Abstract
Agglomeration-triggered deactivation of supported platinum electrocatalysts markedly hinders their application in methanol oxidation reaction (MOR). In this study, graphene-supported nickel–iron layered double hydroxide (NiFe-LDH/rGO), in which Fe3+ was introduced to replace Ni2+ partially in the Ni(OH)2 lattice to provide stronger metal–support bonding sites, was utilized to immobilize Pt nanoparticles (NPs). Given the optimized metal–support interfacial contact (Fe3+-O(H)-Pt) between Pt NPs and NiFe-LDH/rGO nanosheets for Pt/NiFe-LDH/rGO electrocatalysts, the Pt/NiFe-LDH/rGO electrocatalysts displayed dramatically enhanced durability than that of Pt/Ni(OH)2/rGO counterpart as well as commercial Pt/C, and 86.5% of its initial catalytic activity can be maintained even after 1200 cycles of cyclic voltammetry (CV) tests during MOR. First-principle calculations toward the resultant M-O(H)-Pt (M = Fe3+, Ni2+) interfacial structure further corroborates that the NiFe-LDH nanosheets can provide stronger bonding sites (via the Fe3+-O(H)-Pt bonds) to immobilize Pt NPs than those of Ni(OH)2 nanosheets (via the Ni2+-O(H)-Pt bonds).
Similar content being viewed by others
Introduction
Graphene-supported Pt nanoparticles (NPs) have attracted sustained attention due to their distinct electrocatalytic performance in methanol oxidation reaction1. However, given the high surface energy, Pt NPs easily agglomerate and grow during catalytic reactions, especially for Pt NPs with a few nanometers in size2,3. Moreover, Pt-based catalysts are readily poisoned with chemisorbed intermediates generated during operations4,5. To overcome these two severe drawbacks, long-lived and anti-poisoned Pt electrocatalysts should be developed. The selection of proper supported materials and loading method are key points in constructing ideal Pt electrocatalysts6,7,8. Graphene modified with metal hydroxides or oxides is commonly selected as support to immobilize Pt NPs because graphene can transport electrons efficiently; in addition, metal hydroxides or oxides can facilitate water dissociation to generate oxygen-contained species, thereby accelerating the removal of poisoning carbonaceous species on adjacent Pt sites9,10,11.
Among a variety of metal hydroxides and oxides, layered double hydroxides (LDHs) present more advantages because of their distinct structures and highly synergetic with Pt NPs12,13,14,15. As a class of 2D anionic clays, LDHs possess positively charged brucite-like layers and intercalated anions12. Normally, the M2+ cations in the host structure of hydroxides are partially replaced with M3+ cations, and the excessive cationic charge generated by M3+ cations is balanced with anionic intercalation between different hydroxide layers13. Benefiting from the atomic-scale uniform distribution of site-specific and edge-sharing MO6 octahedra in the host structure, LDHs exhibit considerable potential as supports to immobilize Pt NPs due to the strong contact between metal and site-specific supports14. Recently, Chen and co-workers15 demonstrated that Pt NPs, covered with an atomic layer thickness of NiFe–LDH, are highly durable and efficient for the catalytic oxidation of carbon monoxide by taking advantage of the strong interfacial effect. Additionally, the combination of iron and nickel can significantly increase the water splitting ability by taking advantage of synergistic metal–metal interactions16,17,18,19,20. Furthermore, LDHs are more favorable toward water dissociation than that of metal oxides in alkaline electrolytes16. For example, NiFe–LDH nanosheets present comparable water splitting activity to that of scarce and expensive materials, such as IrO2 and RuO219. Despite some achievements, two fundamental limitations which have plagued the full use of LDH supported Pt nanocatalysts still remain: (i) in the contact interfaces between Pt NPs and LDH support, which part (M2+O6 or M3+O6 octahedra) is decisive in immobilizing Pt NPs is still not clear; (ii) traditional solution reduction method, reduce Pt precursors with reductant in liquids, cannot ensure that all Pt nanosrystals directly grown on the LDH supports.
In the present work, we selected the graphene-supported NiFe-LDH (NiFe-LDH/rGO) nanosheets as support materials to synthesize stable Pt electrocatalysts. Ultrafine Pt nanocrystals were directly grown on the NiFe-LDH/rGO nanosheets via photo-assisted in situ reduction of the adsorbed PtCl42− precursor solution. The as-prepared Pt x /NiFe-LDH/rGO electrocatalysts present significantly enhanced durability compared with that of their Pt y /Ni(OH)2/rGO counterparts for MOR. First-principle calculations reveal that the enhanced durability of Pt/NiFe-LDH/rGO electrocatalysts originates from the optimized interfacial contact between Pt NPs and site-specific NiFe-LDH support, in which the Fe3+-O(H)-Pt bonds can more efficiently immobilize Pt NPs than the Ni2+-O(H)-Pt bonds on the Ni(OH)2 support.
Result and Discussion
Strategy for synthesizing Pt/NiFe-LDH/rGO nanocomposites
Pt/NiFe-LDH/rGO electrocatalysts were synthesized by two steps. Primarily, negatively charged GO surface (ζ = −44.3 mV, Figure S1) was used as the template to adsorb Ni2+ and Fe3+ cations; urea was used as a precursor to provide a slow-, sustained-release source of hydroxyl21. Under hydrothermal reaction, GO can be reduced to rGO, and urea will decompose into hydroxyl, ammonia, and carbon dioxide (Equation 1). The hydroxyl formation provides an alkaline environment, which directly promotes the nucleation and growth of 2D NiFe-LDH on rGO (Equation 2)22.
The as-prepared NiFe-LDH/rGO and Ni(OH)2/rGO nanosheets were washed with deionized water and ethanol for several times. The zeta potentials of NiFe-LDH/rGO and Ni(OH)2/rGO nanosheets were +9.91 and −17.4 mV, respectively (Figure S2). Notably, given the positively charged surface originating from the unsaturated Fe3+O sites, the NiFe-LDH/rGO nanosheets showed better PtCl42− adsorption capacity than that of Ni(OH)2/rGO (Figure S3). Subsequently, as illustrated in Fig. 1, NiFe-LDH/rGO-supported Pt NPs were synthesized through photoassisted in situ reduction of adsorbed PtCl42−. The growth of Pt NPs on NiFe-LDH can be expressed by the following formulas:
-
(a)
Adsorption of PtCl42− on the surface of NiFe-LDH
$$\text{NiFe} \mbox{-} \text{LDH}+{{\rm{PtCl}}}_{4}^{2-}\to \text{NiFe} \mbox{-} \text{LDH}-{({{\rm{PtCl}}}_{4}^{2-})}_{{\rm{ad}}}$$(3) -
(b)
Creation of photogenerated electron–hole pairs
$$\text{NiFe} \mbox{-} \text{LDH}+{hv}\to {e}^{-}+{h}^{+}$$(4) -
(c)
Growth of Pt NPs on NiFe–LDH support
The ICP-AES result showed that the Ni2+ concentration in NiFe-LDH/rGO (79.125 μg mL−1) was close to that in Ni(OH)2/rGO (70.425 μg mL−1). In addition, a small amount of Fe3+ replaced Ni2+ in the Ni(OH)2 lattice in the preparation of NiFe-LDH/rGO (atomic ratio, Ni:Fe = 11.87:1). Notably, under the same Pt precursor addition, the loading amount of Pt NPs on NiFe-LDH/rGO was two times higher than that on Ni(OH)2/rGO after a trace amount of Fe3+ was introduced into the Ni(OH)2 lattice (Table S1). Two main factors are responsible for this result. First, the positively charged NiFe-LDH/rGO nanosheet surface possessed stronger PtCl42− adsorption capacity than that of Ni(OH)2/rGO nanosheets. This result indicated that a larger amount of adsorbed PtCl42− on NiFe-LDH/rGO nanosheets can participate in the photoreduction process than that on the Ni(OH)2/rGO supports. Second, the highly dispersed FeO6 octahedra in the LDHs matrix can hinder electron–hole recombination and promote the transfer of light-generated electrons to adsorbed reactants23,24. Therefore, more PtCl42− was reduced to Pt NPs on NiFe-LDH/rGO than that on Ni(OH)2/rGO.
Characterization of Pt/NiFe-LDH/rGO nanocomposites
Figure 2a shows the representative TEM image of Pt0.54/NiFe-LDH/rGO nanocomposite. NiFe-LDH closely grew on the rGO nanosheets, and Pt nanocrystals with an average diameter or width of 2 nm were loaded on the surface of NiFe-LDH (Fig. 2b). Moreover, Pt nanocrystals displayed clear lattice fringes with an interplanar distance of 0.227 nm (Fig. 2c), which was in good agreement with the Pt (111) crystallographic plane. The corresponding HAADF image and EDS elemental mapping images (Fig. 2d) of the Pt0.54/NiFe-LDH/rGO nanocomposite indicated that Pt nanocrystals uniformly dispersed on NiFe-LDH/rGO without agglomeration and Fe atoms homogeneously dispersed in the Ni(OH)2 matrix. C signals covered the whole vision of the image index to the rGO, thereby demonstrating that rGO acted as a template for NiFe-LDH growth. As a comparison sample, Pt0.12/Ni(OH)2/rGO (Figure S4) showed similar morphology with Pt0.54/NiFe-LDH/rGO and a good dispersity of Pt NPs on Ni(OH)2/rGO.
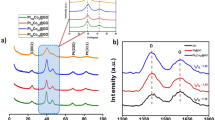
All diffraction peaks shown in the XRD patterns (Fig. 3a) of both NiFe-LDH/rGO and Ni(OH)2/rGO nanosheets matched well with the α-phase Ni(OH)2 (JCPDS No. 38-0715). The high–resolution XPS spectrum of Ni 2p in the NiFe-LDH/rGO (Fig. 3b) displayed two main peaks located at 856.09 and 873.74 eV, which suggested the +2 oxidation states of Ni25. In theory, Fe3+ is preferable than Fe2+ in fabricating NiFe-LDH because its ionic radius is similar to that of Ni2+ 26. As shown in Fig. 3c, the Fe 2p3/2 peaks overlapped with the Ni Auger peaks (near 706 and 712 eV). Thus, the satellite peak (718.80 eV) near the Fe 2p3/2 peak was selected as an indicator of the Fe3+ valence state16,27. The distances between the satellite peak (718.80 eV) and Fe 2p1/2 (724.70 eV) and Fe 2p3/2 (711.01 eV) were 5.90 and 7.79 eV, respectively. Quantitative analysis proved the +3 oxidation state of Fe28. In Fig. 3d, three peaks located at 284.78, 286.10, and 288.01 eV corresponded to C-C, C-O, and C=O bonds, respectively. The C-O and C=O peaks were evidently weaker than those of GO, thereby suggesting that GO was reduced after the hydrothermal reaction (Figure S5)29. Additionally, the emerging peak at 289.50 eV originated from the intercalated carbonate molecule (CO32−) in the NiFe-LDH nanosheets30. For comparison, the chemical compositions of the Ni(OH)2/rGO were also corroborated by XPS, and results are summarized in Figure S6.
Electrochemical performance of catalysts
Figure 4a illustrates the typical CV curves of Pt0.54/NiFe-LDH/rGO, Pt0.12/Ni(OH)2/rGO and commercial Pt/C. On the basis of integrated hydrogen desorption region31, the calculated result showed that the ECSA values of Pt catalysts on Pt0.54/NiFe-LDH/rGO (609.01 cm2 mg−1) and Pt0.12/Ni(OH)2/rGO (767.62 cm2 mg−1) were higher than that of commercial Pt/C (498.86 cm2 mg−1). And given that the comparatively low loading amount and higher dispersity of Pt NPs, as verified by the ICP-AES analysis, Pt0.12/Ni(OH)2/rGO (Pt concentration, 28.53 µg mL−1) ternary hybrids showed slightly higher electrocatalytic activity than that of Pt0.54/NiFe-LDH/rGO (Pt concentration, 146.80 µg mL−1) during MOR. Also, both two hydroxides supported Pt catalysts displayed higher peak current than the commercial Pt/C electrocatalysts (Fig. 4b). Figure 4c presents the CA curves of Pt0.54/NiFe-LDH/rGO, Pt0.12/Ni(OH)2/rGO and Pt/C at the potential of −0.2 V for 3600 s. This potential corresponded to the methanol oxidation peaks during forward sweep. The current density of Pt0.54/NiFe-LDH/rGO surpassed Pt0.12/Ni(OH)2/rGO catalyst after 830 s, and then showed the highest current density during the prolonged CA test. These results illustrated that the Pt0.54/NiFe-LDH/rGO electrocatalysts may possess better stability than that of its Pt0.12/Ni(OH)2/rGO and Pt/C counterparts. To evaluate the overall durability of Pt x /NiFe-LDH/rGO and Pt y /Ni(OH)2/rGO electrocatalysts during MOR testing and figure out which part (Ni2+O6 or Fe3+O6 octahedra in hydroxides supports) is decisive in immobilizing Pt NPs, long-term CV tests were applied at a potential ranging from −0.8 V to 0.2 V at a scan rate of 50 mV s−1. According to Fig. 5a and b, both types of electrocatalysts presented a volcanic-type trend. The mass activity of Pt x /NiFe-LDH/rGO and Pt y /Ni(OH)2/rGO increased with the increasing amount of loaded Pt initially but decreased when excessive amount of Pt precursor was reduced on the hydroxide support (Figures S7 and S8). Notably, after 600 cycles of CV test in methanol and potassium hydroxide mixture, each Pt x /NiFe-LDH/rGO electrocatalyst can maintain more than 93% of their catalytic activity. However, all the Pt y /Ni(OH)2/rGO electrocatalysts showed a considerable loss of catalytic activity during MOR, and less than 40% initial catalytic activity of Pt y /Ni(OH)2/rGO can be retained after 600 cycles of CV tests. Among these samples, Pt0.54/NiFe-LDH/rGO and Pt0.12/Ni(OH)2/rGO presented the optimal peak current density for Pt x /NiFe-LDH/rGO and Pt y /Ni(OH)2/rGO, respectively. In particular, the loading amount of Pt on Pt0.54/NiFe-LDH/rGO was 4.5 times higher than that on Pt0.12/Ni(OH)2/rGO. Commonly, the high loading amount of Pt was likely to cause the aggregation and deactivation of Pt32,33. Nevertheless, after 600 cycles of CV tests, 97.6% initial peak current density of the Pt0.54/NiFe-LDH/rGO electrocatalyst can be maintained (from 711.04 mA mg−1 to 694.28 mA mg−1), whereas the peak current density of Pt0.12/Ni(OH)2/rGO decreased from 724.28 mA mg−1 to 188.75 mA mg−1 (only 26.1% initial peak current density was retained) (Figs 5c,d and S9). Moreover, after 1200 cycles of CV tests, the peak current density of Pt0.54/NiFe-LDH/rGO still remained at 86.5% (Figure S10). By comparison, the commercial Pt/C electrocatalyst can only retain 61.3% of its initial peak current (Figure S11). Amazingly, after the introduction of Fe3+ into Ni(OH)2 lattice, the overall performance of Ptx/NiFe-LDH/rGO electrocatalysts were dramatically enhanced than Pty/Ni(OH)2/rGO as well as other literature reported results (Table S2). We ascribed such encouraging result, especially the durability of Pt x /NiFe-LDH/rGO, to the effect of ferric sites in Ni(OH)2 lattice.
Cyclic voltammetry (CV) curves of Pt0.54/NiFe-LDH/rGO, Pt0.12/Ni(OH)2/rGO and commercial Pt-C (a) in N2-saturated KOH (1 M) at a scan rate of 50 mV s−1 and (b) in KOH (1 M)/CH3OH (1 M) at a scan rate of 50 mV s−1. (c) Chronoamperometry (CA) curves of Pt0.54/NiFe-LDH/rGO, Pt0.12/Ni(OH)2/rGO and commercial Pt-C in KOH (1 M) + CH3OH (1 M).
To further understand the morphology and structure change of Pt x /NiFe-LDH/rGO and Pt y /Ni(OH)2/rGO after MOR cycling tests, systematical TEM characterization and size distribution of Pt NPs were carried out to study the change in the size of as-obtained electrocatalysts before and after the long-term MOR tests. Prior to MOR tests, both the Pt NPs loaded on NiFe-LDH/rGO and Ni(OH)2/rGO gradually grew with a quantitatively increasing addition of Pt precursors (Figure S12 and S13). Differently, the Pt NPs loaded on NiFe-LDH/rGO attached to one another and gradually grew into worm-like structures due to the high loading amount of Pt. By contrast, the Pt NPs loaded on Ni(OH)2/rGO always maintained a granular morphology. For consistency, the width of worm-like Pt and the diameter of particle-like Pt presented on NiFe-LDH/rGO were counted together when the size distribution of Pt nanocrystals was surveyed.
First, the most remarkable samples, namely, Pt0.54/NiFe-LDH/rGO and Pt0.12/Ni(OH)2/rGO, were selected to clarify the variety in the size of Pt NPs before and after 600 cycles of CV test. As shown in Fig. 6, the average size of Pt NPs in Pt0.12/Ni(OH)2/rGO increased from 1.56 nm to 4.12 nm. The Pt nanocrystal in Pt0.54/NiFe-LDH/rGO showed a slight increase from 2.1 nm to 2.5 nm in average size. In general, the surface energy of Pt NPs is closely linked to its size, and the initial Pt NPs of two contrasting samples should be similar in size34. Therefore, Pt0.36/NiFe-LDH/rGO and Pt0.24/Ni(OH)2/rGO were selected as contrasting samples because of their same average size of Pt nanocrystals prior to MOR testing (1.89 nm vs. 1.84 nm). After 600 cycles of CV tests, as given in Figure S14, the average size of Pt NPs on Pt0.24/Ni(OH)2/rGO increased markedly to 4.46 nm. Pt0.36/NiFe-LDH/rGO showed only a 0.29 nm increment in mean size (from 1.89 nm to 2.18 nm). These results confirmed that NiFe-LDH support can efficiently anchor Pt NPs, whereas the Ni(OH)2 support cannot. Consequently, severe agglomeration of Pt NPs occurred on the Ni(OH)2 support during MOR, which caused the quick deactivation of Pt x /Ni(OH)2/rGO electrocatalysts.
TEM image and Pt nanoparticle (NP) size distribution histogram of (a) initial Pt0.54/NiFe-LDH/rGO, (b) Pt0.54/NiFe-LDH/rGO after 600 cycles of CV test, (c) initial Pt0.12/Ni(OH)2/rGO, and (d) Pt0.12/Ni(OH)2/rGO after 600 cycles of CV test. CV tests were performed in1 M KOH+ 1 M CH3OH at a scan rate of 50 mv s−1.
In addition to the size effect, two main factors, namely, the instability of support and weak metal–support contact, are also closely linked to the agglomeration of Pt NPs on supports35,36. Relevant literature demonstrated that in an alkaline electrolyte, α-Ni(OH)2 is prone to convert into β-Ni(OH)2 during long-term electrochemical process37. The phase-transition-induced instability of support may result in the aggregation of Pt NPs, and this process can be detected by ongoing CV tests38. Thus, to evaluate the electrochemical stability of Ni(OH)2/rGO and NiFe-LDH/rGO supports, long-term CV tests were conducted in KOH solution (1 M), which contained methanol (1 M). Figure S15 shows that after 600 cycles of CV test, the latest CV curves of Ni(OH)2/rGO and NiFe-LDH/rGO supports exhibited almost no change compared with their initial ones. The favorable electrochemical stability of both supports is mainly due to the introduction of rGO39. The above results indicated that the aggregation of Pt NPs was not caused by the phase transition of hydroxides support.
On the basis of above analysis, first-principle calculations were performed to analyze the contact interface between Pt NPs and supports (Fig. 7), in which the metal–support interface may be directly linked to the stability of Pt NPs40. The adsorption energy (Ead) of Pt atom adsorbed on the sheets is defined as Ead = Etot − Esheet − EPt, where Etot is the total energy of system with sheets and adsorbed Pt atoms, Esheet is the energy of the optimized pure sheets, and EPt is the energy of one Pt atom in the vacuum. Therefore, a remarkably negative Ead value indicated strong the interaction of Pt atoms with Ni(OH)2 sheets or NiFe-LDH sheets. The adsorption energy Ead of Pt atom adsorbed on the NiFe-LDH sheets (via the Fe3+-O(H)-Pt bonding) was −2.87 eV, which was lower than that of Pt atom adsorbed on Ni(OH)2 sheets (via the Fe3+-O(H)-Pt bonding, −2.08 eV). The theoretical calculation revealed that the interaction of Pt atom with site-specific NiFe-LDH sheets was markedly stronger than that of Pt atom with Ni(OH)2 sheets. Thus, Pt/NiFe-LDH/rGO electrocatalysts were markedly more stable than Pt/Ni(OH)2/rGO electrocatalysts.
Conclusion
This work synthesized 2D NiFe-LDH/rGO and Ni(OH)2/rGO supports via a graphene-templated and urea-assisted hydrothermal route. Afterward, ultrafine Pt nanocrystals were loaded on NiFe-LDH/rGO and Ni(OH)2/rGO supports through photo-assisted in situ reduction of adsorbed PtCl42−. Both ternary composites displayed ultrafine Pt nanocrystals anchored on graphene-supported hydroxide nanosheets. Long-term MOR tests showed that after the introduction of ferric sites in Ni(OH)2 lattice, the Pt/NiFe-LDH/rGO nanocatalysts displayed dramatically enhanced durability compared with that of Pt/Ni(OH)2/rGO as well as commercial Pt/C electrocatalysts. The systematical TEM characterization and size distribution of Pt NPs of as-obtained electrocatalysts before and after long-term MOR tests, together with the chemical stability tests of NiFe-LDH/rGO and Ni(OH)2/rGO supports, confirmed that Fe3+ partially replaced Ni2+ in Ni(OH)2 lattice can efficiently enhance the stability of supported Pt NPs. First-principle calculations of the resultant M-O(H)-Pt (M = Fe3+, Ni2+) interfacial structure further corroborated that the NiFe-LDH nanosheets can provide stronger bonding sites (via the Fe3+-O(H)-Pt bonds) to immobilize Pt NPs than that of Ni(OH)2 nanosheets (via the Ni2+-O(H)-Pt bonds). Our results suggested that the excellent stability of Pt/NiFe-LDH/rGO was attributed to the strong contact between Pt NPs and site-specific NiFe-LDH support. In a broad sense, the strong contact between Pt NPs and site-specific supports elucidated in this work may provide a general strategy to synthesize site-specific anchoring Pt electrocatalysts with long-term stability and high catalytic activity.
Experimental Section
Chemical reagents and materials
All reagents used in the present experiments were of analytical grade and applied without further purification. Graphite powder was purchased from Tianjin Guangfu Fine Chemical Research Institute. GO was prepared from graphite with a modified Hummers method41. Commercial 20% Pt/C was purchased from Alfa Aesar. Deionized water (resistance, 18 MΩ cm−1) was used throughout all experiments.
Synthesis of NiFe-LDH/rGO and Ni(OH)2/rGO
The 2D NiFe-LDH/rGO hybrids were synthesized with a graphene-templated, urea-assisted hydrothermal method. Briefly, 30 mg of Ni(NO3)2·6H2O, 1.07 mg of Fe(NO3)3·9H2O, and 120 mg of urea were subsequently dissolved in 15 mL of GO aqueous solution (0.25 g L−1) under ultrasonic conditions. The obtained uniform solution was transferred into a Teflon cup in a stainless steel-lined autoclave. The autoclave was maintained at 100 °C for 12 h and cooled to room temperature naturally. The final products were washed with deionized water for several times and redispersed in 15 mL of aqueous solution. The 2D Ni(OH)2/rGO composite material was prepared using the same procedure but without the addition of ferric salt.
Synthesis of Pt x /NiFe-LDH/rGO and Pt y /Ni(OH)2/rGO
The 2D NiFe-LDH/rGOs or Ni(OH)2/rGO-supported Pt NPs were prepared via photoreduction of the Pt precursor solution. Prior to illumination, 2 mL of as-prepared NiFe-LDH/rGO or Ni(OH)2/rGO hybrids and a certain amount of sodium chloroplatinate solution (Na2PtCl4·4H2O, 4 mg mL−1) were added into a 50 mL quartz tube containing 30 mL of deionized water. After ultrasonic treatment for 30 min, the mixtures were stirred intensely and illuminated by a 300 W mercury lamp for 90 min with a distance of 10 cm at room temperature. The black precipitates were collected by centrifugation and washed with deionized water for five times. The final products were redispersed in 4 mL of ionized water for electrochemical measurements. A series of electrocatalysts was obtained and labeled as Pt x /NiFe-LDH/rGO (x = 0.17, 0.24, 0.36, 0.54, 0.57) and Pt y /Ni(OH)2/rGO (y = 0.07, 0.12, 0.15, 0.24) by adjusting the amounts of added Na2PtCl4·4H2O solution (0.5, 1.0, 1.5, 2.0, and 2.5 mL) and without changing any other parameter. The x or y values were obtained by the molar ratios of Pt and M (M = Ni or Fe). The mass concentrations of Ni, Fe, and Pt in different prepared catalysts were determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis.
Characterization
The phase structure of the prepared catalysts was analyzed through X-ray diffraction (XRD) with a Philips X’Pert system with Cu Ka radiation (λ = 0.15419 nm). The surface chemical constituents of the prepared catalysts were analyzed by X-ray photoelectron spectroscopy (XPS, Thermo ESCACLB 250). The surface zeta potentials of GO, NiFe-LDH/rGO, and Ni(OH)2/rGO were measured by a Malvern instrument (Nano-zs90). Transmission electron microscopy (TEM) images, high-angle annular dark-field (HAADF) scanning TEM images, and energy-dispersive spectroscopy (EDS) elemental mapping images were captured by a FEI Tecnai TF20 operated at 200 kV.
Electrochemical measurements
Prior to electrode preparation, the catalyst was ultrasonicated for 30 min to form a uniform suspension. A glassy carbon electrode (3 mm in diameter) was polished to a mirror finish with alpha alumina powder (0.05 μm) and ultrasonically cleaned in ethanol for 3 min. Subsequently, 5 μL of the as-prepared catalyst suspension was drop-casted onto a working electrode and dried at ambient temperature. When the slurry was dried, 10 μL of 0.05 wt.% Nafion solution was covered onto the dried sample. All electrochemical experiments were examined at room temperature (25 ± 1 °C). A standard three-electrode electrochemical workstation (Zahner IM6ex) was used for all electrochemical experiments. A Pt wire and an Ag/AgCl electrode were adopted as the counter and reference electrodes, respectively. All potentials in this study were reported with respect to Ag/AgCl electrode. The weight of Pt on each working electrode was calculated through ICP-AES tests. Prior to any electrochemical measurement, electrocatalysts were preactivated in N2-saturated KOH aqueous solution (1 M) by cyclic voltammetry (CV) cycling at the potential ranging from −1.0 V to 0.2 V at 100 mV s−1 for 30 cycles until the curve stabilized. Afterward, the CV curves were collected at a slow scan rate of 50 mV s−1 to calculate the electrochemically active surface area (ECSA) of different electrocatalysts. To evaluate the methanol oxidation performance, both the CV (from −0.8 V to 0.2 V, 50 mV s−1) and chronoamperometry (CA, 3600 s) tests were performed in a hybrid solution of KOH (1 M) containing CH3OH (1 M). Long-term CV cycling tests were also applied to assess the stability of each catalyst.
Theoretical calculation
First-principle calculations were conducted to evaluate the adsorption energy of Pt on the pure Ni(OH)2 sheets and NiFe-LDH sheets. Structure relaxation and total energy calculations were carried out using density functional theory within the generalized gradient approximation, as instructed in the VASP 5.4 package42. Electronic exchange and correlation are described by Perdew-Burke-Ernzerhof function43. All electron plane-wave basis sets with the projector augmented wave potentials were adopted with 2s22p4, 3d84s2, and 3d74s1, which were treated as valence electron configuration for O, Ni, and Fe, respectively. The cutoff energy was 500 eV. A sufficiently dense k-point sampling was evaluated with energy tolerance in 1 meV/atom. A vacuum higher than 15 Å thick was inserted in each model to avoid interaction with imaging free sheets. The ground state geometries were obtained by minimizing the forces on each atom to less than 0.01 eV/Å. The determined magnetic configuration of Ni(OH)2 sheets were antiferromagnetic.
References
Liu, M. M., Zhang, R. Z. & Chen, W. Graphene-Supported Nanoelectrocatalysts for Fuel Cells: Synthesis, Properties, and Applications. Chem. Rev. 114, 5117–5160 (2014).
Munnik, P., de Jongh, P. E. & de Jong, K. P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 115, 6687–6718 (2015).
Hodnik, N., Dehm, G. & Mayrhofer, K. J. J. Importance and Challenges of Electrochemical in Situ Liquid Cell Electron Microscopy for Energy Conversion Research. Acc. Chem. Res. 49, 2015–2022 (2016).
Lu, J. L., Li, Y. H., Li, S. L. & Jiang, S. P. Self-assembled platinum nanoparticles on sulfonic acid-grafted graphene as effective electrocatalysts for methanol oxidation in direct methanol fuel cells. Sci. Rep. 6, 21530 (2016).
Chen, A. C. & Holt-Hindle, P. Platinum-Based Nanostructured Materials: Synthesis, Properties, and Applications. Chem. Rev. 110, 3767–3804 (2010).
Yang, Z. H. & Nakashima, N. A simple preparation of very high methanol tolerant cathode electrocatalyst for direct methanol fuel cell based on polymer-coated carbon nanotube/platinum. Sci. Rep. 5, 12236 (2015).
Liu, X. et al. Oxide-Nanotrap-Anchored Platinum Nanoparticles with High Activity and Sintering Resistance by Area-Selective Atomic Layer Deposition. Angew. Chem. 129, 1670–1674 (2017).
Zhu, H. Y., Gu, C. D., Ge, X. & Tu, J. P. Targeted Growth of Pt on 2D Atomic Layers of Ni-Al Hydroxide: Assembly of the Pt/Exfoliated Ni-Al Hydroxide sheet/Graphene Composite as Electrocatalysts for Methanol Oxidation Reactions. Electrochim. Acta 222, 938–945 (2016).
Wu, S. L. et al. Photo-Excited in situ Loading of Pt Clusters onto rGO Immobilized SnO2 with Excellent Catalytic Performance Toward Methanol Oxidation. Nano Energy 26, 699–707 (2016).
Huang, W. J. et al. Highly Active and Durable Methanol Oxidation Electrocatalyst Based on the Synergy of Platinum-Nickel Hydroxide-Graphene. Nat. Commun. 6, 10035 (2015).
Li, Z. H. et al. Fast Electrosynthesis of Fe-Containing Layered Double Hydroxide Arrays Toward Highly Efficient Electrocatalytic Oxidation Reactions. Chem. Sci. 6, 6624–6631 (2015).
Yu, J., Wang, Q., O’Hare, D. & Sun, L. Preparation of Two Dimensional Layered Double Hydroxide Nanosheets and Their Applications. Chem. Soc. Rev. 46, 5950–5974 (2017).
Han, Y. F. et al. Preparation of Ni2+-Fe3+ Layered Double Hydroxide Material with High Crystallinity and Well-Defined Hexagonal Shapes. Chem. Mater. 20, 360–363 (2008).
Zhao, J. W. et al. A Hierarchical Heterostructure Based on Pd Nanoparticles/Layered Double Hydroxide Nanowalls for Enhanced Ethanol Electrooxidation. J. Mater. Chem. A 1, 5840–5846 (2013).
Chen, G. X. et al. Interfacial Effects in Iron-Nickel Hydroxide-Platinum Nanoparticles Enhance Catalytic Oxidation. Science 344, 495–499 (2014).
Gong, M. et al. An Advanced Ni-Fe Layered Double Hydroxide Electrocatalyst for Water Oxidation. J. Am. Chem. Soc. 135, 8452–8455 (2013).
Smith, R. D., Prévot, M. S., Fagan, R. D., Trudel, S. & Berlinguette, C. P. Water Oxidation Catalysis: Electrocatalytic Response to Metal Stoichiometry in Amorphous Metal Oxide Films Containing Iron, Cobalt, and Nickel. J. Am. Chem. Soc. 135, 11580–11586 (2013).
Wang, J. Y., Ji, L. L. & Chen, Z. F. In Situ Rapid Formation of a Nickel-Iron-Based Electrocatalyst for Water Oxidation. ACS Catal. 6, 6987–6992 (2016).
Wang, J. Y., Ji, L. L., Zuo, S. S. & Chen, Z. F. Hierarchically Structured 3D Integrated Electrodes by Galvanic Replacement Reaction for Highly Efficient Water Splitting. Adv. Energy Mater. 7, 1700107 (2017).
Dong, C. Q., Kou, T. Y., Gao, H., Peng, Z. Q. & Zhang., Z. H. Eutectic-Derived Mesoporous Ni-Fe-O Nanowire Network Catalyzing Oxygen Evolution and Overall Water Splitting. Adv. Energy Mater. 7, 1701347 (2017).
Lu, Z. Y. et al. Three-Dimensional NiFe Layered Double Hydroxide Film for High-Efficiency Oxygen Evolution Reaction. Chem. Commun. 50, 6479–6482 (2014).
Yoon, K. J. et al. Nano-Tailoring of Infiltrated Catalysts for High-Temperature Solid Oxide Regenerative Fuel Cells. Nano Energy 36, 9–20 (2017).
Nayak, S., Mohapatra, L. & Parida, K. Visible Light-Driven Novel g-C3N4/NiFe-LDH Composite Photocatalyst with Enhanced Photocatalytic Activity Towards Water Oxidation and Reduction Reaction. J. Mater. Chem. A 3, 18622–18635 (2015).
Fu, Q. et al. Interface-Confined Ferrous Centers for Catalytic Oxidation. Science 328, 1141–1144 (2010).
Long, X. et al. A Strongly Coupled Graphene and FeNi Double Hydroxide Hybrid as an Excellent Electrocatalyst for the Oxygen Evolution Reaction. Angew. Chem. 126, 7714–7718 (2014).
Cavani, F., Trifirò, F. & Vaccari, A. Hydrotalcite-Type Anionic Clays: Preparation, Properties and Applications. Catalysis today 11, 173–301 (1991).
Yamashita, T. & Hayes, P. Analysis of XPS Spectra of Fe2+ and Fe3+ Ions in Oxide Materials. Appl. Surf. Sci. 254, 2441–2449 (2008).
Ma, R. Z., Liang, J. B., Takada, K. & Sasaki, T. Topochemical Synthesis of Co-Fe Layered Double Hydroxides at Varied Fe/Co Ratios: Unique Intercalation of Triiodide and Its Profound Effect. J. Am. Chem. Soc. 133, 613–620 (2011).
Wu, S. L. et al. Highly Dispersed Ultrafine Pt Nanoparticles on Reduced Graphene Oxide Nanosheets: In Situ Sacrificial Template Synthesis and Superior Electrocatalytic Performance for Methanol Oxidation. ACS Appl. Mater. Interfaces 7, 22935–22940 (2015).
Tang, C. et al. Spatially Confined Hybridization of Nanometer-Sized NiFe Hydroxides into Nitrogen-Doped Graphene Frameworks Leading to Superior Oxygen Evolution Reactivity. Adv. Mater. 27, 4516–4522 (2015).
Qiu, Z. et al. NbC Nanowire-Supported Pt Nanoparticles as a High Performance Catalyst for Methanol Electrooxidation. J. Phys. Chem. C 117, 13770–13775 (2013).
Prieto, G., Zecevic, J., Friedrich, H., De Jong, K. P. & De Jongh, P. E. Towards Stable Catalysts by Controlling Collective Properties of Supported Metal Nanoparticles. Nat. Mater. 12, 34–39 (2013).
Holby, E. F., Sheng, W. C., Yang, S.-H. & Morgan, D. Pt Nanoparticle Stability in PEM Fuel Cells: Influence of Particle Size Distribution and Crossover Hydrogen. Energy Environ. Sci. 2, 865–871 (2009).
Lee, I., Delbecq, F., Morales, R., Albiter, M. A. & Zaera, F. Tuning Selectivity in Catalysis by Controlling Particle Shape. Nat. Mater. 8, 132–138 (2009).
Cheng, N. C. et al. Atomic Scale Enhancement of Metal-Support Interactions Between Pt and ZrC for Highly Stable Electrocatalysts. Energy Environ. Sci. 8, 1450–1455 (2015).
Cargnello, M. et al. Control of Metal Nanocrystal Size Reveals Metal-Support Interface Role for Ceria Catalysts. Science 341, 771–773 (2013).
Xia, D. D. et al. Facilely Synthesized α Phase Nickel-Cobalt Bimetallic Hydroxides: Tuning the Composition for High Pseudocapacitance. Electrochim. Acta 156, 108–114 (2015).
Li, T. et al. Large-Scale Self-Assembly of 3D Flower-like Hierarchical Ni/Co-LDHs Microspheres for High-Performance Flexible Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 8, 2562–2572 (2016).
Wang, H. L., Casalongue, H. S., Liang, Y. Y. & Dai, H. J. Ni(OH)2 Nanoplates Grown on Graphene as Advanced Electrochemical Pseudocapacitor Materials. J. Am. Chem. Soc. 132, 7472–7477 (2010).
Liu, J.-C., Wang, Y.-G. & Li, J. Toward Rational Design of Oxide-Supported Single-Atom Catalysts: Atomic Dispersion of Gold on Ceria. J. Am. Chem. Soc. 139, 6190–6199 (2017).
Hummers, W. S. Jr. & Offeman, R. E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 80, 1339 (1958).
Kresse, G. & Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B. 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Acknowledgements
This work was financially supported by the National Basic Research Program of China (2014CB931704), the Instrument Developing Project of the Chinese Academy of Sciences (YZ201627), the National Natural Science Foundation of China (NSFC, No. 51371166, 51571186, 51401206, 11504375, 11674321, 11604320) and the Anhui Provincial Natural Science Foundation (1508085QA21).
Author information
Authors and Affiliations
Contributions
Yechuang Han, Jun Liu and Changhao Liang designed the experiments. Yechuang Han performed the synthesis and majority of electrochemical tests. Pengfei Li carried out the theoretical calculation. Yixing Ye fabricated the GO nanosheets. Shouliang Wu, Yixing Ye and Zhenfei Tian provided significant advice in data processing and analysis to understand the relationship between the structure and performance of as-prepared catalysts.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, Y., Li, P., Liu, J. et al. Strong Fe3+-O(H)-Pt Interfacial Interaction Induced Excellent Stability of Pt/NiFe-LDH/rGO Electrocatalysts. Sci Rep 8, 1359 (2018). https://doi.org/10.1038/s41598-018-19876-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-19876-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.