Abstract
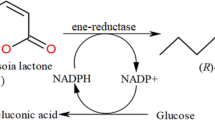
Lactone 2a of a bicyclo[4.3.0]nonane structure is a good starting material for synthesis of many attractive compounds. Enantiomerically enriched (−)-(3aR,7aS)-lactone 2a is produced by whole cells of bacteria. In order to examine the impact of the absolute configuration on biological activity we evaluated the process affording the opposite isomer. To this purpose Candida pelliculosa ZP22 characterized by high dehydrogenase activity was used. The goal of presented work was to perform bioreactor scale microbial one-pot oxidation of diol with selected yeast strain C. pelliculosa ZP22 to obtain chiral (+)-(3aS,7aR)-lactone 2a. The idea was to influence on alcohol dehydrogenase activity by increasing the activity of pro-(+)-ADH and simultanously diminishing the activity of pro-(−)-ADH. The optimization of biotransformation conditions involved the manipulation of the nutritional and physical parameters. Selection of the optimal medium in order to improve yield and process enantioselectivity was based on a two-level factorial design methodology. We have also studied the relationship between microbial growth and biosynthesis of lactone 2a. Preparative oxidation of diol 3a (400 mg/L, 2.9 mM) catalyzed by C. pelliculosa ZP22 in an optimized conditions afforded enantiomerically enriched (+)-(3aS,7aR)-isomer of lactone 2a with the isolated yield (30%).
Similar content being viewed by others
Introduction
Asymmetric transformations catalyzed by whole cells of microorganisms or isolated enzymes have become an attractive alternative for traditional methods leading to optically pure compounds, which derive from either natural sources or by organic synthesis1,2,3,4. It is particularly important in the synthesis of biologically active compounds in which biological activity usually depends on the absolute configuration in a molecule5,6. Therefore, a growing need to find new biocatalysts for synthesis of optically pure molecules with various biological activities is of high importance in the current chemistry. To achieve this purpose in the field of the synthesis of lactones two biocatalytic strategies: kinetic resolutions7,8,9 and stereoselective reactions10,11,12,13,14 were applied so far.
Previously Boratyński12,14,15,16 presented a one-pot biotransformation of diols to chiral lactones. This method involves an asymmetric synthetic value leading to lactones that are well-known to be attractive chiral building blocks. Currently we are particularly interested in the development of a stereoselective biooxidation, which will be significant in the multi-step synthesis of optically active lactones of a bicyclo[4.3.0]nonane structure.
Compounds of such structure represent a large group of natural phtalide derivatives17. They have been isolated from plants of the Apiaceae family Lindl. (Ligusticum officinale (Loveroot, old English Lovage), L. chuanxiong, L. wallichii (Chinese Lovage), Angelica sinensis (Chinese Angelica), Apium graveolens (celeriac) and Petroselinum crispum (parsley)) used in herbal medicine, especially in Chinese folk medicine. More than 70 structures of these lactones were documented. The advantage of this group of compounds is a broad spectrum of biological activity, such as insecticidal18,19, fungicidal18, fragrance20, antioxidant21 and anticoagulant22, anti-proliferative23, cytotoxic24. However, obtaining these valuable natural compounds directly from a plant material is inefficient and thus uneconomic. Chemical synthesis, although efficient, does not recommend by the green chemistry. Alternative approach providing to the optically pure isomers is biocatalysis.
It is worth mentioning that whole cells of yeast are well-known biocatalysts. They catalyze reduction reactions of a carbonyl group25,26,27,28 and a carbon-carbon double bond29,30, hydrolase reaction31 and formation of a carbon-carbon double bond32,33. Additionally, reports published on oxidation reactions performed by yeast are not commonly encountered. Yeast alcohol oxidases were proved to be responsible for the oxidation reaction of some primary34 and secondary alcohols35, sulfides36, racemization37 and deracemization reactions38. It is a well-known fact that whole-cell yeast are highly applicable due to the numerous advantages of their application. Yeast cells are mainly nonpathogenic, inexpensive and can be stored in dried form for a very long time. Yeast, compared to other biocatalysts, are simple to grow (higher increase in biomass) on cheap carbon sources (lower nutritional requirements). One of the major advantages of biotransformation via whole-cells is the availability of all necessary cofactors so it makes ineffective to apply a cofactor-regeneration system. Furthermore, whole-cells yeasts are well-protected within their natural cellular environment, which makes the catalytic system more stable. However, employing wild-type yeast strains as whole-cell biocatalysts also imputes some limitations one of which is the presence of a large number of different dehydrogenases, which quite often overlap in substrate specificity.
Lactone 2a of a bicyclo[4.3.0]nonane structure is a good starting material for synthesis of many attractive compounds. Olejniczak39,40 synthesized a wide range of biologically active racemic derivatives, among them phtalide lactones, epoxy lactones and derivatives as well as lactams and their derivatives (Fig. 1). Many of them indicate high fungistatic activity against Aspergillus glaucus, Botrytis cinerea and Penicillum citrinum.
With application of enzymes Walczak39 obtained (−)-(3aR,7aS)-lactone 2a and its chiral derivatives, which exhibit higher fungistatic activity than its racemates. Boratyński15 results showed that microbial oxidation of meso diol 3a involving bacterial whole cells afforded enantiomerically enriched (−)-(3aR,7aS)-lactone 2a as well. Micrococcus sp. DSM 30771 was the most effective (lactone 2a isolated yield 38%, ee = 94%) among tested biocatalysts. However, in order to examine the impact of the absolute configuration on biological activity we need to evaluate the process affording the opposite isomer. Among tested yeast Candida pelliculosa ZP22 was selected to produce (+)-(3aS,7aR)-isomer of lactone 2a with modest enantioselectivity41. The goal of this study is to carry out the bioreactor scale yeast transformations focusing on process enantioselectivity and (+)-(3aS,7aR)-lactone 2a yield improving. Development of stereoselective biooxidation step will be crucial in synthesis of chiral lactones as chiral building blocks in various asymmetric syntheses.
Many methods were employed in order to improve the selectivity of whole cell yeast biotransformation. These methods involved modifications in cultivation conditions with the application of different carbon and nitrogen sources and the use of two-phase systems42 or application of ionic liquids43. It stands to reason that the optimization processes are crucial for industrial production scale. Thus we have taken up research on improvement in productivity of the microbial secondary metabolite via manipulation of both nutritional and physical parameters. In our present study, based on a fractional factorial experimental design44,45, the cultivation of C. pelliculosa ZP22 was optimized. We have been also studied the relationship between microbial growth and biosynthesis of lactone.
Materials and Methods
Analysis
1H NMR and 13C NMR spectra were recorded in CDCl3 solutions on a Bruker AvanceTM 600 (600 MHz, Billerica, MA, USA) spectrometer. IR spectra were determined on a FT-IR Thermo-Nicolet IR300 (Waltham, Ma, USA) infrared spectrometer. Molecular mass was confirmed on a Varian Chrompack GC MS CP-3800 Saturn 2000 GC/MS/MS with ionization energy of 70 eV, using HP-1 column (crosslinked methyl silicone gum, 25 m × 0.32 mm × 0.25 μm film thickness) and HRMS analysis were conducted on a micrOTOF-Q Bruker. Gas chromatography analysis (GC, FID, carrier gas H2) was carried out on an Agilent Technologies 7890 N (GC System, Santa Clara, CA, USA) with application of a chiral column CP7502 Chirasil-Dex CB (25 m × 0.25 mm × 0.25 μm) with the following temperature program: 80 °C, 150 °C (4 °C/min), 200 °C (20 °C/min) (1 min). The total run time was 21 min. The following retention times of each enantiomers of lactone 2a were established: t R (+)-(3aS,7aR)-2a = 15.77 min and t R (−)-(3aR,7aS)-2a = 15.86 min. Optical rotation was measured on an Autopol IV automatic polarimeter (Rudolph, Hackettstown, NJ, USA) in chloroform solutions (concentration: g/100 mL). Analytical TLC technique (SiO2, DC-Alufolien Kieselgel 60 F254, Merck) were performed by using methylene chloride:methanol (95:5). A solution of 1% Ce(SO4)2 and 2% phosphoromolybdenic acid in 10% H2SO4 was used as a visualizing agent. Preparative column chromatography (SiO2, Kieselgel 60, 230–400 mesh, 40–63 μm, Merck) was performed by using methylene chloride:methanol (95:5).
Chemicals
cis-4-Cyclohexene-1,2-dicarboxylic anhydride (1a), CelLyticTM Y and LiAlH4 were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
Reduction of anhydride 1a
A solution of cis-4-cyclohexene-1,2-dicarboxylic anhydride (1a) (6 mmol) in a mixture of diethyl ether (20 mL) and tetrahydrofuran (10 mL) was added dropwise to LiAlH4 (8 mmol) in diethyl ether (20 mL). The mixture was stirred for 16 hours under reflux. When the reaction was completed (controlled by GC, TLC), water was added to decompose the excess of LiAlH4. The mixture was then acidified with 0.1 M HCl and the products were extracted with chloroform. Then the extract was washed with saturated NaCl and dried over anhydrous MgSO4. The crude products were purified by column chromatography (silica gel, methylene chloride:methanol, 95:5) resulting 73.1% of cis-4,5-bis(hydroxymethyl) cyclohexene (3a) and 7.5% of cis-3a,4,7,7a-tetrahydro-1(3 H)-isobenzofuranone (±)-(2a). The spectral data of obtained products were presented below.
cis-3a,4,7,7a-Tetrahydro-1(3 H)-isobenzofuranone (±)-(2a): 1H NMR (500 MHz, CDCl3) δ: 1.80–1.96 (m, 1 H, one of CH2–4), 2.19–2.54 (m, 3 H, one of CH2-4, H-3a, one of CH2-7), 2.55–2.81 (m, 2 H, one of CH2-7, H-7a), 4.00 (dd, 1 H, J = 8.8, 2.0 Hz, one of CH2-3), 4.30 (dd, 1 H, J = 8.8, 5.1 Hz, 1 H, one of CH2-3), 5.66–5.78 (m, 2 H, H-6, H-5); 13C NMR (151 MHz, CDCl3) δ: 21.9 (CH2-4), 24.6 (CH2-7), 31.9 (CH-7a), 37.2 (CH-3a), 72.7 (CH2-3), 124.8 (CH-5), 125.1 (CH-6), 179.1 (C=O); IR (film, cm−1): 1771 (s); GC-EIMS: 138 (M + 1).
cis-4,5-Bis(hydroxymethyl)cyclohexene (3a): 1H NMR (500 MHz, CDCl3) δ: 1.96-2.08 (m, 4 H, CH2-6, CH2-3), 2.09-2.17 (m, 2 H, CH-1, CH-2), 3.18 (s, 2 H, 2xOH), 3.57 (m, 2 H, CH2-OH), 3.70 (m, 2 H, CH2-OH), 5.59 (s, 2 H, CH-5, CH-4); 13C NMR (151 MHz, CDCl3) δ: 26.92 (CH2-3, CH2-6), 37.79 (CH2-1, CH2-2), 64.03 (CH2-OH), 125.52 (CH-5, CH-4).
Microorganism
Candida pelliculosa ZP22 came from Department of Biotechnology and Food Microbiology at Wroclaw University of Environmental and Life Sciences (Poland). It was maintained at 4 °C on Sabouraud agar slants containing peptone (10 g), glucose (40 g) and agar (15 g) dissolved in water (1 L) at pH 5.5.
Growth conditions
The composition of culture media (g/L H2O): P: 30 g glucose, 10 g peptone; A: 40 g glucose, 15 g (NH4)H2PO4, 7 g KH2PO4, 0.8 g MgSO4 × 7H2O, 0.1 g NaCl, 0.06 g ZnSO4 × 7H2O, 5 × 10−3 g CuSO4 × 5H2O, 0.01 g MnSO4 × 4H2O; B: 50 g glucose, 7 g (NH4)H2PO4, 3.5 g KH2PO4, 0.12 g ZnSO4 × 7H2O, 1 g MgSO4 × 7H2O, 0.025 g NaCl, 0.02 g MnSO4 × 4H2O, 0.01 g CuSO4 × 5H2O.
Conditions of experiments performed in preparative scale: P25: 25 °C, P medium; A25: 25 °C, A medium; A30: 30 °C, A medium; A35: 35 °C, A medium; B35: 35 °C, B medium.
Media optimization
The values of the gradient shown in Table 1 were used to conduct experimental optimization. At this stage, eight experiments were performed (Table 2). The starting point in this stage of research was the middle point of the previous plan, where variables were 0 (control). In the second stage of optimization, experiments in the directions designated by the gradients of each function were performed (Table 3). The way in which values of the variables were accepted, was analogous to initial experiment, where the 0 value comprised the medium composition, in which the highest conversion was observed (Table 4). The concentration plan of each component assumed either an increase or a decrease in the output value of ±50% (or +100% in cases of small amounts of several salts). The hydrated sulfate salts were treated together as one component.
The experiments for media optimization were performed in shaken flasks containing sterile culture medium (50 mL) with the composition prepared according to Tables 2 and 4. After inoculation by C. pelliculosa ZP22, they were incubated in an orbital shaker (140 rpm, 25 °C) until late exponential phase, followed by induction of 3a (0.04 g/mL). The biotransformation progress was followed by gas chromatography applied with chiral column. The control experiments, without microorganism, of diol 3a and lactone 2a, indicating their stability in aqua solution, were performed.
The cultivation of C. pelliculosa ZP22
The reactor used to run the fermentations was a 3 L New Brunswick Scientific BioFlo III (Brunswick, Ramsey, MN, USA). The temperature and agitation were maintained at 25 °C or 35 °C and 600 rpm, respectively. A Broadley James D100 Series Oxyprobe was used to track the dissolved oxygen level to ensure the system was not oxygen transfer limited. The air flow rate into the reactor was 1 L/min and was passed through a sterile 0.2 μm hydrophobic fluoropore PTFE filter. The reactor containing medium was sterilized at 121 °C for 25 minutes prior to use.
A 250 mL Erlenmeyer flask containing sterile culture medium (100 mL), was inoculated by C. pelliculosa ZP22 and incubated in an orbital shaker (140 rpm, 25 °C) until late exponential phase. The content of pre-culture flask was aseptically poured into a final volume of 1500 mL and the culture was grown until the biomass concentration had reached OD600 0.4-0.6. With respect to the screening scale experiments performed previously, 0.6 g of diol 3a diluted in 5 mL of acetone was used in the preparative method. The substrate 3a, was supplied through a port on the reactor lid using sterile pipet tips to reduce the possibility of contamination. The air flow was continuously supplied throughout the duration of the transformation. The cell growth was monitored every 2 hours (in lag and mid exponential growth phase) and every 24 hours (in late exponential and stationary growth phase) by measuring OD600. The 11 day biotransformation progress was followed by gas chromatography applied with chiral column. The reaction mixture was divided in three portions (3 × 500 mL), acidified by 0.1 M HCl, washed with brine and extracted overnight with ethyl acetate (3 × 500 mL) on laboratory shaker. After extraction, centrifuged (10,000 rpm, 20 mins) and evaporated. The crude product was purified by column chromatography using a mixture of hexane/acetone (3:1) as a mobile phase. Oxidation of diol 3a (0.6 g) after 11 days in experiment B35 gave 0.177 g (30% yield) of (+)-(3aS,7aR)-2a, ee = 70% (\({[{\rm{\alpha }}]}_{589}^{25}=+55.4^\circ \) (c = 1.0, CHCl3), ref.23 \({[{\rm{\alpha }}]}_{589}^{25}=-67.1^\circ \) (c = 1.0, CHCl3), ee = 100%). The spectroscopic and chromatographic data, which confirm lactone 2a structure were attached as supporting information (Supplementary Figures S1–S5).
Biomass determination
Determination of the living yeast cells on the basis of serial dilution count method was conducted. Briefly, 1 mL of each sample was serially diluted tenfold in 9 mL of sterile physiological saline. Dilutions from 10−9 to 10−16 were spread in triplicates on Sabouraud agar medium. All inoculated plates were incubated at 25 °C for 48 hours. After incubation, colonies appeared on each plate were counted taking into considerations their morphological characteristics.
Total cellular growth was determined by measuring optical density (OD600). The optical density was previously correlated with the quantity of the dry cells, data not shown. To monitor the number of cells and strain, physiological data automated instrument Scepter™ cell counter was applied as well.
The crude extract preparation
Samples (10 mL) from the bioreactor were withdrawn every 2 or 24 hours. Cells from culture were harvested by centrifugation for 10 min at 15.000 rpm and washed with 100 mM Tris-HCl buffer (pH 8.5). The pellets were suspended in 10 mL of the same buffer containing 1 mM DDT and 100 μM PMSF, mixed and centrifuged for 10 min at 15.000 rpm. In order to extract the proteins cell, lysis agent CelLyticTM Y was applied. Cell debris was removed by centrifugation for 10 min at 15.000 rpm. The supernatant was used for further studies.
Protein determination
Protein concentration was determined according to Bradford method46 using bovine serum albumin as calibration standard.
Activity assay based on reduction of NAD+
Dehydrogenase activity was determined by following the increase in absorbance at 340 nm using 1 mM of 3a as substrate dissolved in 100 mM Tris-HCl buffer (pH 8.5). Enzyme activity was calculating by measuring the formation of NADH-H+ at 340 nm. One unit of the enzyme activity was defined as the amount of enzyme required to reduce 1 μmol of NAD+ per minute.
Activity assay based on reduction of TTC
Dehydrogenase activity was determined by the reduction of a colorless 2,3,5-triphenyltetrazolium chloride (TTC) to a colorful 1,3,5-triphenyltetrazolium formazan (TPF) at 485 nm by following a modified TTC assay47. Activity was expressed in units, where one unit corresponds to the release of 1 μmol of TPF protein per minute.
Results and Discussion
Biological properties of chiral compounds are many a time related to absolute configuration. Therefore, we are especially interested in elaboration of biotechnological methods of synthesis both enantiomers of lactone (Fig. 2). In this microbial oxidation both alcohol dehydrogenases (pro-(+)-ADH and pro-(−)-ADH) take part. Our idea was to increase the activity of pro-(+)-ADH and simultanously diminish activity of pro-(−)-ADH by manipulation of C. pelliculosa ZP22 biotransformations conditions. Modifications of performed experiments concerned mainly cultivation conditions, particularly type of medium and process temperature. The effect of the culture medium composition optimized in a two-level factorial design on the conversion rate and enantiomeric excess of lactone 2a was studied. We analyzed the effect of temperature on abovementioned parameters as well. Moreover the amount of living cells and enzyme activity in order to accurately investigate the whole process was also monitored.
The substrate for biotransformation meso diol 3a was obtained in a good yield in the reduction process with lithium aluminum hydride of the corresponding low-cost anhydride 1a (Fig. 3). Additionally, a small amount of lactone 2a in the racemic form was isolated and used in a gas chromatography analysis to establish retention time of both enantiomers of lactone 2a.
The effect of biotransformation conditions on content and enantiomeric excess of lactone 2a
Transformations of diol 3a by C. pelliculosa ZP22 were conducted for 11 days since enantiomeric excess of lactone 2a was higher than ee = 60%. Bioreactor scale experiments were performed with the following standardized parameters: medium volume (1.5 L), aeration rate (1 v/m), stirring speed (600 rpm) and pH. Samples from bioreactor were withdrawn every 24 hours in order to monitor the progress and enantioselectivity of the biotransformation by chiral gas chromatography (CGC) and to determine both the level of biomass and enzyme activity.
Initially the bioxidation process was performed in two types of media. It is sometimes observed that minimal medium induces microorganisms to produce enzymes able to transform xenobiotics. Firstly, to test scaling-up methodology, the biotransformation was performed in Sabouraud medium in 25 °C (P25) in the same conditions as in the screening scale. The second experiment (A25) was set up in enriched medium (A) chosen on the basis of the literature data describing the growth of yeast of the Candida genera48.
As it can be observed in Fig. 4 lactone 2a was formed from the 3rd day of biotransformation in the P25 as opposed to A25, where the product was formed just after substrate addition. The conversion of diol 3a in both experiments was low. Application of different media did not affect on diol 3a conversion significantly. Nevertheless, in the P25 the modest enantiomeric excess (ee = 78.8%) of lactone 2a was observed (Fig. 5). It is worth pointing out that the enantioselectivity of the whole process was at constant level. In contrast, a decrease of enantioselectivity from ee = 90% to ee = 52% in A25 was observed. Our goal was to obtain lactone 2a with the highest purity, so we decided to activate pro-(+)-ADH in enriched medium (A) since we initially observed high enantioselectivity (ee = 90%) of biotransformation.
Significantly low amount of lactone 2a in both experiments (P25 and A25) encouraged us to investigate whole process. Therefore preparative biotransformation A30 was conducted for 18 days unless no more lactone 2a was formed (Fig. 6). Increasing the time of oxidation caused higher conversion of diol 3a however with lower optical purity of lactone 2a (ee = 33%) in comparison to other experiments (P25, A25, A35, B35). Such low enantiomeric excess of lactone 2a has proven that both, pro-(+)-ADH and pro-(−)-ADH were active during biotransformation.
From our experience appears that generally higher temperature of microbial transformations causes decrease in enantioselectivity, however it can stimulate the biomass growth. In the next experiment (A35) the temperature of biotransformation was increased from 25 °C to 35 °C. As expected, higher temperature applied in A35 caused more than a twofold increase in the amount of lactone 2a (Fig. 4). Unfortunately, despite the initially sustained level of enantiomeric excess (ee = 70%) between 3-7 day, the further conversion of diol 3a caused increase of pro-(−)-ADH activity, which afford in decrease of process enantioselectivity (ee = 60%) (Fig. 5).
The last modification (B35) concerned the application of the improved culture medium (B). The choice of the optimal culture medium was performed in the screening scale experiments on the basis of a two-level factorial design. In the experiment with optimized medium enantiomerically enriched lactone 2a (ee = 70%) (Fig. 5) with 30% of isolated yield (Fig. 4) was achieved. The spectroscopic and chromatographic data of obtained lactone 2a were attached as supporting information (Supplementary Figures S1–S5).
The effect of biotransformation conditions on the number of cells
To determine the influence of the type of medium and the process temperature on C. pelliculosa ZP22 growth a series of experiments, allowing measurements of the number of cells, were provided. The total cell number was measured on the basis of optical density measurements (OD600) and the use of ScepterTM cell counter, which provided additional data about cell size and potential contamination of the yeast culture. Measurements of living cells were based on a serial dilution method, which assumes inoculation of petri dishes and subsequent counting of grown colonies. The samples were taken just after substrate addition from the late logarithmic growth phase.
The total number of cells was the highest in P25 (Fig. 7b). However, the number of living cells was at significantly lower level (Fig. 7a) by having compared it to all experiments carried out in enriched media. Until the 8th day the cells amount was 109 and then increased to 1011. In contrary, cultivation on enriched media (A25, A35, B35) caused a steady increase of the number of living cells from 109 to 1017.
A slight increase in cells number in P25 and an inconsiderable increase of the amount of lactone 2a were observed. On the other hand, an increase of the number of cells in the A media, particularly in A35 and B35, improved biotransformations significantly. Initially, when the number of cells was below 1013 (1–3 days), 2a was not observed. Only when a further increase in biomass occurred, diol 3a was converted into lactone 2a. Stabilization of cells number caused the lack of further conversion of 3a. In A25 the relationship between cells number (Fig. 7) and an increase of 2a (Fig. 4) showed that the amount of lactone 2a doubled between 6–10 day, which was correlated with an increase of biomass from 1013 to 1016.
The conversion of diol 3a depended on microbial growth, which was directly related to the composition of the cultivation medium. The life-time of Candida cells in P medium was shorter than in enriched A medium. Therefore, the more living cells produced in A medium, the higher conversion of diol 3a was observed. The temperature of the biotransformation had less influence on the amount of living cells. Nevertheless, the cells in B35 grew more rapidly, which afforded the highest quantity of the biomass.
The effect of biotransformation conditions on the enzyme activity
The next part of the study involved measurements of the enzyme activity in different culture conditions and its correlation with the progress of the biotransformation. In this study two spectrophotometrical methods for dehydrogenases activity determination were used. The first one was based on measurements of the changes in absorbance during the reduction of the coenzyme NAD+ to NADH as a result of oxidation of diol 3a to lactone 2a. This selective method only defines the activity of dehydrogenases capable to catalyze the particular transformation. The second method involved the incubation of yeast biomass with a colorless substrate 2,3,5-triphenyltetrazolium chloride (TTC), which is enzymatically reduced to a colorful 1,3,5-triphenyltetrazolium formazan (TPF). This method was used to measure the total amount of all dehydrogenases.
Initially, the measurements of the enzyme activity were performed with both methods (Fig. 8a,b). In the method with a TTC the highest activity was observed between 4–9 day of the biotransformation, while in the method with a NAD+ the highest activity was between 1–4 day and then a decrease was subsequently observed at the end of the process. Differences in enzymatic activity can be explained by methods selectivity. In the first method all dehydrogenases produced by microorganism were measured, while in the second method only dehydrogenases responsible for oxidation of diol 3a. In further experiments only a selective method with NAD+ was applied.
No correlation between initially high dehydrogenase activity (Fig. 8a) and low content of lactone 2a established by GC (Fig. 4) was due to the formation of hydroxyaldehyde as an intermediate product in oxidation of diol 3a to lactone 2a. The low content of lactone 2a in P25 can be explained by decrease of activity of dehydrogenases responsible for the second step of oxidation. As Boratyński12,14 evaluated previously the pathway of microbial one-pot synthesis of lactones consists of two oxidation steps. In the first one, hydroxyl group of diol is oxidized and hydroxyaldehyde is formed. Further oxidation of hydroxyaldehyde, proceeding via hemiacetals, leads directly to lactone.
The increase of temperature and the use of optimized medium B35 had a positive effect on the enzymatic activity (Fig. 8c). It should be pointed out that long-term and balanced distribution of activity in B35 had its significant influence on the higher conversion of 3a. The observed increase in cells number during the biotransformation, partially influenced the activity that increased to day 4 and then gradually decreased, but did not disappear in the last stage as it did in P25. It is also worth mentioning that in P25 with the fewer number of cells, the activity was higher compared to B35 in which cells number was higher with lower activity. A similar relationship between the activity of dehydrogenases and the number of cells was observed in the culture media optimization experiments performed on the basis of a two-level factorial design.
Optimisation of culture media on the basis of a two-level factorial design
A two-level factorial experiment was applied to evaluate the effects of independent variables, namely the concentrations of components of medium A: glucose, (NH4)H2PO4, KH2PO4, MgSO4 × 7H2O, NaCl, and minerals (ZnSO4 × 7H2O, CuSO4 × 5H2O, MnSO4 × 4H2O) on lactone 2a bioproduction (Table 1). In the first part of the experiment the highest conversion of 3a was obtained in modifications of biotransformation according to variants 2, 6 and 7 (Table 2). The results of enzymatic activity also confirmed the proper choice of the selected concentrations. Based on the results obtained after the first round of biotransformation in 8 different variants concentrations (Table 2), the medium composition was narrowed (Table 3) and subsequently the number of biotransformations carried out (Table 4). The reduced concentration of (NH4)H2PO4, KH2PO4, NaCl and the increased amount of glucose and other minerals components improved the biosynthesis of lactone 2a. However, for bioreactor scale process further slight modifications in the concentration of medium components were done. The final cultivation medium applied in B35 composed of (NH4)H2PO4 (7 g), KH2PO4 (3.5 g), glucose (50 g), NaCl (0.025 g), MgSO4 (1 g), CuSO4 (0.01 g), ZnSO4 (0.12 g), MnSO4 (0.02 g) per 1 L of H2O.
Conclusion
In conclusion, the microbial stereoselective oxidation catalyzed by whole cell yeast was studied. Based on our previous results, C. pelliculosa ZP22 was selected to catalyze biotransformation of diol 3a into (+)-(3aS,7aR)-isomer of lactone 2a. A significant number of modifications concerning the temperature and composition of culture media were performed in order to improve lactone biosynthesis in the C. pelliculosa ZP22 culture in a bioreactor scale. Selection of the optimal medium was based on a two-level factorial design method. In all preparative experiments performed in a bioreactor, parameters like biomass, protein and enzymatic activity were under continuous control. The cultivation of C. pelliculosa ZP22 in a selected conditions (optimized medium composition and temperature 35 °C) applied in B35 experiment afforded enantiomerically enriched (+)-(3aS,7aR)-isomer of 2a (ee = 70%) in the 30% of isolated yield. Modification of medium composition and higher temperature of biotransformation increased the activity of pro-(+)-ADH in tested strain.
References
Brenna, E., Fuganti, C., Gatti, F. G. & Serra, S. Biocatalytic methods for the synthesis of enantioenriched odor active compounds. Chem. Rev. 111, 4036–4072 (2011).
Muschiol, J. et al. Cascade catalysis - strategies and challenges en route to preparative synthetic biology. Chem. Commun. 51, 5798–5811 (2015).
Hollmann, F., Arends, I. W. C. E., Buehler, K., Schallmey, A. & Buhler, B. Enzyme-mediated oxidations for the chemist. Green Chem. 13, 226–265 (2011).
Hollmann, F., Arends, I. W. C. E. & Holtmann, D. Enzymatic reductions for the chemist. Green Chem. 13, 2285–2314 (2011).
Dams, I., Białońska, A., Ciunik, Z. & Wawrzeńczyk, C. Lactones 38: Synthesis and odoriferous properties of p-menthane lactones. Flavour Frag. J. 27 (2012).
Nawrot, J., Dams, I. & Wawrzeńczyk, C. Feeding deterrent activity of terpenoid lactones with a p-menthane system against stored-product pests. J. Stored Prod. Res. 45, 221–225 (2009).
Olejniczak, T. & Ciunik, Z. Enantioselective hydrolysis of d-acetoxy-g-lactones. Tetrahedron: Asymmetr. 15, 3743–3749 (2004).
Olejniczak, T. & Wawrzeńczyk, C. In Studies in Surface Science and Catalysis Vol. 130 (eds Francisco V. Melo Sagrario Mendioroz Avelino Corma & G. Fierro José Luis) 3387–3392 (Elsevier, 2000).
Fajkowska, M., Obara, R. & Wawrzeńczyk, C. Lactones 29. Enzymatic resolution of racemic g-lactones. Biocatal. Biotransfor. 25, 79–83 (2007).
Olejniczak, T., Gawroński, J. & Wawrzeńczyk, C. Lactones. 6. Microbial lactonization of γ,δ-epoxy esters. Chirality 13, 302–307 (2001).
Olejniczak, T., Mironowicz, A. & Wawrzeńczyk, C. Lactones 12. - Enzymatic lactonization of g,d-epoxy esters by the apple fruit and Jerusalem artichoke bulb. Bioorg. Chem. 31, 199–205 (2003).
Boratyński, F., Kiełbowicz, G. & Wawrzeńczyk, C. Lactones 34 [1]. Application of alcohol dehydrogenase from horse liver (HLADH) in enantioselective synthesis of d- and e-lactones. J. Mol Catal B: Enzym. 65, 30–36 (2010).
Ratuś, B., Gładkowski, W. & Wawrzeńczyk, C. Lactones 32: New aspects of the application of Fusarium strains to production of alkylsubstituted e-lactones. Enzym.Microb.Technol. 45, 156–163 (2009).
Boratyński, F., Smuga, M. & Wawrzeńczyk, C. Lactones 42. Stereoselective enzymatic/microbial synthesis of optically active isomers of whisky lactone. Food Chem. 141, 419–427 (2013).
Boratyński, F. et al. Microbial alcohol dehydrogenase screening for enantiopure lactone synthesis: Down-stream process from microtiter plate to bench bioreactor. Process Biochem. 49, 1637–1646 (2014).
Boratyński, F., Dancewicz, K., Paprocka, M., Gabryś, B. & Wawrzeńczyk, C. Chemo-Enzymatic Synthesis of Optically Active γ- and δ-Decalactones and Their Effect on Aphid Probing, Feeding and Settling Behavior. PLoS ONE 11, e0146160 (2016).
Beck, J. J. & Chou, S. C. The Structural Diversity of Phthalides from the Apiaceae. J. Nat. Prod. 70, 891–900 (2007).
Momin, R. A. & Nair, M. G. Mosquitocidal, Nematicidal, and Antifungal Compounds from Apium graveolens L. Seeds. J. Agric. Food Chem. 49, 142–145 (2001).
Chu, S. S., Jiang, G. H. & Liu, Z. L. Insecticidal compounds from the essential oil of Chinese medicinal herb Atractylodes chinensis. Pest Manag. Sci. 67, 1253–1257 (2011).
Bartschat, D., Beck, T. & Mosandl, A. Stereoisomeric Flavor Compounds. 79. Simultaneous Enantio- selective Analysis of 3-Butylphthalide and 3-Butylhexahydro- phthalide Stereoisomers in Celery, Celeriac, and Fennel. J. Agric. Food Chem. 45, 4554–4557 (1997).
Wang, C. Y. et al. Dl-3-n-butylphthalide-induced upregulation of antioxidant defense is involved in the enhancement of cross talk between CREB and Nrf2 in an Alzheimer’s disease mouse model. Neurobiol. Aging 38, 32–46 (2016).
Yang, J.-Y. et al. Advances in Studies on Pharmacological Functions of Ligustilide and their Mechanisms. Chinese Herb. Med. 4, 26–32 (2012).
Kan, W. L., Cho, C. H., Rudd, J. A. & Lin, G. Study of the anti-proliferative effects and synergy of phthalides from Angelica sinensis on colon cancer cells. J. Ethnopharmacol. 120, 36–43 (2008).
Hu, Y., Bi, X., Zhao, P., Zheng, H. & Huang, X. Cytotoxic Activities, SAR and Anti-Invasion Effects of Butylphthalide Derivatives on Human Hepatocellular Carcinoma SMMC7721 Cells. Molecules (Basel, Switzerland) 20, 20312–20319 (2015).
Cvjetko Bubalo, M., Mazur, M., Radošević, K. & Radojčić Redovniković, I. Baker’s yeast-mediated asymmetric reduction of ethyl 3-oxobutanoate in deep eutectic solvents. Process Biochem. 50, 1788–1792 (2015).
Deasy, R., Riordan, N. & Maguire, A. Baker’s Yeast Mediated Reduction of 2-Acetyl-3-methyl Sulfolane. Catalysts 4, 186 (2014).
Dehli, J. R. & Gotor, V. Dynamic Kinetic Resolution of 2-Oxocycloalkanecarbonitriles: Chemoenzymatic Syntheses of Optically Active Cyclic β- and γ-Amino Alcohols. J. Org. Chem. 67, 6816–6819 (2002).
Soni, P. & Banerjee, U. C. Biotransformations for the production of the chiral drug (S)-Duloxetine catalyzed by a novel isolate of Candida tropicalis. Appl. Microbiol. Biot. 67, 771–777 (2005).
Stuermer, R., Hauer, B., Hall, M. & Faber, K. Asymmetric bioreduction of activated C=C bonds using enoate reductases from the old yellow enzyme family. Curr. Opin. Chem. Biol. 11, 203–213 (2007).
Kawai, Y., Inaba, Y. & Tokitoh, N. Asymmetric reduction of nitroalkenes with baker’s yeast. Tetrahedron: Asymmetr. 12, 309–318 (2001).
Glänzer, B. I., Faber, K. & Griengl, H. Microbial resolution of O-acetylpantoyl lactone. Enzym. Microb. Technol. 10, 689–690 (1988).
Csuk, R. & Glaenzer, B. I. Baker’s yeast mediated transformations in organic chemistry. Chem. Rev. 91, 49–97 (1991).
Crout, D. H. G., Dalton, H., Hutchinson, D. W. & Miyagoshi, M. Studies on pyruvate decarboxylase: acyloin formation from aliphatic, aromatic and heterocyclic aldehydes. J. Chem. Soc. Perk. T. 1, 1329–1334 (1991).
Tani, Y., Miya, T., Nishikawa, H. & Ogata, K. The Microbial Metabolism of Methanol. Agric. Biol. Chem. 36, 68–83 (1972).
Patel, R. N., Hou, C. T., Laskin, A. I., Derelanko, P. & Felix, A. Oxidation of secondary alcohols to methyl ketones by yeasts. Appl. Environ. Microb. 38, 219–223 (1979).
Beecher, J., Brackenridge, I., Roberts, S. M., Tang, J. & Willetts, A. J. Oxidation of methyl p-tolyl sulfide with bakers’ yeast: preparation of a synthon of the mevinic acid-type hypocholestemic agents. J. Chem. Soc. Perk. T. 1, 1641–1643 (1995).
Nestl, B. et al. Biocatalytic racemization of sec-alcohols and α-hydroxyketones using lyophilized microbial cells. Appl. Microbiol. Biot. 76, 1001–1008 (2007).
Titu, D. & Chadha, A. Preparation of optically pure alkyl 3-(hetero-2-yl)-3-hydroxypropanoates by Candida parapsilosis ATCC7330 mediated deracemisation. J. Mol. Catal. B: Enzym. 52–53, 168–172 (2008).
Walczak, P., Pannek, J., Boratyński, F., Janik-Polanowicz, A. & Olejniczak, T. Synthesis and Fungistatic Activity of Bicyclic Lactones and Lactams against Botrytis cinerea, Penicillium citrinum, and Aspergillus glaucus. J. Agric. Food Chem. 62, 8571–8578 (2014).
Olejniczak, T., Boratyński, F. & Białońska, A. Fungistatic activity of bicycle [4.3.0]-g-lactones. J. Agric. Food Chem. 59, 6071–6081 (2011).
Boratyński, F., Szczepańska, E., Pannek, J. & Olejniczak, T. Microbial Stereoselective One-Step Conversion of Diols to Chiral Lactones in Yeast Cultures. Catalysts 5, 2068 (2015).
D’Arrigo, P., Fantoni, G. P., Servi, S. & Strini, A. The effect of absorbing resins on substrate concentration and enantiomeric excess in yeast reduction. Tetrahedron: Asymmetr. 8, 2375–2379 (1997).
Pfruender, H., Jones, R. & Weuster-Botz, D. Water immiscible ionic liquids as solvents for whole cell biocatalysis. J. Biotechnol. 124, 182–190 (2006).
DeMeo, M., Laget, M., Phan-Tan-Luu, R., Mathieu, D. & Dumenil, G. Application of experimental designs for optimization of medium and culture conditions in fermentation. BioScience 4, 99–102 (1985).
Geetha, K. & Gunasekaran, P. Optimization of nutrient medium containing agricultural waste for xylanase production by Bacillus pumilus B20. Biotechnol Bioproc E 15, 882–889 (2010).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72, 248–254 (1976).
Friedel, J. K., Mölter, K. & Fischer, W. R. Comparison and improvement of methods for determining soil dehydrogenase activity by using triphenyltetrazolium chloride and iodonitrotetrazolium chloride. Biol. Fert. Soils 18, 291–296 (1994).
Kitayama, T. Microbial asymmetric syntheses of 3-alkylphthalide derivatives. Tetrahedron: Asymmetr. 8, 3765–3774 (1997).
Acknowledgements
This work was financed by National Science Centre, Grant No. 2011/03/B/NZ9/05005. Publication supported by Wroclaw Centre of Biotechnology, programme The Leading National Research Centre (KNOW) for years 2014-2018 (http://know.wroc.pl).
Author information
Authors and Affiliations
Contributions
F.B. and T.O. designed the experiments. F.B., A.J.-P. and T.O. performed the experiments. F.B. and E.S. analyzed the data. F.B. wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boratyński, F., Janik-Polanowicz, A., Szczepańska, E. et al. Microbial synthesis of a useful optically active (+)-isomer of lactone with bicyclo[4.3.0]nonane structure. Sci Rep 8, 468 (2018). https://doi.org/10.1038/s41598-017-18876-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-18876-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.