Abstract
Although the cognitive impairment in Alzheimer’s disease (AD) is believed to be caused by amyloid-β (Aβ) plaques and neurofibrillary tangles (NFTs), several postmortem studies have reported cognitive normal subjects with AD brain pathology. As the mechanism underlying these discrepancies has not been clarified, we focused the neuroprotective role of astrocytes. After examining 47 donated brains, we classified brains into 3 groups, no AD pathology with no dementia (N-N), AD pathology with no dementia (AD-N), and AD pathology with dementia (AD-D), which represented 41%, 21%, and 38% of brains, respectively. No differences were found in the accumulation of Aβ plaques or NFTs in the entorhinal cortex (EC) between AD-N and AD-D. Number of neurons and synaptic density were increased in AD-N compared to those in AD-D. The astrocytes in AD-N possessed longer or thicker processes, while those in AD-D possessed shorter or thinner processes in layer I/II of the EC. Astrocytes in all layers of the EC in AD-N showed enhanced GLT-1 expression in comparison to those in AD-D. Therefore these activated forms of astrocytes with increased GLT-1 expression may exert beneficial roles in preserving cognitive function, even in the presence of Aβ and NFTs.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is primarily responsible for dementia, and its dramatic increase in the last decade is a worldwide problem1. The neurodegeneration caused by AD is believed to be triggered by an accumulation of amyloid-β (Aβ) plaques and neurofibrillary tangles (NFTs)2,3. Aβ plaques induce synaptic dysfunction and neuronal loss due to their toxicity, and also enhance the phosphorylation of tau4,5. NFTs are formed by aggregated phosphorylated tau and are involved in neuronal impairment and cell death6. Glutamate transmission at synapses is disturbed by the deposition of Aβ and NFTs, and the subsequent elevation in glutamate at axon terminals is associated with cognitive impairment7.
Although Aβ plaques and NFTs are hallmarks of AD, several postmortem studies have shown the presence of cognitive normal subjects with AD-specific pathological changes in the brain8,9. For example, the Medical Research Council Cognitive Function and Aging Study (MRC CFAS) revealed that 34.7% of subjects with massive neuritic plaques in the neocortex showed no dementia10. Further, the Nun study found that 32% of subjects with moderate NFT deposition in the neocortex showed no dementia11. The reason why AD-specific pathological changes do not cause cognitive impairment is based on differences in the length of education, occupation and mental status in childhood12,13,14; however, the detailed brain mechanisms involved in these discrepancies remain unclear.
Recently it has been reported that cognitive impaired subjects with an AD-specific pathology show a decreased number of neurons and synapses in the hippocampus and cerebral cortex, while cognitive normal subjects with an AD-specific pathology show a constant number of neurons and synaptic integrity in these brain regions15,16. In addition, an increased number of astrocytes or microglia was found in cognitive impaired subjects with an AD-specific pathology15,16, while no such increase was apparent in cognitive normal subjects with an AD-specific pathology. Astrocytes play a number of roles in supporting neurons and maintaining synaptic transmission17; however, an accumulation of Aβ plaques stimulate astrocyte conversion to a reactive type that release pro-inflammatory cytokines18. In contrast to such harmful reactive astrocytes, beneficial reactive astrocytes that play neuroprotective roles or aid in Aβ clearance in the aged brain were also found19,20.
We hypothesized that astrocytes in the brain from cognitive normal subjects with an AD-specific pathology might be of the beneficial type that play a neuroprotective role against Aβ and NFT deposition. To clarify this hypothesis, we examined the characteristics of astrocytes in postmortem brains from both cognitive normal and impaired subjects with an AD-specific pathology. The brains were obtained from bodies donated to Sapporo Medical University, and included a variety of cognitive states. We were particularly interested in the amyloid and tau hypothesis as it does not sufficiently explain AD based on the fact that drugs targeting Aβ are not effective in treating cognitive impairment21. The results of the present study led us to first propose the brain mechanism involved in maintaining cognitive function despite the progression of AD-specific pathological changes.
Results
Classification of AD neuropathological changes and cognitive function
After elimination of brains on the basis of the exclusion criteria, 51 brains were obtained for this study. We investigated age, years of education, Thal Aβ stage, Braak NFT stage, CERAD neuritic plaque score, and ABC score for each donor and brain (Table 1). For evaluation of neuropathological changes, the “A” and “B” in the “ABC score” were determined from the Aβ deposit- and NFT-affected regions, respectively, as described in Methods. Representative images of each A and B score are shown in Fig. 1a and b, respectively. “C” in the “ABC score” was evaluated from the density of plaques in the neocortex, as described in Methods. Representative images of each C score are shown in Fig. 1c. The overall AD pathological change was diagnosed from a combination of the three scores according to the NIA-AA Regan criteria. Based on these criteria, “Not” and “Low” were regarded as showing no AD pathological change, while “Intermediate” and “High” were regarded as showing AD neuropathological changes (Table 1).
AD neuropathological diagnosis. Representative images of each score for the AD neuropathological changes. (a) The “A” score was determined after evaluation of the Thal Phase for Aβ plaques. The figure shows an example of “A0” in which no Aβ deposits are observed in any area, “A1” in which deposits are observed in the temporal area, “A2” in which deposits are observed in the temporal area and basal ganglia, and “A3” in which deposits are observed in the temporal area, basal ganglia and cerebellum (Bar, 100 µm). (b) The “B” score was determined after evaluation of the Braak NFT stage. The figure shows an example of “B1” in which the entorhinal region is PHF-tau positive, “B2” in which the entorhinal region and temporal area are PHF-tau positive, and “B3” in which the entorhinal region, and temporal and occipital areas are PHF-tau positive (Bar, 500 µm). (c) The “C” score was determined after evaluation of the CERAD neuritic plaque score in the neocortex area by Bielshowsky silver staining. The figure shows an example of “C0” with no neuritic plaques, “C1” with 1 to 5 plaques per 1 mm2, “C2” with 6 to 20 plaques per 1 mm2, and “C3” with over 20 plaques per 1 mm2 (Bar, 100 µm). (d) Each score of Thal Aβ phase, Braak NFT stage and CERAD neuritic plaque score is significantly higher in the AD-N and AD-D groups in comparison with the N-N group. However, no differences are observed between the AD-N and AD-D groups. **P < 0.01, Kruskal-Wallis test and Mann-Whitney U test. Values are means ± SD (N-N: n = 19, AD-N: n = 10, AD-D: n = 18).
Cognitive function was assessed by postmortem evaluation using the CDR scale. The questions on the CDR were answered by members of the bereaved family or other acquaintances of the donor (Supplementary Table 1). ‟No dementia” was determined as a CDR score of 0 or 0.5, and “Dementia” was determined as a CDR score of 1, 2 or 3 for convenience as previously reported22,23,24. Based on the AD neuropathological changes and CDR scores, the 51 brains were classified into 4 groups: (1) No AD neuropathological changes with No dementia; N-N, (2) No AD neuropathological changes with Dementia; N-D, (3) AD neuropathological changes with No dementia; AD-N, and (4) AD neuropathological changes with Dementia; AD-D. As the aim of this study was to reveal the neuropathological features that distinguish the AD-N group from the AD-D group, we excluded the N-D group (4 of 51 brains) as this group may have included other neurological deficits such as dementia with Lewy bodies or cerebrovascular dementia. We also evaluated the activities of daily living using the Katz Index and I-ADL score.
We found that 19 of 47 brains (41%) belonged to the N-N group, 10 of 47 (21%) to the AD-N group, and 18 of 47 (38%) to the AD-D group (Table 1). Average age at death was 81.9 ± 7.8 in the N-N group, 87.9 ± 9.1 in the AD-N group, and 91 ± 8.8 in the AD-D group (Table 1). Age at death was significantly higher in the AD-D group than in the N-N group (P = 0.006). However, no difference was found between the AD-N and AD-D groups. The causes of death in each group are shown in Supplementary Table 1. Years of education were significantly higher in the N-N group (12.9 ± 3.3) than in the AD-N (9.7 ± 3.5, P = 0.04) and AD-D groups (9.5 ± 2.2, P = 0.01) (Table 1).
Thal Aβ phase, Braak NFT stage, and CERAD neuritic plaque score were significantly higher in the AD-N and AD-D groups than in the N-N group (Fig. 1d). However, no differences were found between the AD-N and AD-D groups (Fig. 1d). Details of the percentage of each ABC score (A as Thal Aβ phase, B as Braak NFT stage, and C as CERAD neuritic plaque score) in demented and non-demented subjects are shown in Supplementary Table 3. As the entorhinal cortex (EC) is an important area for the evaluation of cognitive function, we investigated neuropathological changes in this area.
The area of the EC
Coronal sections of the brain that cut through the lateral geniculate body and hippocampus are shown in Fig. 2, and the area of the EC (enclosed by red dotted lines in Fig. 2a) was measured. The area was significantly decreased in the AD-D group compared to the N-N group (Fig. 2b). However, no difference was found between the AD-N and AD-D groups (Fig. 2b).
The area of the EC. (a) Scanned images of the coronal sections of the brain that cut through the lateral geniculate body and hippocampus are shown (Bar, 10 mm). (b) The area of the EC enclosed by red dotted lines is significantly lower in the AD-D group compared to that in the N-N group. No difference is observed between the AD-N and AD-D groups. **P < 0.01, one-way ANOVA, Tukey post-test. Values are means ± SD (N-N: n = 19, AD-N: n = 10, AD-D: n = 18).
Aβ and PHF-tau in the EC
Aβ-positive area in the EC was significantly higher in the AD-N and AD-D groups than in the N-N group. Likewise, the PHF-tau-positive area in the EC was significantly higher in the AD-N and AD-D groups than in the N-N group (Fig. 3a). However, no differences were found in Aβ- and PHF-tau-positive areas between the AD-N and AD-D groups (Fig. 3a).
Immunohistochemical analysis of Aβ, PHF-tau, NeuN and synaptophysin in layer I-VI of the EC. (a) The Aβ- and PHF-tau-positive area is significantly higher in the AD-N and AD-D groups than in the N-N group. No difference is observed between the AD-N and AD-D groups (Bar, 500 µm). (b) The number of NeuN-positive cells in the EC is lower in the AD-D group compared to those in the N-N and AD-N groups. No difference is observed found in the number of NeuN-positive cells between the N-N and AD-N groups (Bar, 500 µm). (c) The synaptophysin-positive area is lower in the AD-D group than in the N-N and AD-N groups. No difference is observed between the N-N and AD-N groups (Bar, 500 µm). *P < 0.05, **P < 0.01, one-way ANOVA, Tukey post-test. Values are means ± SD (N-N: n = 19, AD-N: n = 10, AD-D: n = 18).
The number of NeuN-positive cells and the synaptophysin-positive area in the EC
The number of NeuN-positive cells in the EC was lower in the AD-D group than in the N-N and AD-N groups (Fig. 3b). However, no difference was found in the number of NeuN-positive cells between the N-N and AD-N groups (Fig. 3b). The synaptophysin-positive area was lower in the AD-D group than in the N-N and AD-N groups, however no difference was found between the N-N and AD-N groups (Fig. 3c).
The GFAP-positive area, the number of GFAP-positive astrocytes and astrocytic processes in layer I/II of the EC
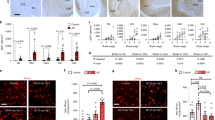
The GFAP-positive area in layer I/II of the EC was higher in the AD-N and AD-D groups than in the N-N group, however no difference was found between the AD-N and AD-D groups (Fig. 4a). The number of GFAP-positive astrocytes in layer I/II of the EC was higher in the AD-D group than in the N-N and AD-N groups (Fig. 4a). However, no difference was found in the number of GFAP-positive astrocytes between the N-N and AD-N groups (Fig. 4a). On the other hand, the number of GFAP-positive astrocytic processes was lower in the AD-D group compared to the N-N and AD-N groups (Fig. 4a). No difference was found in the number of GFAP-positive astrocytic processes between the N-N and AD-N groups (Fig. 4a). More characteristically, prominent morphological differences were observed in the astrocytic processes between the AD-N and AD-D groups. The GFAP-positive astrocytes in the AD-N group possessed long, thick, and bushy cytoplasmic processes, while those in the AD-D group possessed relatively short, thin cytoplasmic processes (Fig. 4b). In both the AD-N and AD-D groups, the perikarya was enlarged in comparison to that in the N-N group. In the N-N group, astrocytes were observed as an intermediate or mixed type between those in the AD-N and AD-D groups (Fig. 4b).
Immunohistochemical analysis of GFAP and GLT-1 in layer I/II of EC. (a) Images of GFAP-positive astrocytes shown at different magnifications (Bar = 100 µm). The GFAP-positive area is higher in the AD-N and AD-D groups than in the N-N group, while no difference is observed between the AD-N and AD-D groups. The number of GFAP-positive astrocytes is higher in AD-D group than in the N-N and AD-N groups, while no difference is observed between the N-N and AD-N groups. Astrocytic processes in the AD-N group appear extremely long, thick, and bushy, while those in the AD-D group appear short and thin. (b) The number of astrocytic processes observed 20 μm away from the soma is lower in the AD-D group than in the N-N and AD-N groups. (c) GLT-1 images are shown at different magnifications (Bar = 100 µm). The GLT-1-positive area is lower in the AD-D group than in the N-N and AD-N groups, while no difference is observed between the N-N and AD-N groups. The expression of GLT-1 is strong in both the proximal and distal astrocytic processes in the N-N and AD-N groups, while GLT-1 expression is weaker, especially in the distal astrocytic processes, in the AD-D group. **P < 0.01, one-way ANOVA, Tukey post-test. Values are means ± SD (N-N: n = 19, AD-N: n=10, AD-D: n = 18).
The GFAP-positive area, the number of GFAP-positive astrocytes and astrocytic processes in layer III-VI of the EC
The GFAP-positive area in layer III-VI of the EC was higher in the AD-N and AD-D groups than in the N-N group, however no difference was found between the AD-N and AD-D groups (Fig. 5a). The number of GFAP-positive astrocytes and the number of astrocytic processes in layer III-VI of the EC was higher in both the AD-N and AD-D groups than in the N-N groups (Fig. 5a). However, no difference was found in the number of GFAP-positive astrocytes and astrocytic processes between the AD-N and AD-D groups (Fig. 5a). In both the AD-N and AD-D groups, GFAP-positive astrocytes showed enlarged perikarya and thick processes compared to that in the N-N group.
Immunohistochemical analysis of GFAP and GLT-1 in layer III-VI of EC. (a) Images of GFAP-positive astrocytes shown at different magnifications (Bar = 100 µm). The GFAP-positive area is higher in the AD-N and AD-D groups than in the N-N group, while no difference is observed between the AD-N and AD-D groups. The number of GFAP-positive astrocytes is higher in AD-N group and AD-D than in the N-N group, while no difference is observed between the AD-N and AD-D groups. The number of astrocytic processes observed 20 μm away from the soma is higher in the AD-N and AD-D groups than in the N-N group. (b) GLT-1 images are shown at different magnifications (Bar = 100 µm). The GLT-1-positive area is lower in the AD-D group than in the AD-N groups. The expression of GLT-1 is strong in both the proximal and distal astrocytic processes in the AD-N groups, while GLT-1 expression is weaker, especially in the distal astrocytic processes, in the AD-D group. *P < 0.05, **P < 0.01, one-way ANOVA, Tukey post-test. Values are means ± SD (N-N: n=19, AD-N: n = 10, AD-D: n = 18).
In contrast, no significant difference was found in the number of S100b-positive cells in layer III-VI of the EC, and no significant difference was found in the number of processes of S100b-positive cells among the three groups (Supplementary Figure 4a). Moreover, morphological differences in cell structures between the three groups were not found (Supplementary Figure 4a).
The GLT-1-positive area and mRNA expression in the EC
The GLT-1-positive area in layer I/II of the EC was lower in the AD-D group than in the N-N and AD-N groups (Fig. 4c). However, no difference was found between the N-N and AD-N groups (Fig. 4c). The mRNA expression for GLT-1 in layer I/II of the EC was lower in the AD-D group than in the AD-N group (Supplementary Figure 3). However, no difference was found between the AD-D and N-N groups (Supplementary Figure 3).
The GLT-1-positive area in layer III-VI of the EC was lower in the AD-D group than in the AD-N group (Fig. 5b). However, no difference was found between the AD-D and N-N group (Fig. 5b). The percentage of GLT-1-positive cells in S100b-positive cells in layer III-VI of the EC was higher in the AD-N group than in the N-N and the AD-N groups (Supplementary Figure 4b). However, no difference was found between the AD-D and N-N groups (Supplementary Figure 4b). The GLT-1-positive area in each GLT-1/S100b-positive cell in layer III-VI of the EC was lower in the AD-D group than in the AD-N group (Supplementary Figure 4b). However, no difference was found between the AD-D and N-N groups (Supplementary Figure 4b).
The staining pattern of GLT-1-positive astrocytes varied among the three groups. In the N-N and AD-N groups, the expression of GLT-1 was strong in both the proximal and distal astrocytic processes in layer I/II and layer III-VI, while GLT-1 expression was weaker, especially in the distal astrocytic processes, in the AD-D group (Figs 4c, 5b and Supplementary Figure 4b).
Factors correlated with CDR-SOB
Good correlations were found between the CDR-SOB and Katz index and IADL score as shown in Supplementary Figure 1. In addition, the GLT-1-positive area and the number of GFAP-positive astrocytic processes in the layer I/II showed good correlations with the CDR-SOB, while other factors, including age, area of the EC, Aβ-positive area, number of NeuN-positive cells, synaptophysin-positive area, the GLT-1-positive area and the number of GFAP-positive astrocytic processes in the layer III-VI were moderately or lower correlated with the CDR-SOB (Supplementary Figure 2).
Discussion
To our knowledge, this is the first study to show that reactive astrocytes expressing GLT-1 in the EC are associated with the maintenance of cognitive function in spite of the progression of AD-specific pathological changes. Compared to the AD-D group, astrocytes in layer I/II of the EC from the AD-N group showed an increased number of astrocytic processes and longer or thicker forms with higher GLT-1 expression, while astrocytes in layer III-VI of the EC from the AD-N group showed higher GLT-1 expression. These activated astrocytes in the AD-N group may exert beneficial effects to protect neuronal transmission from the neurotoxicity associated with Aβ and NFT deposition.
We examined brains obtained from bodies aged over 70 years at death donated to Sapporo Medical University. The brain samples used covered various levels of cognitive function and were obtained from donors who died from a variety of causes. After careful exclusion of brains with non-AD neurological diseases such as cerebrovascular dementia or Parkinson’s disease-related dementia based on past medical history, we evaluated AD pathology based on the NIA-AA criteria “ABC score” composed of Thal Phase for Aβ plaques, Braak NFT stage and CERAD neuritic plaque score. These criteria are widely accepted as they reflect two important factors associated with AD pathology, Aβ and tau protein deposition25. Based on this report, “Not” and “Low” were evaluated as non-AD pathological change, while “Intermediate” and “High” were evaluated as AD pathological change.
To assess cognitive function, the CDR questionnaire was distributed to members of the bereaved families or other acquaintances. CDR is a common method of assessing cognitive function in the elderly, and can be used not only for diagnosis during life but also for postmortem diagnosis26,27. The validity and reliability of CDR for postmortem diagnosis were confirmed in a previous report28, and several clinico-pathological studies have applied this method15,26. We evaluated cognitive functions by questionnaires completed by family members but not by clinical physicians, as “No dementia” subjects are less likely to take cognitive tests given by clinical physicians when they were alive. In the present study, the cognitive level at 6 months before death was evaluated with CDR 0 (absent) and CDR 0.5 (questionable) classified as “No dementia”, and CDR 1, 2, and 3 classified as “Dementia,” as previously reported22,23,24. We also evaluated the activities of daily living using the Katz index and I-ADL, and both scores showed a good correlation with CDR, as reported previously27,29,30. Moreover, we confirmed the good inter-rater reliability of CDR between two the raters (EK and MN). Thus, the results of CDR were considered reliable.
In this study, the percentage of non-demented subjects with AD pathology was larger than that in previous studies. With regard to the Braak stage, 48% of the subjects with stage III + IV(12 of 25 cases) and 17% of the subjects with stage V + VI (1 of 6 cases) showed no dementia in this study (Supplementary Table 3). On the other hand, 32% of the subjects with stage III + IV and 8% of the subjects with stage V + VI showed no dementia in the Nun study11. With regard to the CERAD score, 40% of the subjects with moderate or frequent neuritic plaques (C2 or 3) showed no dementia in this study (10 of 25 cases) (Supplementary Table 3), while 34.7% of the subjects with C2 or 3 showed no dementia in the MRC CFAS study10. The discrepancies between the present and previous studies regarding the percentages of subjects with no dementia but with AD pathology might be due to the fact that 57% of the subjects (27 of 47 cases) lived at home during the final 6 months before death and had frequent contact with their family members (Supplementary Table 1, Supplementary Table 2), as a lack social interaction is closely related to the onset of dementia in the elderly30.
In our study, there were no differences in age or length of education between the AD-N and AD-D groups, and no differences were found in Thal Aβ phase, Braak NFT stage or CERAD score between the two groups. These results indicate that the cognitive impairment in the AD-D group might not be explained by aging, length of education, or deposition of Aβ plaques or tau accumulation in the brain. Therefore, we examined factors other than Aβ plaques and tau accumulation that may cause cognitive impairment in subjects with AD pathology.
The results revealed no differences in the accumulation of Aβ or PHF-tau in the EC between the AD-N and AD-D groups; however, the number of NeuN-positive cells and the area positive for synaptophysin were higher in the AD-N group than in the AD-D group. These results were in agreement with the findings of previous studies in which preserved neuronal number and synaptic density were shown in non-demented subjects with AD pathology15,16. As for the hippocampal volume, previous studies have shown that the hippocampal volume is correlated with the neuronal number31, and a reduction in volume, as assessed by MRI, is used for the diagnosis of AD32. Our results showed that the two-dimensional area of the EC was unchanged between the AD-N and AD-D groups, suggesting that the two-dimensional cross-section of the EC, at least, is not correlated with cognitive function.
The most characteristic findings were the differences in the morphology of the astrocytes in layer I/II of the EC between the AD-N and AD-D groups, although both groups exhibited reactive astrocytes with a larger GFAP-positive area than did the N-N group. Astrocytes in the AD-N group possessed longer or thicker processes while those in the AD-D group possessed shorter or thinner processes. Astrocytes located in layers I/II are known as “interlaminar astrocytes” and are characterized by the presence of millimeter-long projections terminating in layer III to IV33. It has been reported that in AD patients, disruption of the processes of these interlaminar astrocytes is correlated with dysfunction in neuronal transmission support34. In the present study, the longer or thicker astrocytic processes found in layer I/II extended to layer III to IV, where the astrocytic processes may play important roles in the synaptic transmission of neurons connected to the CA1 region35,36.
In contrast to astrocytes in layer I/II, there were no differences in the morphology of the astrocytes observed in layer III-VI between the AD-N and AD-D groups, although both groups exhibited a larger GFAP-positive area than did the N-N group. Astrocytes in layer III-VI, known as “protoplasmic astrocytes,” are spherical and possess several main processes which are accompanied by very thin branches17. Therefore, it remains possible that GFAP-positive reactions do not always detect the whole branches of very thin astrocytic processes in layer III-VI. In contrast to GFAP positive astrocytes, there were no differences in both the number of cells and processes in S100b positive astrocytes in layer III-VI. The subpopulation of astrocytes has been reported to be positive for S100b but negative for GFAP37, and S100b-positive reaction is localized around the perikarya of astrocytes38. Therefore, GFAP staining seems to be more suitable for detecting the reactive astrocytes which possess processes than S100b staining.
Reactive astrocytes have also been shown to have diphasic functions39, including both beneficial effects, such as the formation of a barrier to confine lesions, promotion of blood-brain barrier repair, inhibition of synaptic loss and slowing of neurodegenerative disease progression, and harmful effects, such as the production of reactive oxygen species, secretion of cytokines and inhibition of synapse and axon regeneration40. In previous reports, the expression of inflammatory cytokines, such as IL-1β, was higher in demented patients with AD pathology, compared to the non-demented patients with AD pathology41. Moreover, a single astrocyte in the human brain is associated with around 2 million synapses via its processes, which support memory formation42. Therefore, a diminished number of astrocytic processes leads to reduced glutamate transport and insufficient communication across gap junctions43.
To clarify the beneficial function of reactive astrocytes in the AD-N group, we examined the expression of GLT-1, which is a major glutamate transporter that uptakes excess glutamate to prevent neuronal excitotoxicity44,45, in the astrocytes in each group. The GLT-1 expression was detected by immunohistochemistry and its validity was confirmed by a quantitative RT-PCR. We observed a higher level of GLT-1 expression in astrocytes in the AD-N group compared to that in the AD-D group in both layer I/II and layer III-VI, suggesting that astrocytes in the AD-N group play beneficial roles in neuronal functions. The discrepancies in GLT-1 expression and morphology between astrocytes in the AD-N and AD-D groups in layer III-VI can be explained by the fact that astrocytes in the AD-N groups express high levels of GLT-1 in the very thin processes, but they were not detected completely by GFAP immunohistochemistry46. The higher expression of GLT-1 in astrocytes in layer III-VI from AD-N group was also confirmed by the overlapping staining of GLT-1 and S100b. In fact, the percentages of GLT-1-positive cells in S100b-positive cells and GLT-1 expression in each cell were higher in the AD-N group than in the N-N and the AD-N groups. Previous studies have shown that a reduction in GLT-1 is related with the cognitive impairment in AD47,48, and functional abnormalities in GLT-1 induce synaptic loss49. In the present study, reactive astrocytes in the AD-D group showed weak GLT-1 expression in spite of a higher number of cells, suggesting that these reactive astrocytes may not function adequately in the brain.
Various factors are known to influence the number of astrocytic processes as well as GLT-1 expression; for example, the ramification of astrocytic processes is increased by physical exercise or an enriched environment50,51, and GLT-1 expression is up-regulated by treadmill training52. Furthermore, GLT-1 expression and the number of astrocytic processes are increased through the memory formation processes53. Although the details of daily life and intellectual activity of the donors in the present study were not known, the potential for reactive astrocytes to be converted from non-activated to activated type and the maintenance of GLT-1 expression might be key factors in the preservation of normal cognitive function, even in the presence of AD pathology.
In conclusion, activated forms of astrocytes with higher GLT-1 expression are associated with cognitive normal subjects with the AD pathology in the brain. Identification of methods to convert reactive astrocytes into this beneficial type appears to afford a promising approach to the prevention of AD.
Methods
Subjects
All brains were obtained from the body donation program run by the Sapporo Medical University (Shiragiku-kai). This program consists of members aged 70 years or older who donate their bodies for medical education and research after death. The donation was agreed to by the members while they are alive. There were no prisoners in the subjects of this study. The protocols were approved by the Sapporo Medical University Ethics Committee and informed consent was obtained from all participants. All study methods were performed in accordance with the relevant guidelines and regulations of Sapporo Medical University.
Cognitive function was assessed by postmortem evaluation of informant questionnaires as reported previously28. The questionnaires consisted of the Japanese version of the Clinical Dementia Rating Scale (CDR)22,24,54, Katz Index55 and I-ADL56. Details of CDR are described in Supplementary information.
AD neuropathological diagnosis
For evaluation of AD neuropathological changes, we applied three assessment scales: the Thal Phase for Aβ plaques57, Braak NFT stage58,59 and CERAD neuritic plaque score60. Details of evaluation of each score are described in Supplementary information.
After assessing each score of the “ABC”, we classified each brain as “Not”, “Low”, “Intermediate” or “High” based on a combination of the three scores according to guidelines of National Institute on Aging-Alzheimer’s Association25. “Not” and “Low” corresponded to no AD neuropathological change, while “Intermediate” and “High” corresponded to AD neuropathological change. These procedures were applied to each brain with the assessors blinded to the donor.
Immunohistochemistry
Whole brain samples were fixed in 10% formalin. Blocks of the target area were cut, and then immersed in 15% sucrose solutions. For immunohistochemical analysis, coronal sections were cut into 20 μm thick frozen sections and obtained every 100 μm. A standard avidin-biotin complex (ABC) method was used for the analysis. Details of antibodies are described in Supplementary information.
Statistical analysis
One-way ANOVA followed by Tukey post hoc comparison was used to detect differences among the three groups for variables with normal distributions. Non-parametric Kruskal-Wallis test and Mann-Whitney U test were used to detect differences among groups for variables with non-normal distributions. Bartlett’s test was used to confirm the data distributions. Spearmann’s correlation coefficient was used to confirm correlations between the CDR-SOB and Katz Index, I-ADL, age, area of the EC, Aβ-positive area, PHF-tau-positive area, number of NeuN-positive cells and synaptophysin-positive area. Statistical significance was set at 5% and data are presented as mean ± standard deviation (SD). All statistical analyses were performed using R software (version 3.3.2).
References
Cornutiu, G. The Epidemiological Scale of Alzheimer’s Disease. J Clin Med Res 7, 657–666 (2015).
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
Querfurth, H. W. & LaFerla, F. M. Alzheimer’s disease. N Engl J Med 362, 329–344 (2010).
Mucke, L. & Selkoe, D. J. Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med 2, a006338 (2012).
Shankar, G. M. & Walsh, D. M. Alzheimer’s disease: synaptic dysfunction and Abeta. Mol Neurodegener 4, 48 (2009).
Iqbal, K., Liu, F., Gong, C. X. & Grundke-Iqbal, I. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res 7, 656–664 (2010).
Danysz, W. & Parsons, C. G. Alzheimer’s disease, beta-amyloid, glutamate, NMDA receptors and memantine–searching for the connections. Br J Pharmacol 167, 324–352 (2012).
Katzman, R. et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol 23, 138–144 (1988).
Price, J. L. & Morris, J. C. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 45, 358–368 (1999).
Neuropathology, G. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet 357, 169–175 (2001).
Snowdon, D. A. Healthy aging and dementia: findings from the Nun Study. Ann Intern Med 139, 450–454 (2003).
Stern, Y. et al. Influence of education and occupation on the incidence of Alzheimer’s disease. Jama 271, 1004–1010 (1994).
Tucker, A. M. & Stern, Y. Cognitive reserve in aging. Curr Alzheimer Res 8, 354–360 (2011).
Whalley, L. J. et al. Childhood mental ability and dementia. Neurology 55, 1455–1459 (2000).
Andrade-Moraes, C. H. et al. Cell number changes in Alzheimer’s disease relate to dementia, not to plaques and tangles. Brain 136, 3738–3752 (2013).
Perez-Nievas, B. G. et al. Dissecting phenotypic traits linked to human resilience to Alzheimer’s pathology. Brain 136, 2510–2526 (2013).
Sofroniew, M. V. & Vinters, H. V. Astrocytes: biology and pathology. Acta Neuropathol 119, 7–35 (2010).
Medeiros, R. & LaFerla, F. M. Astrocytes: conductors of the Alzheimer disease neuroinflammatory symphony. Exp Neurol 239, 133–138 (2013).
Belanger, M. & Magistretti, P. J. The role of astroglia in neuroprotection. Dialogues Clin Neurosci 11, 281–295 (2009).
Mathur, R. et al. A reduced astrocyte response to beta-amyloid plaques in the ageing brain associates with cognitive impairment. PLoS One 10, e0118463 (2015).
Doody, R. S. et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med 370, 311–321 (2014).
Hughes, C. P., Berg, L., Danziger, W. L., Coben, L. A. & Martin, R. L. A new clinical scale for the staging of dementia. Br J Psychiatry 140, 566–572 (1982).
Jellinger, K. A. Clinicopathological analysis of dementia disorders in the elderly–an update. J Alzheimers Dis 9, 61–70 (2006).
Morris, J. C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43, 2412–2414 (1993).
Montine, T. J. et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123, 1–11 (2012).
Farfel, J. M. et al. Very low levels of education and cognitive reserve: a clinicopathologic study. Neurology 81, 650–657 (2013).
Ferretti-Rebustini, R. E. et al. Validity of the Katz Index to assess activities of daily living by informants in neuropathological studies. Rev Esc Enferm USP 49, 946–952 (2015).
Ferretti, R. et al. Post-Mortem diagnosis ofdementia by informant interview. Dement Neuropsychol 4, 138–144 (2010).
Marra, T. A., Pereira, D. S., Faria, C. D., Tirado, M. G. & Pereira, L. S. Influence of socio-demographic, clinical and functional factors on the severity of dementia. Arch Gerontol Geriatr 53, 210–215 (2011).
Glei, D. A. et al. Participating in social activities helps preserve cognitive function: an analysis of a longitudinal, population-based study of the elderly. Int J Epidemiol 34, 864–871 (2005).
Zarow, C. et al. Correlates of hippocampal neuron number in Alzheimer’s disease and ischemic vascular dementia. Ann Neurol 57, 896–903 (2005).
Du, A. T. et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. J Neurol Neurosurg Psychiatry 71, 441–447 (2001).
Vasile, F., Dossi, E. & Rouach, N. Human astrocytes: structure and functions in the healthy brain. Brain Struct Funct 222, 2017–2029 (2017).
Yassa, M. A., Muftuler, L. T. & Stark, C. E. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proc Natl Acad Sci USA 107, 12687–12691 (2010).
McCall, M. A. et al. Targeted deletion in astrocyte intermediate filament (Gfap) alters neuronal physiology. Proc Natl Acad Sci USA 93, 6361–6366 (1996).
Sovrea, A. S. & Bosca, A. B. Astrocytes reassessment - an evolving concept part one: embryology, biology, morphology and reactivity. J Mol Psychiatry 1, 18 (2013).
Steiner, J. et al. Evidence for a wide extra-astrocytic distribution of S100B in human brain. BMC Neurosci 8, 2 (2007).
Boyes, B. E., Kim, S. U., Lee, V. & Sung, S. C. Immunohistochemical co-localization of S-100b and the glial fibrillary acidic protein in rat brain. Neuroscience 17, 857–865 (1986).
Pekny, M. & Pekna, M. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev 94, 1077–1098 (2014).
Hamby, M. E. & Sofroniew, M. V. Reactive astrocytes as therapeutic targets for CNS disorders. Neurotherapeutics 7, 494–506 (2010).
Morimoto, K. et al. Expression profiles of cytokines in the brains of Alzheimer’s disease (AD) patients compared to the brains of non-demented patients with and without increasing AD pathology. J Alzheimers Dis 25, 59–76 (2011).
Zorec, R., Horvat, A., Vardjan, N. & Verkhratsky, A. Memory Formation Shaped by Astroglia. Front Integr Neurosci 9, 56 (2015).
Pekny, M., Wilhelmsson, U. & Pekna, M. The dual role of astrocyte activation and reactive gliosis. Neurosci Lett 565, 30–38 (2014).
Danbolt, N. C. Glutamate uptake. Prog Neurobiol 65, 1–105 (2001).
Rothstein, J. D. et al. Localization of neuronal and glial glutamate transporters. Neuron 13, 713–725 (1994).
Sosunov, A. A., Guilfoyle, E., Wu, X., McKhann, G. M. 2nd & Goldman, J. E. Phenotypic conversions of “protoplasmic” to “reactive” astrocytes in Alexander disease. J Neurosci 33, 7439–7450 (2013).
Masliah, E., Alford, M., DeTeresa, R., Mallory, M. & Hansen, L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer’s disease. Ann Neurol 40, 759–766 (1996).
Mookherjee, P. et al. GLT-1 loss accelerates cognitive deficit onset in an Alzheimer’s disease animal model. J Alzheimers Dis 26, 447–455 (2011).
Zumkehr, J. et al. Ceftriaxone ameliorates tau pathology and cognitive decline via restoration of glial glutamate transporter in a mouse model of Alzheimer’s disease. Neurobiol Aging 36, 2260–2271 (2015).
Saur, L. et al. Physical exercise increases GFAP expression and induces morphological changes in hippocampal astrocytes. Brain Struct Funct 219, 293–302 (2014).
Viola, G. G. et al. Morphological changes in hippocampal astrocytes induced by environmental enrichment in mice. Brain Res 1274, 47–54 (2009).
Wang, X. et al. Pre-ischemic treadmill training alleviates brain damage via GLT-1-mediated signal pathway after ischemic stroke in rats. Neuroscience 274, 393–402 (2014).
Choi, M. et al. Hippocampus-based contextual memory alters the morphological characteristics of astrocytes in the dentate gyrus. Mol Brain 9, 72 (2016).
Sugishita, M. & Furukawa, K. Clinical Dementia Rating (CDR). Nihon Rinsho 69 Suppl 8, 413–417 (2011).
Katz, S., Ford, A. B., Moskowitz, R. W., Jackson, B. A. & Jaffe, M. W. studies of illness in the aged. the index of adl: a standardized measure of biological and psychosocial function. Jama 185, 914–919 (1963).
Lawton, M. P. & Brody, E. M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9, 179–186 (1969).
Thal, D. R., Rub, U., Orantes, M. & Braak, H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58, 1791–1800 (2002).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82, 239–259 (1991).
Braak, H., Alafuzoff, I., Arzberger, T., Kretzschmar, H. & Del Tredici, K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112, 389–404 (2006).
Mirra, S. S. et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41, 479–479 (1991).
Acknowledgements
The authors would like to thank Kozue Kamiya, Yuko Hayakawa, and Tatsuya Shiraishi for technical support, Natsumi Hashimoto, Mami Yamaguchi and Michitoshi Kimura for support with cutting paraffin sections and Bielschowsky staining, Kota Iwamura for support of brain fixation. This work was supported by JSPS KAKENHI Grant Number JP16K19317 and LEOC Co., Ltd. There is no financial relationship to disclose.
Author information
Authors and Affiliations
Contributions
E.K., M.N. and M.F. designed the study and wrote the paper. S.M. assisted brain removal and fixation. K.K., N.H., T.C., M.O., Y.M. and K.N. helped analysis of the data.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kobayashi, E., Nakano, M., Kubota, K. et al. Activated forms of astrocytes with higher GLT-1 expression are associated with cognitive normal subjects with Alzheimer pathology in human brain. Sci Rep 8, 1712 (2018). https://doi.org/10.1038/s41598-018-19442-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-19442-7
This article is cited by
-
The concept of resilience to Alzheimer’s Disease: current definitions and cellular and molecular mechanisms
Molecular Neurodegeneration (2024)
-
The glutamatergic system in Alzheimer’s disease: a systematic review with meta-analysis
Molecular Psychiatry (2024)
-
Developmental dynamics of the single nucleus regulatory landscape of pig hippocampus
Science China Life Sciences (2023)
-
Music compensates for altered gene expression in age-related cognitive disorders
Scientific Reports (2023)
-
Cyclic multiplex fluorescent immunohistochemistry and machine learning reveal distinct states of astrocytes and microglia in normal aging and Alzheimer’s disease
Journal of Neuroinflammation (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.