva in frass of the convergent lady beetle. Plant Dis. 83, 806–809, https://doi.org/10.1094/PDIS.1999.83.9.806 (1999).
Hatcher, P. E. Three‐way interactions between plant pathogenic fungi, herbivorous insects and their host plants. Biol. Rev. 70, 639–694, https://doi.org/10.1111/j.1469-185X.1995.tb01655.x (1995).
Altomare, C., Norvell, W. A., Bjorkman, T. & Harman, G. E. Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295–22. Appl. Environ. Microbiol. 65, 2926–2933 (1999).
Liddell, C. M. Abiotic factors and soilborne diseases. In: Soilborne diseases of tropical crops (eds. Hillocks, R.J. and Waller, J.M.) 365–376 (CAB International, 1997).
Tedersoo, L. et al. Global diversity and geography of soil fungi. Science 346, 1256688, https://doi.org/10.1126/science.1256688 (2014).
Jones, A. M. & Harrison, R. M. The effects of meteorological factors on atmospheric bioaerosol concentrations–a review. Sci. Total Environ. 326, 151–180, https://doi.org/10.1016/j.scitotenv.2003.11.021 (2004).
Doyle, J. J. & Doyle, J. L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19, 11–15 (1987).
White, T. J., Bruns, T., Lee, S. & Taylor, J. W. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics in PCR Protocols: A Guide toMethods and Applications (eds Innis, M. A., Gelfand, D. H., Snisky, J. J. and White, T. J.)315–322 (Academic Press Inc., 1990).
Shmitz, A. & Riesner, D. Purification of nucleic acids by selective precipitation with polyethylene glycol 6000. Anal. Biochem. 354, 311–313 (2006).
Tamura, K. et al. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739, https://doi.org/10.1093/molbev/msr121 (2011).
Tamura, K. & Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10, 512–526, https://doi.org/10.1093/oxfordjournals.molbev.a040023 (1993).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing (2015). Aviable at: http://www.r-project.org/ (November, 2016).
Acknowledgements
We thank Ivan Francisco Dressler da Costa from the “Departamento de Defesa Fitossanitária, Clínica Fitossanitária (CCR/UFSM)” for identification of Macrophomina phaseolina, Ricardo Harakava from the “Instituto Biológico de São Paulo, Laboratório de Bioquímica Fitopatológica”, for identification of Fusarium complex, Zaida Inês Antoniolli and Daiana Bortolluzzi Baldoni for assistance in molecular phylogenetic analysis. To the “Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG)” and the “Programa Cooperativo sobre Proteção Florestal (PROTEF) do Instituto de Pesquisas e Estudos Florestais (IPEF)” for the finantial support. Dr. Phillip Villani (University of Melbourne) revised and corrected the English language used in this manuscript.
Author information
Authors and Affiliations
Contributions
G.S.-N., M.A.B.V., J.V.C.G., M.F.B.M., E.B.; C.F.W., B.M.C.C., A.P.-R. and J.C.Z. designed the research; G.S.-N., M.A.B.V., J.V.C.G., M.F.B.M., E.B.; C.F.W., B.M.C.C., A.P.-R. and J.C.Z. performed the experiments; G.S.-N., M.A.B.V., J.V.C.G., M.F.B.M., E.B.; C.F.W., B.M.C.C., A.P.-R. and J.C.Z. analyzed the data, participated in writing and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salgado-Neto, G., Vaz, M.A.B., Guedes, J.V.C. et al. Dispersion of the soybean root rot by Cycloneda sanguinea (Coleoptera: Coccinellidae). Sci Rep 8, 2409 (2018). https://doi.org/10.1038/s41598-018-20587-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-20587-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.