Abstract
To tailor the properties of colloidal materials, precise control over the self-assembly of their constituents is a prerequisite. Here, we govern the assembly of silica particles by functionalization with supramolecular moieties which interact with each other via directional and reversible hydrogen bonding. Through a generally applicable synthesis protocol, two different types of self-complementary hydrogen bonding moieties, BTA- and UPy-derivatives, are anchored to silica particles. Their self-assembly is initiated by the UV-induced removal of a photolabile protecting group, allowing the formation of hydrogen bonds between tethered molecules. The light-induced assembly of BTA- and UPy-decorated colloids in single-component dispersions and colloidal self-sorting in mixed dispersions is studied. Furthermore, we demonstrate that UPy-colloids can dissasemble upon addition of traces of a competitive binder (NaPy). This work provides further insight into the utility of supramolecular handles to orchestrate the assembly of micron-sized colloids via non-oligonucleotide hydrogen-bonding units.
Similar content being viewed by others
Introduction
The ability to control molecular self-assembly via non-covalent interactions is an emerging bottom-up strategy to create complex metamaterials. In turn, well-defined molecular recognition can be used as a powerful tool to direct the assembly of micron-sized1,2,3 colloids or even macroscopic objects4 and create larger aggregated structures with emergent optical5,6,7, mechanical8 or catalytic properties9,10. Molecular control over the assembly of micron-sized particles can be realized through particle surface-grafting of molecules exhibiting dipole-dipole11,12, metal coordination13, hydrophobic forces14,15 and/or hydrogen bonding (H-bonding)16,17,18. Ideally, the tethered motifs should be easy to synthesize, responsive, externally addressable and provide directional, dynamic and specific bonding within the assemblies. Moreover, to facilitate colloidal assembly, a precise control between the interplay of non-specific surface forces (attractive and/or repulsive) is crucial19.
A popular strategy in the field is the functionalization of colloids with complementary DNA strands20,21. Great advances in insight and structural complexity have been achieved in the last 20 years, enabling the assembly of colloids into both disordered and exotic ordered states22,23, in a reversible22,24,25 or irreversible26 manner and at the nano-27,28 and mesoscale25,29. Notwithstanding, DNA-technology has its limitations, such as high cost and restricted environmental conditions27,30 because the strands only assemble in water at sufficiently high ionic strengths and at low temperature, precluding material synthesis and processing from organic solvents. Furthermore, up to now, only temperature has been used as external trigger of assembly, while recent advances towards light sensitive DNA-strands can be utilized in future to develop dual-responsive systems sensitive to both temperature and light31. A promising alternative strategy exploits the potential of synthetic self-assembling molecules to fabricate multiresponsive functional materials. The incorporation of photoresponsive motifs, such as azobenzenes for example, has been proposed to guide particle assembly at the nano- and mesoscale5,11,32. Azobenzenes can be reversibly switched between two isomers, only one of which has a significant dipole moment and thus gives rise to intermolecular dipolar interactions. Interestingly, the behaviour of the azobenzenes is tuneable by molecular design; i.e. modification of the substituents in the aromatic ring. In this manner one can modulate which isomer (cis or trans) has a large dipole moment and (red) shift the wavelength of photo-isomerization33,34. Disadvantageously, however the induced dipole-dipole interactions are not specific. Azobenzene-mediated colloidal assembly can however be made selective, when used in combination with cyclodextrins15,35,36.
Aiming to explore the possibilities and limitations of molecular control over colloidal assembly further, we here introduce a versatile route, built upon a previous work16, towards light-activated selective assembly of silica microparticles via H-bonding. We select H-bonding for colloidal assembly, since it is specific, directional and tuneable in strength. We utilize two different types of photo-activatable motifs that interact through H-bonds in an orthogonal fashion (i.e., exclusively with the same motif) to prepare dual-responsive silica microparticles, sensitive to light and temperature. Here we coat silica microparticles with either o-nitrobenzyl protected benzene-1,3,5-tricarboxiamide (BTA) derivatives or o-nitrobenzyl protected 2-ureido-4[1 H]-pyrimidinone (UPy) derivatives (Fig. 1) via a mild coupling reaction. Both BTAs and UPys motifs can readily be functionalized with a variety of side chains for easy coupling reactions to the colloids37, enhanced solubility in aqueous17 and organic media and thermo- and light-responsiveness16. When molecularly dissolved in a mixed solution, these motifs preferentially self-assemble in organic media of low polarity via H-bonds into single component stacks and homodimers, respectively38, this phenomenon is so-called narcissistic aggregation. We profit from this specific selectivity at the molecular level, to prepare two-component dispersions of silica microparticles, which spontaneously self-sort into single-specie clusters upon UV-illumination, hence increasing the generality and modularity of our approach. Interestingly, colloidal gels with this arrested complex architecture7,37,38,39,40 and other similar illustrative examples33,41 have shown promising mechanical properties and could serve as a model to understand biological phenomena. The exploitation of our approach based on the high specificity and fidelity of H-bonding supramolecular moieties, has resulted for the first time in a light-activated colloidal self-sorting of identical particles into single-species clusters.
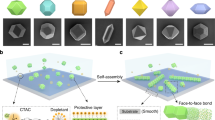
Chemical structures of the (A) BTA-, (B) UPy- and (C) NaPy derivatives. Schematic representation of the behavior of (D) BTA- and E) UPy-colloids. Upon UV-irradiation, the labile o-nitrobenzyl group is cleaved off and the H-bonds become active, triggering colloidal clustering. (E) Addition of NaPy disrupts clusters of UPy-colloids.
In summary, two types of supramolecular colloids, BTA- and UPy-colloids, were designed and synthesized to photo-initiate colloidal assembly in single component dispersions and colloidal self-sorting in mixed dispersions. These results demonstrate that the intrinsically responsive nature of small molecules can be harnessed effectively to program the mesoscale assembly of silica microparticles whose properties can be dynamically modulated, which is an essential stepping stone for the bottom-up manufacturing of complex colloidal metamaterials in aqueous and organic milieu.
Results and Discussion
Efficient coupling of supramolecular moieties under mild conditions
To regulate colloidal assembly via supramolecular chemistry, a mild, efficient and generally applicable two-step coupling strategy was developed. Herein, hydrophobic silica colloids are first produced and subsequently post-functionalized with the supramolecular moieties at room temperature, thus preventing molecular degradation (Fig. 2A). The two-step functionalization begins with a simultaneous coupling of stearyl alcohol (a C18 spacer) and a NVOC-C11-OH linker to the particles. Next, the NVOC group is cleaved by light (λmax = 354 mm) from the linker to afford primary amines for an efficient coupling of various functional groups under mild reaction conditions, like o-nitrobenzyl protected benzene-1,3,5-tricarboxamide (BTA) and o-nitrobenzyl protected 2-ureido-4[1 H]-pyrimidinone (UPy) derivatives. The stearyl alcohol plays several important roles. First it makes the particles hydrophobic, allowing them to be dispersed in organic solvents. Second, it enhances colloidal stability since it acts as a steric barrier inhibiting direct contact between two or more particles. And lastly, it suppresses the attractive Van der Waals interactions by decreasing the refractive index mismatch between the colloids and the solvent37. This classical colloidal almost hard-sphere-like model system of stearyl alcohol coated silica beads in cyclohexane, thus serves as an excellent starting point for supramolecular control over colloidal assembly, as other attractions are effectively minimized.
(A) Representative scheme of the functionalization of particles with BTA. Spectra of silica colloids at the different functionalization steps: (B) bare silica colloids, (C) NVOC-C11- functionalized, (D) amine- functionalized, (E) BTA- functionalized and (F) UPy-functionalized colloids. (G) BTA and (H) UPy molecules before coupling to the colloids.
The different functionalization steps were monitored by infrared spectroscopy (IR) on small functionalized colloids (D = 26 nm) to maximize the intensity of the peaks corresponding to vibrations of the different tethered moieties. Figure 2 shows the spectra of plain (B), NVOC-C11- (C) and amine-functionalized (D) colloids together with the spectra of the two final products, i.e. supramolecular BTA- (E) and UPy-colloids (F). As a reference, the spectra of the supramolecular moieties prior to attachment are also shown (Fig. 2G,H). The peaks at 2920 (I) and 2850 (II) cm−1 present in all the spectra of functionalized particles are mainly attributed to the stretching bands of the -CH2 and -CH3 groups of the alkyl chains. NVOC-C11-functionalized colloids (C) exhibit a peak at 1520 cm−1 (III), which corresponds to the stretching band of the N-O group from the NVOC functional group. The lack of this band in the IR spectrum of the washed deprotected colloids (D), together with the appearance of small peaks around 1550 cm−1, corresponding to the stretching band of primary amines, suggested that the NVOC group was efficiently cleaved off, hence yielding the desired amine-functionalized particles, onto which supramolecular moieties can be coupled. As expected, the spectra of BTA-functionalized colloids (E) and the BTA moiety (G) bear strong similarities, displaying broad peaks of the C=O and C-N stretching bands at 1740 (IV) and 1370 (V) cm−1, respectively. In addition, the N-O peak III from the protecting o-nitrobenzyl group was also observed. Similarly, UPy-colloids display, although weak, the bands at 1520 (III), 1740 (IV) and 1445 (VI) cm−1 from the N-O, C=O and -CS-NH of the functional groups, respectively, also present in the spectrum of the UPy moiety (H). Thus the IR results indicate successful coupling of both molecules onto the silica colloids as the IR spectra taken during the synthesis of the supramolecular colloids display the characteristic vibrations of the starting (Fig. 2B,C,G,H), intermediate (Fig. 2D) and final product (Fig. 2E,F) of the reaction.
To validate the IR results and estimate the maximum number of BTA and UPy molecules that can be coupled to the colloids, the number of primary amines present onto small colloids during the deprotection step was quantified using a UV-spectroscopic essay for bioconjugation as described in the Methods section. Figure 3 shows the evolution of the surface area per amine on the colloids as a function of the deprotection time (t i ). After 60 min of deprotection, a plateau was reached at approximately 46.4 nm2/amine, indicating that the deprotection of the NVOC group onto the colloids was complete. This density corresponds to approximately 24350 amines per large colloid (D = 600 nm)37, which in turn equals the maximal number of supramolecular moieties that can be coupled to the particles.
A parallel kinetic study was carried out for the NVOC-C11-OH linker free in solution and a similar pattern was observed. After 60 min of irradiation, 95% of the NVOC groups were converted to amines (Fig. 3).
Self-assembly of the silica colloids
To confirm that the H-bonds from the tethered molecules can be activated by light, through cleavage of the o-nitrobenzyl protective group, the assembly of BTA- and UPy-colloids was studied by means of confocal microscopy as a function of the irradiation time, t i . The fraction of singlets (f s ) was determined by quantitative image analysis as previously described37. Before irradiation (t i = 0 min) most of the colloids were nicely dispersed as singlets (Fig. 4A,C and E) with f s = 0.63, 0.80 and 0.48 for stearyl alcohol coated particles, BTA-colloids, and UPy-colloids respectively. This confirms on the one hand, that the Van der Waals forces are weak (estimated to be about −0.2 kBT at contact), and on the other hand that the o-nitrobenzyl protecting group effectively blocks H-bond formation prior to irradiation. Notice the somewhat smaller fraction of singlets prior to illumination for UPy-colloids relative to BTA-colloids (f s = 0.48 vs. 0.80), which we tentatively attribute to a less effective inactivation of H-bonds by the o-nitrobenzyl and limited solubility of the UPy derivative in cyclohexane. Upon irradiation, f s decreases drastically within 5 minutes to a vanishingly small value of f s ~0.05 for both BTA- and UPy-colloids (Fig. 4B,D,G, see Figure S3 for more exemplary pictures). Conversely, as expected, the fraction of singlets is independent of irradiation time for stearyl alcohol coated particles (Fig. 4E–G), which are not UV-light responsive. These results clearly demonstrate that the attractive hydrogen bonding interactions between tethered supramolecular moieties can be effectively shielded prior to activation by UV-light, which triggers colloidal assembly as a consequence of the dominant intermolecular association.
Dissolution of UPy clusters upon addition of a hetero-complementary motif
In the above, we have shown that colloidal assembly can be photo-initiated via o-nitrobenzyl protected BTA and UPy derivatives. In a previous work, we demonstrated that the association strength between BTA-colloids can be controlled by temperature16. Here, we explore the use of an external, competitive binder to control the assembly of UPy-colloids. UPy homodimers can be dissolved upon addition of a complementary supramolecular moiety, a 2,7-diamido-1,8-naphthyridines derivative (NaPy), yielding hence UPy-NaPy heterodimers42. Self-assembly is favoured at low NaPy concentrations, while heterodimerization is predominant at high concentrations43. Hence, in principle UPy colloidal clusters can be selectively disrupted upon the addition of the competitive binder NaPy. Based on this, free NaPy was added to a dispersion of UPy colloids in cyclohexane after 20 min of irradiation to test by confocal microscopy whether NaPy can be utilized to break-up clusters of UPy-functionalized colloids (Fig. 5). As expected, light-induced assembly of UPy-colloids was evidenced by a dramatic reduction of the singlet fraction from f s = 0.48 to 0.04 after 20 min illumination and a concomitant increase in the abundance of small and especially large clusters. Addition of a 20-fold excess of NaPy broke these clusters apart, albeit partially, resulting in a singlet fraction f s = 0.13, i. e. substantially lower than prior to UV-illumination. Addition of a larger, 1000-fold excess of NaPy disrupted more clusters and further increased the singlet fraction to f s = 0.25. However, a full recovery to the initial f s = 0.48 (without simultaneously raising the sample temperature) was not attainable, since this 1000-fold excess corresponds to a NaPy concentration near the solubility limit in cyclohexane.
Engineering orthogonality in the colloidal domain
Aiming to investigate whether supramolecular motifs can be used to create self-sorting colloids, we explored the behavior of binary mixtures of supramolecular colloids. As a preliminary test of selectivity, a 0.1 wt% dispersion of stearyl alcohol coated colloids (green) and a 0.1 wt% dispersion of protected BTA-colloids (red) were mixed in equal volumes. Before illumination, most of the colloids were present as singlets (Fig. 6A). Upon irradiation, stearyl alcohol functionalized colloids remained dispersed, while the red BTA-colloids formed clusters (Fig. 6B). This selective assembly again corroborates that H-bonds between supramolecular BTA-colloids are the dominant attractive interactions. To assess whether the orthogonality of the triple- and quadruple H-bonding motifs translates into the colloidal domain, protected BTA-(green) and UPy-colloids (red) were mixed following the same protocol. Once more, most colloids remained in a non-aggregated state prior illumination (Fig. 6C). After irradiation, most of the colloids nicely assembled in an orthogonal fashion, resulting in two types of clusters comprising either BTA or UPy colloids (Fig. 6D, see Figure S4 for more exemplary pictures). These results evidence that the orthogonality of the supramolecular interactions at the molecular level can be effectively transferred to the colloidal domain, introducing a useful tool to build mixed systems with controlled aggregation behavior.
Confocal images of a mix of stearyl-coated colloids (green) and BTA-colloids (red) (A) before and (B) after 30 min irradiation. Confocal images of an equal mixture of BTA-colloids (green) and UPy-colloids (red) (C) before and (D) after 30 min of irradiation (λmax = 354 mm). Inset: scheme of the triple and quadruple H-bonding between BTAs and UPys, where the dashed lines represent the H-bonds between the moieties.
Conclusions
Herein we introduce a simple and modular approach to achieve reversible16 and controlled self-assembly of micron-sized colloids by surface-anchoring of supramolecular moieties, such as BTA and UPy derivatives. As the behaviour of the colloids is governed by light-responsive tethered motifs, their self-assembly can be externally tuned. Upon deprotection with UV-light, supramolecular moieties are ‘activated’, thereby initiating colloidal self-organization via H-bonding. Clusters of UPy-colloids could be dissolved partially by addition of free NaPy derivatives and colloidal self-sorting into single-species clusters was realized in binary mixtures of BTA- and UPy-colloids due to the orthogonality of the non-covalent intermolecular interactions. These findings demonstrate the utility of small molecular guides to control colloidal assembly, which we will exploit in the future to build photo-sensitive gels and Pickering emulsions.
Experimental
Materials
The synthesis of the supramolecular moieties is described in the Supplementary information. Solvents were dried over molsieves prior to use.
Synthesis of the core-shell particles
Based on the Stöber method, 600 nm in diameter core-shell particles with a fluorescein isothiocyanate (FITC) core were synthesized according to ref.37. The same protocol was followed to synthesize particles with a core containing the red dye Rhodamine B isothiocyanate (RITC). Throughout the text these are referred to as green (FITC) and red (RITC) colloids.
Functionalization of core-shell particles
- Synthesis of amine-functionalized particles: a detailed description of the synthesis of NVOC-C11- and stearyl alcohol-functionalized particles with a 20/80 molar ratio has already been reported37. The amine-functionalized particles were obtained upon cleavage of the NVOC group as follows. A dispersion of the NVOC-functionalized particles in chloroform was irradiated with a UV-oven (Luzchem LZC-4V UV oven equipped with 8 × 8 Watt UV-A light bulbs with λmax = 354 mm) for 1 hour under continuous stirring at room temperature. After illumination, the resulting amine-functionalized particles were washed 6 times with chloroform.
- BTA-functionalized particles: 20 mg of green amine-functionalized particles were dispersed in 3 mL of dry chloroform, to this suspension BTA derivative (18 mg, 0.02 mmol), (Benzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate (PyBOP, 10.4 mg, 0.02 mmol) and N,N-Diisopropylethylamine (DIPEA, 17.4 μl, 0.1 mmol) were added. The dispersion was stirred for 16 hours at room temperature under an argon atmosphere. Afterwards, particles were washed 6 times with chloroform and dried overnight in vacuo at 70 °C.
- UPy-functionalized particles: 20 mg of red amine-functionalized particles were dispersed in 3 mL of dry chloroform together with UPy derivative (18.2 mg, 0.04 mmol). The suspension was stirred for 16 hours at room temperature under an argon atmosphere. Afterwards, particles were washed 6 times with chloroform and dried overnight in vacuo at 70 °C.
- Functionalization of commercial Ludox AS-40 particles: small commercial particles of 26 nm in diameter were used for the IR characterization and determination of the deprotection kinetics of the NVOC group on the particles. Their functionalization was carried out as in above-described for the core-shell particles. For the IR experiments, Ludox AS-40 particles were fully functionalized with the NVOC-C11-OH linker only (i.e. no stearyl alcohol) to enhance the intensity of the peaks coming from the different functional groups by maximizing the organic/inorganic matter ratio.
Methods
IR spectroscopy
Functionalization of the colloids at the different steps of the protocol was confirmed by IR. IR spectra of the colloids in the solid state were obtained using a Perkin-Elmer spectrum equipped with a UATR Two sample stage. Over 40 spectra were recorded for each sample.
UV-Vis spectroscopy
To probe the deprotection of the NVOC group onto the colloids, several vials with 20 mg each of small NVOC-C11-colloids dispersed in CHCl3 were irradiated within a UV-oven (Luzchem LZC-4V UV equipped with 8 × 8 Watt UV-A light bulbs with λmax = 354 mm) for different times. While deprotecting, the dispersions were gently stirred with a magnetic stirrer to avoid sedimentation and keep the surface of the colloids always exposed to the light, ensuring a homogenous deprotection and hence, an homogenous amine coverage. The kinetics of deprotection were followed by monitoring the primary amines onto the particles as a function of the exposure times. To do so, a commonly used UV-spectroscopic essay for bioconjugation was chosen and adapted, see reference44 for a detailed description. Briefly, succinimidyl 3-(2-pyridyldithio)propionate (SPDP) molecules were coupled to the amines onto the particles, followed by the cleaving of the UV-active pyridine-2-thione upon the addition of 1,4-Dithiothreitol (DTT). Assuming that all reactions take place, the number of pyridine-2-thiones cleaved corresponds to the number of amines present. Note that the protocol was verified measuring the number of amines from amine-functionalized commercial particles. All experiments were carried out in duplicate.
Confocal microscopy
A Nikon Ti Eclipse laser scanning confocal microscope was used to characterize the clustering process of the supramolecular colloids. Microscopy samples were prepared by dispersing 0.1 wt% of the supramolecular colloids in cyclohexane and sonicate for 20 min. Afterwards the dispersion was irradiated in a UV oven (Luzchem LZC-4V UV equipped with 8 × 8 Watt UV-A light bulbs with λmax = 354 mm). At different deprotecting times, a 25 μl aliquot was withdrawn and placed onto a slide using a spacer (Grace BioLabs Secure seal imaging spacer, Sigma-Aldrich). Tens of images (accounting for hundreds of particles) were analyzed by ImageJ and Matlab according to the protocol described in ref.37.
References
Cantekin, S., De Greef, T. F. A. & Palmans, A. R. A. Benzene-1,3,5-tricarboxamide: A versatile ordering moiety for supramolecular chemistry. Chem. Soc. Rev. 41, 6125–6137 (2012).
Schacher, F. H., Rupar, P. A. & Manners, I. Functional block copolymers: Nanostructured materials with emerging applications. Angew. Chemie - Int. Ed. 51, 7898–7921 (2012).
Elacqua, E., Lye, D. S. & Weck, M. Engineering orthogonality in supramolecular polymers: From simple scaffolds to complex materials. Acc. Chem. Res. 47, 2405–2416 (2014).
Harada, A., Kobayashi, R., Takashima, Y., Hashidzume, A. & Yamaguchi, H. Macroscopic self-assembly through molecular recognition. Nat. Chem. 3, 34–37 (2011).
Klajn, R., Wesson, P. J., Bishop, K. J. M. & Grzybowski, B. A. Writing self-erasing images using metastable nanoparticle ‘inks’. Angew. Chemie - Int. Ed. 48, 7035–7039 (2009).
Hu, C., West, K. R. & Scherman, O. A. Hollow mesoporous raspberry-like colloids with removable caps as photoresponsive nanocontainers. Nanoscale 8, 7840–7844 (2016).
Gentili, D., Ori, G., Ortolani, L., Morandi, V. & Cavallini, M. Cooperative and Reversible Anisotropic Assembly of Gold Nanoparticles by Modulation of Noncovalent Interparticle Interactions. ChemNanoMat https://doi.org/10.1002/cnma.201700212 (2017).
Di Michele, L. et al. Aggregation dynamics, structure, and mechanical properties of bigels. Soft Matter 10 (2014).
Chen, Z. et al. Light controlled reversible inversion of nanophosphor-stabilized pickering emulsions for biphasic enantioselective biocatalysis. J. Am. Chem. Soc. 136, 7498–7504 (2014).
Zhao, H. et al. Reversible trapping and reaction acceleration within dynamically self-assembling nanoflasks. Nat. Nanotechnol. 11, 82–88 (2016).
Klajn, R., Bishop, K. J. M. & Grzybowski, B. A. Light-controlled self-assembly of reversible and irreversible nanoparticle suprastructures. Proc. Natl. Acad. Sci. USA 104, 10305–10309 (2007).
Chovnik, O., Balgley, R., Goldman, J. R. & Klajn, R. Dynamically self-assembling carriers enable guiding of diamagnetic particles by weak magnets. J. Am. Chem. Soc. 134 (2012).
Wang, Y. et al. Patchy particle self-assembly via metal coordination. J. Am. Chem. Soc. 135, 14064–14067 (2013).
Chen, Q., Bae, S. C. & Granick, S. Directed self-assembly of a colloidal kagome lattice. Nature 469, 381–384 (2011).
Han, K., Go, D., Hoenders, D., Kuehne, A. J. C. & Walther, A. Switchable supracolloidal coassembly of microgels mediated by host/guest interactions. ACS Macro Lett. 6, 310–314 (2017).
De Feijter, I., Albertazzi, L., Palmans, A. R. A. & Voets, I. K. Stimuli-responsive colloidal assembly driven by surface-grafted supramolecular moieties. Langmuir 31 (2015).
Van Ravensteijn, B. G. P., Vilanova, N., De Feijter, I., Kegel, W. K. & Voets, I. K. Temperature-Induced, Selective Assembly of Supramolecular Colloids in Water. ACS Omega 2, 1720–1730 (2017).
Celiz, A. D., Lee, T.-C. & Scherman, O. A. Polymer-mediated dispersion of cold nanoparticles: using supramolecular moieties on the periphery. Adv. Mater. 21 (2009).
Gerth, M. & Voets, I. K. Molecular control over colloidal assembly. Chem. Commun. 53, 4414–4428 (2017).
Mirkin, C. A., Letsinger, R. L., Mucic, R. C. & Storhoff, J. J. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382, 607–609 (1996).
Storhoff, J. J. & Mirkin, C. A. Programmed Materials Synthesis with DNA. Chem. Rev. 99, 1849–1862 (1999).
Wang, Y. et al. Crystallization of DNA-coated colloids. Nat. Commun. 6 (2015).
Kim, A. J., Biancaniello, P. L. & Crocker, J. C. Engineering DNA-mediated colloidal crystallization. Langmuir 22, 1991–2001 (2006).
Dreyfus, R. et al. Simple quantitative model for the reversible association of DNA coated colloids. Phys. Rev. Lett. 102 (2009).
Valignat, M.-P., Theodoly, O., Crocker, J. C., Russel, W. B. & Chaikin, P. M. Reversible self-assembly and directed assembly of DNA-linked micrometer-sized colloids. Proc. Natl. Acad. Sci. USA 102, 4225–4229 (2005).
Rogers, W. B. & Manoharan, V. N. Programming colloidal phase transitions with DNA strand displacement. Science (80-.). 347, 639–642 (2015).
Jin, R., Wu, G., Li, Z., Mirkin, C. A. & Schatz, G. C. What controls the melting properties of DNA-linked gold nanoparticle assemblies? J. Am. Chem. Soc. 125, 1643–1654 (2003).
Storhoff, J. J. et al. What controls the optical properties of DNA-linked gold nanoparticle assemblies? J. Am. Chem. Soc. 122, 4640–4650 (2000).
Wang, Y. et al. Colloids with valence and specific directional bonding. Nature 491, 51–55 (2012).
Mognetti, B. M., Leunissen, M. E. & Frenkel, D. Controlling the temperature sensitivity of DNA-mediated colloidal interactions through competing linkages. Soft Matter 8, 2213–2221 (2012).
Yan, Y., Chen, J. I. L. & Ginger, D. S. Photoswitchable oligonucleotide-modified gold nanoparticles: Controlling hybridization stringency with photon dose. Nano Lett. 12, 2530–2536 (2012).
Xue, C., Gutierrez-Cuevas, K., Gao, M., Urbas, A. & Li, Q. Photomodulated self-assembly of hydrophobic thiol monolayer-protected gold nanorods and their alignment in thermotropic liquid crystal. J. Phys. Chem. C 117, 21603–21608 (2013).
Manna, D., Udayabhaskararao, T., Zhao, H. & Klajn, R. Orthogonal Light-Induced Self-Assembly of Nanoparticles using Differently Substituted Azobenzenes. Angew. Chemie - Int. Ed. 54 (2015).
Bandara, H. M. D. & Burdette, S. C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 41, 1809–1825 (2012).
Isenbügel, K., Gehrke, Y. & Ritter, H. Photo-switchable behavior of azobenzene-dye-modified silica nanoparticles and their assembly with cyclodextrin derivatives. Macromol. Chem. Phys. 213, 227–233 (2012).
Stricker, L., Fritz, E.-C., Peterlechner, M., Doltsinis, N. L. & Ravoo, B. J. Arylazopyrazoles as Light-Responsive Molecular Switches in Cyclodextrin-Based Supramolecular Systems. J. Am. Chem. Soc. 138, 4547–4554 (2016).
Vilanova, N., De Feijter, I. & Voets, I. K. Synthesis and characterization of supramolecular colloids. J. Vis. Exp. 2016 (2016).
Mes, T. et al. Network formation in an orthogonally self-assembling system. ACS Macro Lett. 1 (2012).
Varrato, F. et al. Arrested demixing opens route to bigels. Proc. Natl. Acad. Sci. USA 109 (2012).
Di Michele, L. et al. Multistep kinetic self-assembly of DNA-coated colloids. Nat. Commun. 4 (2013).
Han, K. et al. Social Self-Sorting of Colloidal Families in Co-Assembling Microgel Systems. Angew. Chemie - Int. Ed. 56, 2176–2182 (2017).
Teunissen, A. J. P., Van Der Haas, R. J. C., Vekemans, J. A. J. M., Palmans, A. R. A. & Meijer, E. W. Scope and limitations of supramolecular autoregulation. Bull. Chem. Soc. Jpn. 89 (2016).
Ligthart, G. B. W. L., Ohkawa, H., Sijbesma, R. P. & Meijer, E. W. Complementary quadruple hydrogen bonding in supramolecular copolymers. J. Am. Chem. Soc. 127 (2005).
Hermanson, G. T. Bioconjugate Techniques. (Elsevier, 2013).
Acknowledgements
The authors acknowledge A.J.H. Spiering and P.J.M. Stals for the synthesis of BTA and UPy supramolecular moieties, respectively, T.F.E. Paffen for the advice on the NaPy experiments. The authors thank the Netherlands Organization for Scientific research (NWO ECHO-STIP Grant 717.013.005, NWO VIDI Grant 723.014.006) and the Dutch Ministry of Education, Culture and Science (Gravity Program 024.001.035) for financial support. The ICMS Animation Studio (Eindhoven University of Technology) is acknowledged for providing some of the artwork for Figure 1, 2 and 6.
Author information
Authors and Affiliations
Contributions
N.V., I.F. and I.K.V. designed the experiments. N.V. and I.F. performed the experiments and analysed the data. A.J.P.T. synthesized the NaPy derivative and helped to interpret the data of the experiments involving this molecule. N.V. and I.K.V. wrote the article and the other authors reviewed it.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vilanova, N., Feijter, I.d., Teunissen, A.J.P. et al. Light induced assembly and self-sorting of silica microparticles. Sci Rep 8, 1271 (2018). https://doi.org/10.1038/s41598-018-19282-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-19282-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.






 ) on the colloids (expressed as the surface area per amine on the colloids) as a function of the irradiation time (t
i
). Lines are drawn to guide the eye.
) on the colloids (expressed as the surface area per amine on the colloids) as a function of the irradiation time (t
i
). Lines are drawn to guide the eye.

