Abstract
The carbon cycle is significantly affected by Spartina alterniflora invasion through its impact on blue carbon in many salt marshes. To determine the impacts on soil organic carbon (SOC), we studied the vertical and horizontal distribution of SOC. And stable carbon isotopes were used to explore the impact of the age of S. alterniflora invasion on SOC in Chongming Dongtan wetland located in the Yangtze River estuary, China. The results showed that the SOC concentration was higher in the S. alterniflora community than that in the native Phragmites australis community. The age of invasion and the SOC concentration increased with increasing elevation, while the SOC concentration decreased with increasing soil depth. The δ13C value became less negative at greater depth, which was related to the contribution from 13C- enriched carbon sources after 3 years of invasion. After 7 and 10 years, the δ13C value became more negative at greater depth in both communities. S. alterniflora had a positive effect on the soil carbon pool, and its contribution was related to soil depth. In the low tidal marshes, the contribution of S. alterniflora was negatively correlated with soil depth, while it was positively correlated with soil depth in the high tidal marshes. The results from this study will contribute to improved understanding of future ecological consequences.
Similar content being viewed by others
Introduction
‘Blue carbon’, which refers to carbon sequestered in salt marsh, seagrass, and mangrove ecosystems, is important for understanding the link between terrestrial and oceanic carbon cycles1,2,3. Accounting for all sources of carbon and organic matter contributing to coastal ecosystems is imperative for accurate budgeting of the current global carbon cycle and for forecasting changes in carbon cycling induced by global climate change and other anthropogenic impacts4,5, such as land use change. Population changes in native species within the plant community alter the ecosystem structure and the soil carbon pool6. Therefore, alterations in the composition of salt marsh plant species, which are affected by current natural and human activities, and their associated carbon sequestration capacity require further attention4,7,8.
Plant invasion profoundly influences ecosystem functions and soil processes in ecosystems around the world6,9,10. Changes in the dominant species within a plant community may alter ecosystem structures and material cycling processes, which in turn alter above- and belowground carbon pools, net primary productivity, plant growth rates, litter quality and quantity, and nutrient and carbon mineralization rates11. Plant invasions mainly influenced soil organic carbon pool by altering the quantity and quality of litter and roots entering the soil. The effect of invasive plants on soil carbon pools is critically important for understanding changes in carbon cycles. However, compared with soil carbon reservoirs, the changes in soil organic matter, even when significant, are small. Therefore, conventional methods cannot be used to examine the variation in the soil organic carbon (SOC) pool within the short period over which most experiments are conducted.
Spartina alterniflora was introduced to China from North America in 197912. Since then, this invasive species has rapidly expanded in salt marshes along the coast. However, S. alterniflora has negative impacts on native ecosystems, as it continuously encroaches on the habitat of native plant communities, forming a single-dominant plant community13,14. Several studies have focused on the effects of S. alterniflora invasion on wetland total SOC and the horizontal and vertical distribution and seasonal dynamics of SOC15. However, few studies have focused on the effects of S. alterniflora on SOC dynamics using an invasion chronosequence.
Stable carbon isotopes have been employed as tracers of the sources of accumulated organic carbon in salt marsh sediments16,17,18,19,20,21,22,23,24. This technique is based on differences in stable carbon isotope ratios, such as those between the soil carbon inputs of S. alterniflora (C4 source) and Phragmites australis (C3 source). C4 and C3 sources are readily distinguished by their distinctive δ13C values. C3 plants have been reported to exhibit δ13C values between −35 and −20‰, while those of C4 plants range between −19 and −9‰25,26,27. These values are widely used to study the source of soil organic matter. If the climate and environmental conditions are the same for all sampled plots in a given study, the variation in the soil isotope composition can be employed to measure the newly generated SOC after S. alterniflora invasion.
In this study, we hypothesized that time since S. alterniflora invasion would significantly affect the soil carbon pool in different soil fractions in the Chongming Dongtan tidal marsh. Analyzing the stable carbon isotopic composition and carbon concentration in the soil to a depth of 50 cm in areas where the invasive plant S. alterniflora and the native plant P. australis occurred allowed us to better understand the influence of invasive C4 plants on soil carbon. The major purposes of this study were to 1) understand the different vertical and horizontal distributions of the soil carbon pool in this Yangtze River estuary salt marsh; 2) quantify the contribution of S. alterniflora invasion to SOC; and 3) determine the influence of different S. alterniflora invasion times (3, 7 and 10 years) on the soil carbon pool.
Results
Plant biological traits
Along the southern transect, the aboveground biomass of the S. alterniflora community was 3500, 3889 and 3412 g m−2 for the low, middle and high tidal marshes, respectively, but these values did not differ significantly. These biomass values were approximately 1.5 times higher than those recorded for the P. australis community. Along the northern transect, the corresponding aboveground biomass of the S. alterniflora community was 3525, 2800 and 3104 g m−2 for the low, middle, and high tidal marshes, respectively; and these values were more than three times higher than those recorded for the P. australis community (Table 1).
Distribution patterns of SOC in Dongtan wetland
Horizontal distribution of SOC
Along the southern transect, the average SOC concentrations in all soil layers rapidly increased from approximately 2% to 8% across an area about 200 m from the mudflat to the vegetated tidal marsh which the distance reached 1000 m. Then, the SOC values remained at a high level, starting at approximately 8%, increased slightly when the distance reached approximately 1700 m. Along the entire northern transect, the soil total carbon content quickly increased rapidly from the mudflat to the vegetated area (Fig. 1).
Along the southern transect, the SOC of the S. alterniflora community increased during the period of plant invasion, ranging from 3.00% to 7.79%. Compared with the SOC of the mudflat, the SOC of the S. alterniflora-invaded plots (S-L-S) was increased by 128%. Additionally, the SOC of the S. alterniflora-invaded plots increased by 43.25% and 24.00% in the middle and high marshes, respectively, compared with that associated with P. australis at the same elevation. The SOC along the northern transect ranged from 3.87% to 11.16%. Compared with the SOC of the mudflat, the SOC of the S. alterniflora-invaded sample plots (N-L-S) was increased by 55.64%. The SOC measured in the S. alterniflora plots was generally higher than that in the P. australis plots, except in the N-M-S sample plot along the northern transect (Fig. 1b).
Vertical distribution of SOC
The average SOC content in Dongtan wetland over a depth of 0–50 cm increased with increasing distance from the mudflat, but the rate of change varied in each soil layer. At the edge of the coastal marsh along the southern transect, the SOC content remained almost unchanged at a depths of 0–20 cm (P > 0.05) but decreased from 20 to 50 cm (P < 0.05) in the mudflat and the low and middle marshes (Fig. 2a). In the high marsh and near the dike, the SOC content decreased with increasing soil depth. The northern transect showed the same pattern (Fig. 2b). In addition, there was a greater decrease in SOC with depth in the high marsh than that in the low and middle marshes.
Isotope composition and soil carbon stabilization in Dongtan wetland
In this study, the δ13C value of S. alterniflora ranged from −13.68 to −14.5‰ (Table 2). The δ13C value of S. alterniflora leaves was much lower than those of the roots and litter (P < 0.05), while the δ13C values of the roots and litter were similar. The average δ13C value for the leaves, roots, and litter of −13.47‰, is regarded as representative for S. alterniflora.
Along the southern transect, the δ13C value of the S. alterniflora community was less at greater soil depth in the low tidal marsh. The rate of increase of the δ13C value was 0.24 (R2 = 0.91, P < 0.05). In the middle and high tidal marshes, the δ13C value of the S. alterniflora community was more negative at greater soil depth. The rates of increase in the δ13C value were −0.59 (R2 = 0.88, P < 0.05) and −0.56 (R2 = 0.96, P < 0.05) for the middle and high tidal marshes, respectively. There were significant differences in the δ13C values between the S. alterniflora and P. australis communities (P < 0.05). Along the northern transect, the δ13C value of the S. alterniflora community of the mudflat and high tidal marsh exhibited the same pattern as for the southern transect, while the δ13C value of the S. alterniflora community did not change in the middle tidal marsh. In the high tidal marsh, the δ13C value of SOC in the S. alterniflora community was more negative as the stem density of S. alterniflora increased. The rate of decrease in the δ13C value was −1.16 (P < 0.05, R2 = 0.97). However, there were no significant differences in the δ13C values between the P. australis and S. alterniflora communities (Fig. 3).
Contribution of S. alterniflora to the soil carbon pool in Dongtan wetland
Along the southern transect, after 3 years of S. alterniflora invasion, the contribution of this specie to SOC was significantly positively correlated with soil depth (P < 0.01, R2 = 0.82); at soil depths of 0–5, 5–10, 10–20, 20–30 and 30–50 cm, the contribution of S. alterniflora to SOC represented 18.0, 19.8, 23.6, 23.6 and 23.8%, respectively. After 7 years of invasion, the SOC contribution of this specie was negatively correlated with soil depth (P < 0.01, R2 = 0.97); at soil depths of 0–5, 5–10, 10–20, 20–30 and 30–50 cm, the contribution to the SOC content were 35.7, 31.9, 19.6, 5.5 and 9.0%, respectively. After 10 years of invasion, the SOC contribution was significantly negatively correlated with soil depth (P < 0.01, R2 = 0.99); at soil depths of 0–5, 5–10, 10–20, 20–30 and 30–50 cm, the contributions of S. alterniflora to the SOC content were 38.0, 31.6, 25.0, 21.4 and 12.5%, respectively (Fig. 4a).
Along the northern transect, after 3 years of S. alterniflora invasion, the contribution of this species to the SOC content was significantly positively correlated with soil depth (P < 0.01, R2 = 0.97); at depths of 0–5, 5–10, 10–20, 20–30 and 30–50 cm, the contributions of this specie to the SOC content were 13.5, 18.6, 28.1, 37.9 and 52.2%, respectively. After 7 years of invasion, the contribution of S. alterniflora to the SOC content was not correlated with soil depth (P > 0.05, R2 = 0.53); at depths of 0–5, 5–10, 10–20, 20–30 and 30–50 cm, the contributions of this specie to the SOC content were 20.98, 25.68, 22.43, 15.46 and 16.09%, respectively. After 10 years of invasion, the contribution of this specie to the SOC content was significantly negatively correlated with soil depth (P < 0.01, R2 = 0.96); at depths of 0–5, 5–10, 10–20, 20–30 and 30–50 cm, the contributions of this specie to SOC was 48.7, 44.5, 31.1, 10.9 and 3.8%, respectively (Fig. 4b).
Discussion
Plant invasion has been found to alter various components of nutrient cycles in wetland ecosystems28. Our results showed that the seaward invasion of S. alterniflora could play a major role in increasing carbon fixation in Chongming Dongtan wetland (Fig. 1). SOC was generally higher in soil dominated by S. alterniflora than that in soil dominated by native P. australis (Fig. 1). Higher SOC concentrations have been found in S. alterniflora communities due to high levels of rhizome and root biomass and elevated plant residue decomposition rates15. Our results showed that the aboveground biomass of S. alterniflora was significantly higher than that of P. australis (P < 0.05) (Table 1), indicating that S. alterniflora fixed more carbon and potentially provided more residues to the wetland soil6,29,30. In terms of the soil SOC distribution in the S. alterniflora community along the southern transect, the SOC content in the S-M-S plot was the highest but was not significantly different from that in the S-H-S plot (Fig. 2a). This showed that SOC stabilized after 7–10 years of S. alterniflora invasion. Wang et al.31 reported that SOC was saturated after 5–12 years of S. alterniflora invasion in marshes along the coast of Xinyanggang in North Jiangsu Province, China. However, SOC gradually increased with increasing time since invasion along the northern transect (Fig. 2b), similar to the results of previous research. Zhang et al.11 revealed that in Wanggang, Jiangsu Province, the carbon sequestration capacity of the topsoil remained unsaturated after 14 years of S. alterniflora invasion. The differences between the northern and southern transects could be related to the sedimentation conditions and organic carbon input from decomposition (e.g., the tidal influx rate and organic matter content in tidewater), environmental factors (e.g., soil moisture, redox potential, heat, bioturbation, and many associated factors) and the characteristics of species development in different research areas. Long-term monitoring of changes in SOC and controlled experiments are needed in future studies.
In the middle and high marshes, the SOC content was higher in the surface (0~20 cm) than that in the deeper soil layers. However, the highest SOC in the vegetation edge zone, the transition from the mudflat to the tidal marsh, occurred in the subsurface soil layer (5–20 cm) (Fig. 2). These results indicated that a considerable amount of partly decomposed S. alterniflora residue remained within soil depths of 5–20 cm. At the vegetation edge zone, the soil formation process began on the dead S. alterniflora trapped by the tidally transported sediment. Farther from the mudflat, tidal forcing is weak, resulting in less sediment deposited on the surface and a higher SOC content in the near-surface layer. Aboveground parts (i.e., standing desiccated parts) decompose in the air or fall to the ground. The tide carries away decomposed soluble carbon and tiny carbon granules, transporting them inshore, where they are deposited on the soil surface or leach into the soil6,32,33.
The photosynthetic pathways of different plants make different relative contributions to the net primary productivity of surface soil, and the δ13C values of SOC vary8. Therefore, under conditions of long-term stability of plant species, the δ13C value of SOC is strongly influenced by plant δ13C26,33,34,35. Our stable isotopic analysis suggested that the δ13C value of SOC along the soil profile differed between the S. alterniflora and P. australis communities. The δ13C value was less negative at greater depth, which was related to the contribution from 13C-enriched carbon sources, after 3 years of invasion by S. alterniflora (Fig. 3). This finding is consistent with previously reported δ13C distributions in soil profiles36,37. However, after 7 and 10 years, the δ13C value was typically more negative at greater depth in both S. alterniflora and P. australis communities. This result can be explained by the presence of SOC derived from 13C-depleted C3 vegetation32. Cheng et al.15 considered that 13C-depleted C4 S. alterniflora contributed less to SOC than native species in Jiuduansha tidal marsh because of a short invasion (i.e.,<7 years) before which Scirpus maiqueter monoculture was dominant on the island for a 30-year period.
Along the southern transect, the rates of the decrease in δ13C values along the 0–20 cm soil profile in the S. alterniflora community were 0.88 (P < 0.01, R2 = 0.99) and 0.78 (P < 0.05, R2 = 0.80) for the middle and high marshes, respectively. In addition, the rates of the decrease along the 20–50 cm soil profile in the S. alterniflora community were 0.18 (P < 0.05, R2 = 0.80) and 0.33 (P < 0.01, R2 = 0.99), respectively. These results likely occurred because SOC decomposes rapidly in surface soil. The SOC was almost completely decomposed when the δ13C value reached a plateau. Subsequently, the decomposition rate of SOC slowed, as did the change in the δ13C value. In this study, the S. alterniflora residue contributed predominantly to SOC at a depth of 20 cm compared with a lower contribution to surface SOC due to increased oxygenation that resulted in greater decomposition of organic carbon38. S. alterniflora contributed less to deep SOC because of less root exudation and weaker osmosis of soluble organic carbon34.
In the present study, the sediment of the mudflat originated mainly from tidally transported sediment, which is likely why the δ13C value of the mudflat sediments was approximately −24.33‰, which is similar to that of terrestrial C3 plants, such as P. australis. A double-variable hybrid model was used to calculate the contribution of S. alterniflora to surface SOC; with increasing time since S. alterniflora invasion, the content and proportion of the contributed SOC increased (Fig. 5). During the seaward invasion of S. alterniflora, the SOC content and proportion attributed to this species showed a clear positive correlation with time since invasion (P < 0.01), revealing that the SOC contributed by S. alterniflora increased continuously. The proportion of SOC contributed by S. alterniflora over 10 years of invasion reached 27.3 and 33.7% along the southern and northern transects, respectively.
A study conducted in the Jiuduansha tidal marsh in Shanghai showed that after 8 years of seaward invasion by S. alterniflora in a S. mariqueter community, the contribution to surface SOC ranged from 5 to 10%15. In addition, after 14 years of landward invasion by S. alterniflora in a Suaeda salsa community in the Wanggang tidal marsh in Jiangsu Province, the contribution of S. alterniflora to surface SOC was 18.7%31. First of all, soil organic carbon input was affected by the high primary productivity and extensive root system of S. alterniflora. In addition, S. alterniflora had higher primary productivity in Chongming Dongtan, ranging from 2800 to 3889 g·m−2, compared with that in Wanggang and Jiuduansha tidal marshes, which ranged from 1442 to 2596 g·m−2 39,40. It can immobilize carbon from the atmosphere and surrounding areas during growth; this carbon then enters the soil via litter and root secretions, which can directly influence the SOC content. And its well-developed roots, S. alterniflora can weaken the power of the tide, solidifying sediments in the flat and sedimenting tiny granules, which greatly improves adsorption due to the high specific surface area of the tiny granules, facilitating an increase in SOC. Second, our research site is located in the Yangtze River estuary, which carried a large number of organic matters through the tidal action, and deposited in Chongming Dongtan tidal marsh. Wang et al.31 considered the biomass of S. alterniflora and the sediment particle size to be the dominant factors determining the content of SOC. Additionally, the SOC content derived from S. alterniflora showed little correlation with S. alterniflora biomass but presented a clear positive correlation with particle size, which revealed a complicated process between the plant and soil that was greatly affected by soil characteristics.
The organic carbon input of salt marsh ecosystem mainly has two kinds of ways, including endogenous and exogenous input. Endogenous input contains soil organic matter and litter decomposition41. The exogenous inputs include suspended particles and dissolved organic carbon brought by tidal waters42. In Chongming Dongtan, suspended organic matter and sediment entered the soil carbon pool through horizontal flux. S. alterniflora has dense stalks and developed underground roots. Its ability to attenuate wave energy and reduce flow contributes to the sedimentation and deposition of silt43. Therefore, in this study of the effects of invasion on the carbon balance of ecosystem, the lateral flux caused by tidal activity is a component as well.
In this study, the tide had more influence on the contribution on soil carbon pool during the early period of invasion (after 3 years of invasion). With invasion time extension (after 7 years of invasion), the increase of soil carbon pool mainly depends on the plant (Fig. 4). The tidal flow is affected by surface roughness, elevation, and friction from plant stems and leaves44. After 3 years of invasion, the S. alterniflora patch adjacent to the bare flat had less tidal energy consumption and greater biomass loss (such as litter and lodged plants) compared with observations recorded after 7 and 10 years of invasion. In coastal marsh, the frequent cyclical effects of tide brought a non-negligible sea source input to soil carbon pool and showed an influence on the early stage of S. alterniflora invasion45.
Conclusions
In summary, we found that S. alterniflora invasion in Chongming Dongtan wetland over the past 10 years was associated with increased SOC concentrations. SOC was generally higher in the S. alterniflora than in the native P. australis community. The contribution of S. alterniflora to the soil carbon pool exhibited an obvious gradient in terms of its horizontal distribution. In the high marsh, which was invaded 10 years ago, the contribution was positively correlated with soil depth, while in the low marsh, which was invaded by S. alterniflora within the past 3 years, the contribution of S. alterniflora to the soil carbon pool was negatively correlated with depth. This result might have occurred because litter is frequently washed away by the tide in the low marsh. Thus, the main source of SOC is likely the root exudates of S. alterniflora.
Materials and Methods
Study area
This study was conducted in the Shanghai Chongming Dongtan Wetland National Nature Reserve (31°25′–31°38′N, 121°50′–122°05′E) located at the eastern end of Chongming Island. Chongming Dongtan is formed by alluvial silt and soil from the upper reaches of the Yangtze River and is the largest and youngest estuarine tidal marsh in the Yangtze River estuary. Chongming Dongtan has a northern subtropical ocean climate, with average annual precipitation of 1117.1 mm and a mean annual temperature of 15.3 °C (Fig. 6a). The native species Scirpus mariqueter and Phragmites australis have dominanted for over 20 years, while invasive S. alterniflora has rapidly spread in this tidal marsh and is replacing native species due to its considerable seed production and high germination rate46. Currently, P. australis and S. alterniflora now show zonation in their distribution in Chongming Dongtan46.
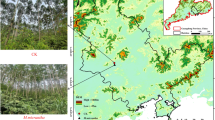
(a) Location of the sampling site in Chongming Dongtan, Shanghai, China. (b) Map of the soil profile sampling sites. Erdas9.2 TM was used to acquire the spatial distribution of plant communities from the purchased remote sensing image, which came from the 2012.5.16 ZY-02C satellite (HRC 2.36 meters), and ArcGIS10.2 TM was subsequently employed to draw these images.
Using ZY-3 satellite high-resolution remote sensing images and GPS coordinates, two transects were established using a combination of factors specific to each site, such as vegetation type, invasion time, water content, soil physical factors (pH, ORP, Salinity) (Table 3). The southern and northern transects were located in southeastern and northeastern Dongtan, respectively. The salt marsh directly landward of the study area was reclaimed in 1998. Both transects were established so that they extended through a variety of plant communities containing S. alterniflora and P. australis. The southern transect–with westernmost geographic coordinates of 31°50′N and 121°13′E and easternmost coordinates of 31°32′N and 121°58′E – was approximately 1800 m long. The northern transect–with westernmost coordinates of 31°91.67ʹN and 121°08.85′E and easternmost coordinates of 31°90.77′N and 121°95.52′E–was approximately 700 m long. Both transects were divided into three parts according to the tidal characteristics, i.e., low, middle and high tidal marshes. Low marsh refers to areas of the saltmarsh below mean high water level of neap tide. Middle marsh refers to areas of the saltmarsh between mean water level and mean high water level. High marsh refers to areas of the saltmarsh above mean high water. According to the elevations of two tidal gauges near Chongmin Dongtan (Jigujiao Gauge and Wusong Gauge), the mean high water level of Chongming Dongtan is 1.46 ± 0.01 m, the mean high water level during neap tide is 0.95 ± 0.05 m, and the mean water level is approximately 0.2 ± 0.03 m. These two chronosequenced transects extended through remnant P. australis and the S. alterniflora communities that replaced P. australis in 2001 (10 years previously), 2004 (7 years) and 2008 (3 years). Plots were established every 200–300 m along the transects. Each plot consisted of two 20 m × 20 m quadrats, and samples were collected from five replicate 0.5 m × 0.5 m subquadrats randomly established within each quadrat (Fig. 6b).
Field sample collection
Soil, leaf, litter, and root samples were collected from the S. alterniflora and P. australis communities. Cores were extracted using a stainless-steel peat core sampler and were divided into 5 cm sections in the field. Each section was sealed in multiple plastic bags with as much air squeezed out as possible and stored on ice or at 4 °C to avoid volatilization losses and minimize microbial activity until analysis.
Laboratory analyses
Plant biomass was sampled in all plots in September 2011, when the aboveground plant biomass was the greatest according to a previous study in a nearby wetland47. All aboveground plant biomass was harvested and then oven-dried at 50 °C to a constant weight.
Plant material and organic debris were first gently removed from the soil samples using forceps, and the leaf, root, litter, and soil samples were then dried at 60 °C to a constant weight and ground to pass through 100-mesh sieves34,48. After thorough mixing, approximately 10 g of dried soil was treated with 0.5 mol L−1 hydrochloric acid (HCl) for 24 h at room temperature to remove any inorganic carbon, and the unhydrolyzed residue was categorized as SOC48. Approximately 3 mg of the leaf, root, and litter subsamples was weighed and analyzed to determine the total carbon contents of the plant materials. Non-HCl-treated soil subsamples (approximately 50 mg) were weighed and analyzed to determine soil total carbon. These measurements were performed using a soil element analyzer (Flash, EA, 1112 Series, USA).
The carbon isotope ratio of organic materials (leaves, roots, litter and HCl-treated soil) was measured using an isotope ratio mass spectrometer (Thermo Fisher, Delta V Advantage, Flash, EA, 1112 Series, USA). The precision of δ13C was ±0.1‰, which was based on repeated measurements of a laboratory working standard. The δ13C value of the organic matter was calculated using the following equation:
where R = 13C/12C, and PDB indicates Pee Dee Belemnite – the belemnite carbonate standard of the Peedee Formation in South Carolina, USA49,50.
Calculation of carbon inputs
The proportion f is the proportion of S. alterniflora-derived carbon in the soil. This calculation was based on the δ13C values of the soil before and after S. alterniflora invasion (δ13Cold and δ13Cnew, respectively) and the δ13C value of S. alterniflora (δ13Cmix), which was the average of the values for the leaves, roots, and litter (Table 2)15,34.
Statistical analyses
Statistical analyses were performed using the statistical software package SPSS version 18 for Windows 7. Data were tested for normality prior to statistical analysis. One-way ANOVA at a 5% significance level was employed to determine the differences in SOC and the biomass, height and density of the plants. Significant differences in plant δ13C values between the two species were analyzed using the t-test and the least significant difference at P < 0.05. δ13C data were arcsine transformed to ensure homoscedasticity before the ANOVAs were conducted. Regression analyses were performed to test the relationships between the accumulative rates of SOC or S. alterniflora-derived carbon and the ages of the plant invasions.
Data availability statement
The datasets generated during the current study are available from the corresponding author upon reasonable request.
References
Deegan, L. A. et al. Coastal eutrophication as a driver of salt marsh loss. Nature 490, 388–392 (2012).
McLeod, E. et al. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 9, 552–560 (2011).
Mitsch, W. J. & Gosselink, J. G. The value of wetlands: importance of scale and landscape setting. Ecol. Econ. 35, 25–33 (2000).
Duarte, C. M., Middelburg, J. J. & Caraco, N. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2, 1–8 (2005).
Hopkinson, C. S., Cai, W. J. & Hu, X. P. Carbon sequestration in wetland dominated coastal systems - a global sink of rapidly diminishing magnitude. Curr. Opin. Environ. Sustain. 4, 186–194 (2012).
Liao, C. et al. Altered ecosystem carbon and nitrogen cycles by plant invasion: a meta-analysis. New Phytologist 177, 706–714 (2008).
Chmura, G. L., Anisfeld, S. C., Cahoon, D. R. & Lynch, J. C. Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycle 17, 1111 (2003).
Gebrehiwet, T., Koretsky, C. M. & Krishnamurthy, R. V. Influence of Spartina and Juncus on saltmarsh sediments. III. Organic geochemistry. Chem. Geol. 255, 114–119 (2008).
Allison, S. D. & Vitousek, P. M. Rapid nutrient cycling in leaf litter from invasive plants in Hawai’i. Oecologia 141, 612–619 (2004).
Ehrenfeld, J. G. Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 6, 503–523 (2003).
Zhang, Y., Ding, W., Luo, J. & Donnison, A. Changes in soil organic carbon dynamics in an Eastern Chinese coastal wetland following invasion by a C4 plant Spartina alterniflora. Soil Biology and Biochemistry 42, 1712–1720 (2010).
Qin, P. & Zhong, C. X. Applied Studies on Spartina. (Ocean Press, 1992).
Bruno, J. F. Facilitation of cobble beach plant communities through habitat modification by Spartina alterniflora. Ecology 81, 1179–1192 (2000).
Peng, S. & Xiang, Y. The invasion of exotic plants and effects of ecosystems. Acta Ecologica Sinica 19, 560–569 (In Chinese with English Abstract) (1999).
Cheng, X. et al. Short-term C4 plant Spartina alterniflora invasions change the soil carbon in C3 plant-dominated tidal wetlands on a growing estuarine Island. Soil Biology and Biochemistry 38, 3380–3386 (2006).
Benner, R., Fogel, M. L. & Sprague, E. K. Diagenesis of belowground biomass of Spartina alterniflora in salt‐marsh sediments. Limnol. Oceanogr. 36, 1358–1374 (1991).
Currin, C. A., Newell, S. Y. & Paerl, H. W. The role of standing dead Spartina alterniflora and benthic microalgae in salt marsh food webs: Considerations based on multiple stable isotope analysis. Mar. Ecol.-Prog. Ser. 121, 99–116 (1995).
Dai, J. H. & Sun, M. Y. Organic matter sources and their use by bacteria in the sediments of the Altamaha estuary during high and low discharge periods. Org. Geochem. 38, 1–15 (2007).
Ember, L. M., Williams, D. F. & Morris, J. T. Processes that influence carbon isotope variation in salt marsh sediments. Mar. Ecol.-Prog. Ser. 36, 33–42 (1987).
Fogel, M. L., Sprague, E. K., Gize, A. P. & Frey, R. W. Diagenesis of organic matter in Georgia salt marshes. Estuar. Coast. Shelf Sci. 28, 211–230 (1989).
Gardner, L. R. Simulation of the diagenesis of carbon, sulfur, and dissolved oxygen in salt marsh sediments. Ecol. Monogr. 60, 91–111 (1990).
Middelburg, J. J., Nieuwenhuize, J., Lubberts, R. K. & van de Plassche, O. Organic carbon isotope systematics of coastal marshes. Estuar. Coast. Shelf Sci. 45, 681–687 (1997).
Zhang, S. P. et al. Organic carbon accumulation capability of two typical tidal wetland soils in Chongming Dongtan, China. J. Environ. Sci. 23, 87–94 (2011).
Boschker, H. T. S., de Brouwer, J. F. C. & Cappenberg, T. E. The contribution of macrophyte-derived organic matter to microbial biomass in salt-marsh sediments: Stable carbon isotope analysis of microbial biomarkers. Limnol. Oceanogr. 44, 309–319 (1999).
Bernoux, M., Cerri, C. C., Neill, C. & de Moraes, J. F. L. The use of stable carbon isotopes for estimating soil organic matter turnover rates. Geoderma 82, 43–58 (1998).
Nyberg, G., Ekblad, A., Buresh, R. J. & Högberg, P. Respiration from C3 plant green manure added to a C4 plant carbon dominated soil. Plant Soil 218, 83–89 (2000).
Staddon, P. L. Carbon isotopes in functional soil ecology. Trends Ecol. Evol. 19, 148–154 (2004).
Ehrenfeld, J. G. Plant-soil interactions. In: Levin, S. (Ed.), Encyclopedia of Biodiversity. 689–707 (Academic Press, 2001).
Long, S. P. Evnironmental responses. In: Sage, R.F., Monson, R.K. (Eds.), C4 Plant Biology. 215–249 (Academic Press, 1999).
Still, C. J., Berry, J. A., Ribas-Carbo, M. & Helliker, B. R. The contribution of C3 and C4 plants to the carbon cycle of a tallgrass prairie: an isotopic approach. Oecologia 136, 347–359 (2003).
Wang, G., Yang, W., Wang, G., Liu, J. e. & Hang, Z. The effects of Spartina alterniflora seaward invasion on soil organic carbon fractions,sources and distribution. Acta Ecologica Sinica 33, 2474–2483 (In Chinese with English Abstract) (2013).
Henderson, D. C., Ellert, B. H. & Naeth, M. A. Utility of C-13 for ecosystem carbon turnover estimation in grazed mixed grass prairie. Geoderma 119, 219–231 (2004).
Hobbie, E. A., Johnson, M. G., Rygiewicz, P. T., Tingey, D. T. & Olszyk, D. M. Isotopic estimates of new carbon inputs into litter and soils in a four-year climate change experiment with Douglas-fir. Plant Soil 259, 331–343 (2004).
Chiang, P. N., Wang, M. K., Chiu, C. Y., King, H. B. & Hwong, J. L. Changes in the grassland-forest boundary at Ta-Ta-Chia long term ecological research (LTER) site detected by stable isotope ratios of soil organic matter. Chemosphere 54, 217–224 (2004).
Osher, L. J., Matson, P. A. & Amundson, R. Effect of land use change on soil carbon in Hawaii. Biogeochemistry 65, 213–232 (2003).
Ehleringer, J. R., Buchmann, N. & Flanagan, L. B. Carbon isotope ratios in belowground carbon cycle processes. Ecol. Appl. 10, 412–422 (2000).
Follett, R. F. et al. Carbon isotope ratios of great plains soils and in wheat-fallow systems. Soil Sci. Soc. Am. J. 61, 1068–1077 (1997).
Chen, X. P. et al. Evaluating the impacts of incubation procedures on estimated Q10 values of soil respiration. Soil Biol. Biochem. 42, 2282–2288 (2010).
Bu NS. Soil CO2, CH4 emissions and carbon dynamic in wetland of Yangtza Estuary. Doctor Disseration, Fudan University, Sahnghai. 25 (2012).
Feng, Z. X. et al. The response of organic carbon content to biomass dynamics in Spartina alterniflora marsh. Acta Ecologica Sinica 35, 2038–2047 (2015).
Hr, H. & Mannino, A. The chemical composition and cycling of particulate and macromolecular dissolved organic matter in temperate estuaries as revealed by molecular organic tracers. Organic Geochemistry 32, 527–542 (2001).
Hemminga, M. A., Cattrijsse, A. & Wielemaker, A. Bedload and nearbed detritus transport in a tidal saltmarsh creek. Estuarine, Coastal and Shelf Science 42, 55–62 (1996).
Liao, C. Z. et al. Invasion of Spartina alterniflora enhanced ecosystem carbon and nitrogen stocks in the Yangtze Estuary, China. Ecosystems 10, 1351–1361 (2007).
Shi, B. W., Yang, S. L., Luo, X. X. & Xu, X. J. A wave attenuation over the transitional zone of mudflat and salt marsh: A case study in the eastern Chongming on the Changjiang delta. Acta Oceanologica Sinica 32(2), 174–178 (2010).
Wang, D., Zhang, R., Xiong, J., Guo, H. Q. & Zhao, B. Contribution of invasive species Spartina alterniflora to soil organic carbon pool in coastal wetland: Stable isotope approach. Chinese Journal of Plant Ecology 39(10), 941–949 (2015).
Li, H., Zhang, L. & Wang, D. Distribution of an exotic plant Spartina alterniflora in Shanghai. Biodiversity Science 14, 114–120 (2006).
Cheng, X. et al. Assessing the effects of short-term Spartina alterniflora invasion on labile and recalcitrant C and N pools by means of soil fractionation and stable C and N isotopes. Geoderma 145, 177–184 (2008).
Lin, G. H., Ehleringer, J. R., Rygiewicz, P. T., Johnson, M. G. & Tingey, D. T. Elevated CO2 and temperature impacts on different components of soil CO2 efflux in Douglas-fir terracosms. Glob. Change Biol. 5, 157–168 (1999).
Desjardins, T., Carneiro, A., Mariotti, A., Chauvel, A. & Girardin, C. Changes of the forest-savanna boundary in Brazilian Amazonia during the Holocene revealed by stable isotope ratios of soil organic carbon. Oecologia 108, 749–756 (1996).
Wooller, M., Smallwood, B., Jacobson, M. & Fogel, M. Carbon and nitrogen stable isotopic variation in Laguncularia racemosa (L.) (white mangrove) from Florida and Belize: implications for trophic level studies. Hydrobiologia 499, 13–23 (2003).
Acknowledgements
We are grateful to Guo Haiqiang, Wu Jianqiang, Ruan Junjie and Tan Juan for their assistance in laboratory and field measurements. This work was supported by the National Key R&D Program of China (2016YFC0502702), the National Science Foundation of China (31100404) and the Natural Science Foundation of Shanghai (11ZR1430700).
Author information
Authors and Affiliations
Contributions
M.W., Q.W. and J.C. conceived and designed the experiments. M.W. and Q.W. performed the experiments. M.W. and C.S. analyzed the data. M.W. and Q.W. wrote the manuscript, and other authors provided editorial advice. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, M., Wang, Q., Sha, C. et al. Spartina alterniflora invasion affects soil carbon in a C3 plant-dominated tidal marsh. Sci Rep 8, 628 (2018). https://doi.org/10.1038/s41598-017-19111-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-19111-1
This article is cited by
-
Spartina alterniflora raised sediment sulfide in a tidal environment and buffered it with iron in the Jiuduansha Wetland
Journal of Soils and Sediments (2024)
-
Litterfall Production and Decomposition in Tropical and Subtropical Mangroves: Research Trends and Interacting Effects of Biophysical, Chemical, and Anthropogenic Factors
Wetlands (2024)
-
Conversion of organic carbon from decayed native and invasive plant litter in Jiuduansha wetland and its implications for SOC formation and sequestration
Journal of Soils and Sediments (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.