Abstract
Epicardial adipose tissue (EAT) is a metabolically active tissue intimately associated with metabolic syndrome and cardiovascular disease. Quantification of EAT volume is an interesting clinical tool for the evaluation of cardiometabolic disease. Nevertheless, current methodology presents serious disadvantages. The soluble form of the receptor LRP1 (sLRP1) is a non-invasive biomarker of EAT in general population. Here, we analysed the potential of circulating sLRP1 as biomarker of EAT volume in patients with type 1 diabetes mellitus (T1DM). The study included a well-characterized cohort of T1DM patients without clinical cardiovascular disease (N = 73). EAT volume was assessed by a multidetector computed tomography (MDCT). sLRP1 and panel of inflammatory and endocrine mediators were measured using commercially available ELISA. EAT volume showed a direct association with circulating sLRP1 (β = 0.398, P = 0.001) in univariate linear regression analysis. This association was higher than that observed for other potential inflammatory and endocrine biomarkers. Using multivariate linear regression analyses, we demonstrated that the association between EAT volume and circulating sLRP1 was independent of potential confounding factors, including age, sex, body mass index, CRP, HbA1c and LDL-C (P < 0.050 for all multivariate linear regression models). In conclusion, sLRP1 is an independent biomarker of EAT in T1DM patients.
Similar content being viewed by others
Introduction
Cardiovascular disease is the main cause of death in patients with type 1 diabetes mellitus (T1DM), being the relative risk of death by coronary artery disease (CAD) ten times greater than in the non-diabetic population, especially in women1,2. The pathophysiology underlying cardiovascular events in patients with T1DM is still unclear and the relative role of conventional cardiovascular risk factors is not well defined. Unlike Type 2 diabetes mellitus (T2DM), T1DM is mainly characterized by insulin deficiency. Nonetheless, insulin resistance and metabolic syndrome are also common pathological derangements contributing to cardiovascular risk in T1DM patients3,4. Epicardial adipose tissue (EAT), the fat depot located between the myocardium and pericardium, is an active tissue involved in the development of metabolic and cardiovascular disease5. EAT is increased in T1DM and has been associated with insulin resistance/metabolic syndrome6 and higher risk of cardiovascular complications7. Thus, assessment of EAT could provide relevant insights into cardiometabolic risk prediction in T1DM.
The impact of EAT in cardiometabolic disease has led to a growing clinical interest in the accurate quantification of this fat depot8. However, the evaluation of EAT using current imaging modalities is impractical for large-scale population screening and presents several disadvantages including radiation exposure, limited availability and elevated cost8. Thus, blood-based biomarkers emerge as non-invasive and early accessible indicators of EAT.
In previous studies, we have demonstrated that the membrane receptor low density lipoprotein receptor-related protein 1 (LRP1) is upregulated in epicardial fat of diabetic patients9, and that its soluble form, sLRP1, is a novel biomarker of EAT volume in general population10. Insulin deficiency, hyperglycaemia, insulin resistance and systemic inflammation could alter the expression of LRP111,12. Consequently, the relation of sLRP1 with EAT volume could be altered in patients with T1DM with respect to the general population. On the basis of these previous findings, the aim of current investigation was to test whether sLRP1 concentration is associated with EAT volume in a well-characterized and well-controlled cohort of patients with T1DM.
Results
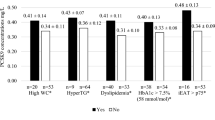
The main clinical and analytical characteristics of the study population are shown in Table 1. The age at diagnosis was 24.8 ± 8.7 years. All patients received intensive insulin therapy with basal-bolus regimen (14.3% with insulin pump) and the average daily dose of insulin was 0.66 ± 0.22 IU / kg / day. The overall mean HbA1c throughout the follow-up was 7.20 ± 0.79% and in 60% it was ≤ 7.5% during the follow-up. 29% received hypotensive treatment and 42.9% statins. 35.1% were smokers, 40.3% were overweight and 20.8% were obese.
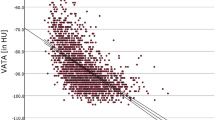
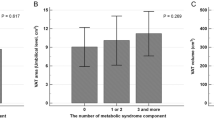
Using univariate linear regression analysis we evaluated the association between the EAT volume and a panel of potential circulating biomarkers in T1DM patients, including sLRP1 (Table 2). EAT volume showed a direct association with leptin (β = 0.304, P-value = 0.011) and sLRP1 (β = 0.398, P-value = 0.001). As expected, an inverse association was observed for EAT volume and adiponectin (β = −0.323, P-value = 0.006). A borderline association with transforming growth factor β (TGF-β) was also reported (β = −0.209, P-value = 0.082). No association was observed between EAT volume and interleukin (IL)–6 (β = 0.076, P-value = 0.539) and IL-10 (β = 0.164, P-value = 0.175). It should be noted that the standardized beta coefficients were numerically higher for sLRP1.
Then, a multivariate linear regression analysis was performed to study in detail the association between the EAT volume and circulating sLRP1 concentration in T1DM patients. Previous publications reported a close association between cardiac adiposity and age, sex or body composition parameters13,14. In order to take into account the potential confounding effect of these variables, the association between EAT volume and sLRP1 concentration was adjusted for age, sex and BMI in model 1. As shown in Table 2, the association between the EAT volume and sLRP1 concentration (β = 0.238, P-value = 0.015) remained statistically significant even after adjusting for these clinical characteristics. Possible confounders such as CRP, HbA1c or LDL-C, which are associated or have been previously associated with EAT volume and/or sLRP1 concentration13,15, were also entered as independent variables into the model 1 (models 2 and 3). The direct association between the EAT volume and circulating sLRP1 concentration remained statistically significant even after adjusting for these confounding factors (β = 0.228, P-value = 0.024 for model 2 and β = 0.251, P-value = 0.049 for model 3). Due to the association observed between the EAT volume and the circulating concentrations of leptin and adiponectin in the univariate regression analysis, the clinical relevance of smoking and the association between sLRP1 and statin use15, an additional model was performed including these four variables. The association between EAT volume and sLRP1 remained statistically significant even after adjusting by these potential confounding factors (β = 0.238, P-value = 0.045).
Discussion
Here, we demonstrated that: i) circulating sLRP1 is a biomarker of EAT volume in a cohort of well-characterized and well-controlled T1DM patients without clinical cardiovascular disease; ii) the direct relation between the EAT volume and circulating sLRP1 concentration is independent of a number of clinical and metabolic confounders; iii) the association reported between EAT volume and sLRP1 concentration is higher than the observed for other potential biomarkers such as leptin, adiponectin or different inflammatory markers.
Since EAT is a modificable factor associated with a higher cardiometabolic risk, therapeutic strategies targeted to reduce EAT (i.e. weight loss or pharmacological interventions) could be implemented in those T1DM patients with high EAT levels5. Thus, identifying T1DM subjects with high levels of EAT could be relevant for patient management. Nonetheless, current evaluation of EAT using imaging techniques is not feasible in large-scale screenings. Supporting previous data on general population15, circulating sLRP1 is an independent biomarker of EAT volume in T1DM patients that provides additional information to current standard risk factors or established clinical indicators. Importantly, sLRP1 could be cost-effectively quantified in clinical laboratories with current technology, which facilitates its applicability. The evaluation of sLRP1 could constitute an easily performed test to provide additional information to clinicians for monitoring EAT in diabetic care settings.
The pathological role of sLRP1 in cardiometabolic disease is unclear. Our group has identified the cellular form of LRP1 as a key receptor for cholesterol accumulation in vascular cells16,17,18,19 and cardiomyocytes20,21,22, and showed that this receptor is strongly upregulated in coronary arteries and myocardium in in vivo models23,24,25 and patients22,26,27,28 exposed to cardiovascular risk factors. LRP1 has also the potential to interact with different ligands involved in inflammation29 and coagulation30. We have also shown that circulating levels of sLRP1 are associated with atherosclerosis in patients with familial hypercholesterolemia15. Here, we could only speculate about the physiological or pathological implications of the association observed between EAT volume and sLRP1. sLRP1 may affect the functionality of cellular receptor ligands, and thus, may influence different mechanisms linked to cardiovascular disease, such as lipid metabolism, coagulation, inflammation or extracellular protease activity, among others31,32,33. This is an interesting and unexplored field that deserves future investigation.
The main limitation of the current study is the cross-sectional design. As explained above, we cannot draw causal conclusion about the nature of the association between EAT and sLRP1. Since LRP1 is a ubiquitous expressed receptor we cannot conclude whether the sLRP1 detected in plasma is released exclusively from EAT. Despite the robustness of the associations observed here the sample size precluded the analysis of the different subgroups of patients. Finally, generalization of the results is somewhat limited by the defining characteristics of the type 1 diabetes population. Indeed, the detailed analysis of the association between EAT and sLRP1 in patients with cardiovascular disease should be performed.
In conclusion, our results demonstrated that circulating sLRP1 is a novel surrogate biomarker of EAT volume in T1DM patients. A test based on the circulating concentration of this soluble receptor could be an easily accessible tool for cardiometabolic risk stratification in T1DM patients. Further studies should validate these results in multicentric studies with larger populations.
Methods
Subjects with T1DM who were diagnosed between 1985 and 1994 and regularly followed up at the Endocrinology and Nutrition Department in the Hospital de la Santa Creu i Sant Pau (n = 130 patients) were offered participation. The exclusion criteria was the presence of clinical cardiovascular disease. Seventy-three patients with T1DM and without established clinical cardiovascular disease agreed to participate and were evaluated. Demographic and anthropometric characteristics, cardiovascular risk factors and clinical history were collected. Venous blood samples were taken after overnight fasting for laboratory determinations. Lipid profile was determined according routine protocols of the Sant Pau Hospital, Spain34. The study protocol was approved by The Ethical Committee of Clinical Investigation (CEIC) in Sant Pau’s Hospital (http://www.recercasantpau.cat/investigacio-clinica/ceic/). All subjects gave their written informed consent before participating in the study. All methods were performed in accordance with the relevant guidelines and regulation of CEIC-Sant Pau’s Hospital.
EAT was measured in a contrast-enhanced scan acquired with a 256-slice multidetector computed tomography (MDCT) scanner (Brilliance iCT 256-slice, Philips Healthcare). This scan was prospectively triggered at 75% of the RR interval using from 100 to 120 kV (120 kV in patients with a body mass index > 30 kg/m2). After that, MDCT studies were analysed on an off-line workstation. The methodology to calculate EAT was done with dedicated software (OsiriX MD, v 6.5, FDA cleared, Pixmeo) as it follows: first, the upper and lower slice limits of pericardium were manually defined using axial views. Then, EAT was marked in each slice by drawing regions of interest with voxel density between −150 to −30 Hounsfield units (corresponding to adipose tissue). After that, a contiguous 3-dimensional volume render (showing EAT volume) was performed and quantified in cubic centimetres (cm3) as well as indexed to body surface area (cm3/m2).
In addition to sLRP1, a panel of endocrine and inflammatory mediators was measured in frozen plasma samples. All mediators were measured using commercial available ELISA: Adiponectin and leptin (R&D), IL-6, IL-10 and TGF-β (Bender MedSystems), and sLRP1 (USCN Life Science).
The statistical software package SPSS 15.0 for Windows (SPSS Inc.) was used for all statistical analyses. Descriptive statistics were used to characterize the study populations and to analyse the studied parameters. Data were presented as the mean ± SD for continuous variables and as frequencies (percentages) for categorical variables. The normality of the data was analysed using the Kolmogorov-Smirnov test. Variables with skewed distribution were log-transformed prior to use as continuous variables. Univariate and multivariate linear regression analyses were performed to examine the associations between EAT volume and circulating sLRP1. The standardized beta coefficient (β) was used to evaluate the strength of the effect of the independent variables. Data are expressed as the standardized beta coefficient (β). Differences were considered statistically significant when P-value < 0.050.
Change history
08 November 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Libby, P. et al. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation. 111, 3489–3493 (2005).
Lind, M. et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 371, 1972–1982 (2014).
Chillaron, J. J. et al. Estimated glucose disposal rate in assessment of the metabolic syndrome and microvascular complications in patients with type 1 diabetes. J Clin Endocrinol Metab. 94, 3530–3534 (2009).
Nadeau, K. J. et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 95, 513–521 (2010).
Iacobellis, G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 11, 363–371 (2015).
Momesso, D. P. et al. Increased epicardial adipose tissue in type 1 diabetes is associated with central obesity and metabolic syndrome. Diabetes Res Clin Pract. 91, 47–53 (2011).
Aslan, A. N. et al. The Relationship between Epicardial Fat Thickness and Endothelial Dysfunction in Type I Diabetes Mellitus. Echocardiography. 32, 1745–1753 (2015).
Britton, K. A. & Fox, C. S. Ectopic fat depots and cardiovascular disease. Circulation. 124, e837–841 (2011).
Nasarre, L. et al. Low density lipoprotein receptor-related protein 1 is upregulated in epicardial fat from type 2 diabetes mellitus patients and correlates with glucose and triglyceride plasma levels. Acta Diabetol. 5, 23–30 (2012).
de Gonzalo-Calvo, D. et al. Circulating levels of soluble low-density lipoprotein receptor-related protein 1 (sLRP1) as novel biomarker of epicardial adipose tissue. Int J Cardiol. 223, 371–373 (2016).
Ceschin, D. G., Sanchez, M. C. & Chiabrando, G. A. Insulin induces the low density lipoprotein receptor-related protein 1 (LRP1) degradation by the proteasomal system in J774 macrophage-derived cells. J Cell Biochem. 106, 372–380 (2009).
Gorovoy, M., Gaultier, A., Campana, W. M., Firestein, G. S. & Gonias, S. L. Inflammatory mediators promote production of shed LRP1/CD91, which regulates cell signaling and cytokine expression by macrophages. J Leukoc Biol. 88, 769–778 (2010).
Ahn, S. G. et al. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart. 94, e7 (2008).
Iozzo, P. et al. Contribution of glucose tolerance and gender to cardiac adiposity. J Clin Endocrinol Metab. 94, 4472–4482 (2009).
de Gonzalo-Calvo, D. et al. Circulating soluble low-density lipoprotein receptor-related protein 1 (sLRP1) concentration is associated with hypercholesterolemia: A new potential biomarker for atherosclerosis. Int J Cardiol. 201, 20–29 (2015).
Llorente-Cortés, V., Martínez-González, J. & Badimon, L. LDL receptor-related protein mediates uptake of aggregated LDL in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 20, 1572–1579 (2000).
Llorente-Cortés, V., Otero-Viñas, M. & Badimon, L. Differential role of heparan sulfate proteoglycans on aggregated LDL uptake in human vascular smooth muscle cells and mouse embryonic fibroblasts. Arterioscler Thromb Vasc Biol. 22, 1905–1911 (2002).
Llorente-Cortés, V., Otero-Viñas, M., Sánchez, S., Rodríguez, C. & Badimon, L. Low-density lipoprotein upregulates low-density lipoprotein receptor-related protein expression in vascular smooth muscle cells: possible involvement of sterol regulatory element binding protein-2-dependent mechanism. Circulation. 106, 3104–3110 (2002).
Llorente-Cortés, V., Otero-Viñas, M., Camino-López, S., Costales, P. & Badimon, L. Cholesteryl esters of aggregated LDL are internalized by selective uptake in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 26, 117–123 (2006).
Castellano, J. et al. Hypoxia exacerbates Ca(2 + )-handling disturbances induced by very low density lipoproteins (VLDL) in neonatal rat cardiomyocytes. J Mol Cell Cardiol. 50, 894–902 (2011).
Cal, R. et al. Low-density lipoprotein receptor-related protein 1 mediates hypoxia-induced very low density lipoprotein-cholesteryl ester uptake and accumulation in cardiomyocytes. Cardiovasc Res. 94, 469–479 (2012).
Cal, R. et al. Low density lipoprotein receptor-related protein 1 expression correlates with cholesteryl ester accumulation in the myocardium of ischemic cardiomyopathy patients. J Transl Med. 10, 160 (2012).
Llorente-Cortés, V. et al. Cholesterol-lowering strategies reduce vascular LRP1 overexpression induced by hypercholesterolaemia. Eur J Clin Invest. 41, 1087–1097 (2011).
Samouillan, V. et al. Cardiomyocyte intracellular cholesteryl ester accumulation promotes tropoelastin physical alteration and degradation: Role of LRP1 and cathepsin S. Int J Biochem Cell Biol. 55, 209–219 (2014).
Revuelta-Lopez, E. et al. Relationship among LRP1 expression, Pyk2 phosphorylation and MMP-9 activation in left ventricular remodelling after myocardial infarction. J Cell Mol Med. 21, 1915–1928 (2017).
Llorente-Cortés, V., Otero-Viñas, M., Berrozpe, M. & Badimon, L. Intracellular lipid accumulation, low-density lipoprotein receptor-related protein expression, and cell survival in vascular smooth muscle cells derived from normal and atherosclerotic human coronaries. Eur J Clin Invest. 34, 182–190 (2004).
Roura, S. et al. Inverse relationship between raft LRP1 localization and non-raft ERK1,2/MMP9 activation in idiopathic dilated cardiomyopathy: Potential impact in ventricular remodeling. Int J Cardiol. 176, 805–814 (2014).
Roura, S. et al. Extracellular vesicles do not contribute to higher circulating levels of soluble low-density lipoprotein receptor-related protein 1 in idiopathic dilated cardiomyopathy. J Cell Mol Med. 21, 3000–3009 (2017).
May, P. The low-density lipoprotein receptor-related protein 1 in inflammation. Curr Opin Lipidol. 24, 134–137 (2013).
van den Biggelaar, M. et al. Factor VIII Interacts with the Endocytic Receptor Low-density Lipoprotein Receptor-related Protein 1 via an Extended Surface Comprising “Hot-Spot” Lysine Residues. J Biol Chem. 290, 16463–16476 (2015).
Scilabra, S. D. et al. Differential Regulation of Extracellular Tissue Inhibitor of Metalloproteinases-3 Levels by Cell Membrane-bound and Shed Low Density Lipoprotein Receptor-related Protein 1. J Biol Chem. 288, 332–342 (2013).
Selvais, C. et al. Metalloproteinase-dependent shedding of low-density lipoprotein receptor-related protein-1 ectodomain decreases endocytic clearance of endometrial matrix metalloproteinase-2 and -9 at menstruation. Endocrinology. 150, 3792–3799 (2009).
Wygrecka, M. et al. Shedding of low-density lipoprotein receptor-related protein-1 in acute respiratory distress syndrome. Am J Respir Crit Care Med. 184, 438–448 (2011).
Sanchez-Quesada, J. L. et al. Impact of the LDL subfraction phenotype on Lp-PLA2 distribution, LDL modification and HDL composition in type 2 diabetes. Cardiovasc Diabetol. 12, 112 (2013).
Acknowledgements
This work was supported by FIS PI14/01729 and FIS PI13/00364 from the Instituto Salud Carlos III, co-financed by the European Fund for Regional Development (E.F.R.D), Fundació Marató TV3 (201521 10) and Ayudas Sociedad Española de Diabetes (SED) de Investigación Básica y Clínica en Diabetes 2011. CIBER Cardiovascular (CB16/11/00403) and CIBERDEM (CB07/08/0016) are Instituto de Salud Carlos III Projects. DdG-C was a recipient of a Sara Borrell grant from the Instituto de Salud Carlos III (CD14/00109). AR-U, MP-C and JLS-Q are members of the Quality Research Group 2014-SGR-0246 and VLL-C, DdG-C are members of the Quality Research Group 2014-SGR-00170 from Generalitat de Catalunya.
Author information
Authors and Affiliations
Contributions
D.d.G., V.L.l.-C. and A.P. conceived the idea and designed research. D.d.G.-C., C.C., D.V., A.R.-U., A.-H.M., M.P.-C. and J.L.S.-Q. collected samples, conducted the research and analysed data. D.d.G.-C., C.C., V.L.l.-C. and A.P. wrote the paper and had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Gonzalo-Calvo, D., Colom, C., Vilades, D. et al. Soluble LRP1 is an independent biomarker of epicardial fat volume in patients with type 1 diabetes mellitus. Sci Rep 8, 1054 (2018). https://doi.org/10.1038/s41598-018-19230-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-19230-3
This article is cited by
-
PCSK9 plasma concentration is associated with epicardial adipose tissue volume and metabolic control in patients with type 1 diabetes
Scientific Reports (2024)
-
Soluble low-density lipoprotein receptor-related protein 1 as a surrogate marker of carotid plaque inflammation assessed by 18F-FDG PET in patients with a recent ischemic stroke
Journal of Translational Medicine (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.