Abstract
Bisphenol A (BPA) is a well-known endocrine disruptor compound reported to have prostate toxicity. This study aimed to assess the effect of BPA on the proliferation of dorsolateral prostate (DLP) and the expression of epithelial–mesenchymal transition (EMT)-related genes in aged rats. Male aged SD rats were treated with BPA (10.0, 30.0, and 90.0 µg/kg i.g., daily) or vehicle (i.g., daily) for 3 months. Treatment with BPA resulted in increased the expression of PCNA, DLP weight and DLP epithelial height compared with the control group (P < 0.01); such effects were more obvious at higher BPA doses. 90 µg/kg BPA significantly increased the estrogen to androgen ratio (P < 0.05). The EMT chip showed the BPA induced upregulation of vimentin, Snail, Twist1, and transforming growth factor beta 1, as well as the downregulation of E-cadherin in the DLP. Immunohistochemical data showed that the expression of vimentin, estrogen receptor subtypes, and androgen receptor increased and the expression of E-cadherin decreased in 30 and 90 µg/kg BPA groups. It was concluded that environmental exposure to low doses of BPA might promote the proliferation of DLP in aged rats by increasing the estrogen to androgen ratio and inducing EMT.
Similar content being viewed by others
Introduction
Benign prostatic hyperplasia (BPH) is a common disease in elderly men. The incidence of BPH has shown a gradual upward trend in recent years with the aging of the population and increase in the number of elderly males1,2. The prostate is an androgen-dependent organ, and androgen plays an important role in BPH occurrence3. Moreover, the effect of estrogen on prostate should not be underestimated. The literature reported that 50 µg/kg estrogen stimulated the development of prostate hyperplasia in Sprague–Dawley (SD) rats4, suggesting that low doses of estrogen could promote BPH. However, in a certain range, estrogen can play a synergistic role in promoting BPH5. In addition to the endogenous estrogen, scholars focus on environmental estrogens, namely environmental endocrine disruptors (EDCs).
Generally, EDCs disrupt the original endocrine system in the form of hormones after aggregating in organisms6. Bisphenol A (BPA), a common xenoestrogen, widely exists in fillers, polymer materials, cosmetics, and plasticizers. A growing evidence suggests that BPA has the potential to cause adverse outcomes in the reproductive system, including prostate. It was found that 235 mg/kg BPA decreased dorsolateral prostate (DLP) weight7 and 3 mg/kg BPA increased ventral prostate weight8, suggesting that the impact of BPA on the prostate was lobe selective, and the biological effects changed with dose. The oral administration of low-dose BPA was found to promote the proliferation of ventral prostate and upregulate the expression of prostaglandin D2 synthase in adult rats9. The biological endocrine system changes with age. BPH is an age-related disease. However, a direct relationship between low-dose BPA and prostate in aged rats has not been demonstrated yet, let alone the DLP.
The exact pathogenesis of BPH is controversial due to various influencing factors. Epithelial–mesenchymal transition (EMT) is an important mechanism that allows the polarized and immotile epithelial cells to convert into motile mesenchymal cells10. A previous study indicated that BPH was not the proliferation of stromal cells, but the accumulation of mesenchymal-like cells derived from the prostatic epithelium11. EMT is extremely common in tumors. Nanomolar concentrations of BPA can promote the metastasis of colon cancer cells through EMT. Since BPA can induce EMT and EMT can induce BPH, it is speculated that BPA can promote BPH by EMT.
The present study aimed to assess the effect of BPA on the proliferation of DLP and its impact on gene expression related to EMT in aged rats.
Results
Body and DLP weight
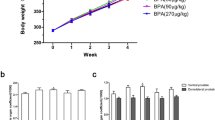
During the 3-month administration of BPA, animal body weight increased slowly with time, reached the maximum in the ninth week, and then decreased slightly (Fig. 1). After treatment, 10–90 μg/(kgday) BPA did not have a significant impact on body weight; however, BPA increased the DLP weight in a dose-dependent manner. BPA had the trend to increase the volume and relative weight of DLP in a dose-dependent manner, and 90 μg/(kg.day) BPA showed a significant difference (P < 0.05, Table 1). However, the volume and relative weight of VP did not change significantly.
Histology
H&E staining showed glandular prostatic hyperplasia with an increase in the size of alveoli and degree of papillary infolding; the glandular cavity was slightly enlarged and increased in BPA-treated groups compared with the control group (Fig. 2). Moreover, BPA significantly increased the height of DLP epithelium (P < 0.01, Fig. 3a); 90 μg/(kg.day) BPA had the most obvious manifestation. However, no significant difference was found among BPA-treated groups. After 3-month treatment, low-dose BPA showed a growth-promoting effect on the DLP.
Histological analysis of dorsolateral prostate in male aged rats treated with BPA for 3 months. The glandular cavity was slightly enlarged and increased in BPA-treated groups. (a–d) Representative sections of comparable regions were shown for vehicle control rats (a), and animals exposed to BPA (10 μg/kg/day) (b), BPA (30 μg/kg/day) (c), and BPA (90 μg/kg/day), (d) (scale bar: 50 μm, x40).
(a) Effect on height of dorsolateral prostatic epithelium. After aged rats were treated with 10–90 μg/kg BPA for 3 months, BPA significantly increased the height of DLP epithelium in a dose-dependent way, *P < 0.01, compared with the vehicle controls. BPA: bisphenol A. (b) The expression of the PCNA in DLP. The expression of PCNA was increased obviously in BPA-treated groups. *P < 0.05: compared with the vehicle controls; *P < 0.01, compared with the vehicle controls. BPA: bisphenol A.
Evaluation of the PCNA expression in the DLP
To better evaluate BPA effect on DLP proliferation, immunohistochemistry for proliferation markers (PCNA) was performed. In the epithelium, positive staining for PCNA in BPA-treated groups was obviously compared with the control group (Fig. 4), the percentage of PCNA-positive epithelial cells in BPA-treated groups was 51%, 60% and 61%, respectively (Fig. 3b).
Serum hormone levels (estradiol, T, and insulin)
All groups treated with BPA had a higher E2 level compared with the control group, especially for 90 μg/(kgday) BPA (P < 0.05). A dose of 10 μg/(kgday) BPA increased the serum T level slightly, followed by a trend of decrease with increasing doses. Beyond that, BPA increased the estrogen to androgen ratio, and the increase was more pronounced at a high-dose BPA. The trend was similar for the insulin level between groups treated with BPA and vehicle. These data are shown in Fig. 4.
EMT gene expression profile of DLP shown by a microarray analysis
Gene expression microarray related to EMT was assayed to understand whether a cause-effect relationship existed between BPA and EMT pathway. Quantitative polymerase chain reaction quality results showed that the purity of the samples could meet the experimental requirements. According to scatter plots and data clustering analysis, the gene expression profile was different between the BPA and control groups (Fig. 5). A total of 89 EMT genes were analyzed and 26 genes upregulated at a cutoff of 2 significantly. Statistical analyses revealed the loss of epithelial marker, E-cadherin, and gain of the mesenchymal marker, vimentin, indicating BPA-induced EMT in DLP tissues. Simultaneously, the expression of Snails, Twist, transforming growth factor beta 1 (TGF-β1), and ERα was upregulated (Table 2).
Effects of BPA on E2, T, and Insulin serum levels in aged male rats. After aged rats were treated with 10–90 μg/kg BPA for 3 months, 90 μg/kg BPA significantly increased the E2 level and the estrogen to androgen ratio; BPA had the trend of decreasing the T level and increasing the insulin level. p < 0.05, compared with the vehicle controls. BPA: bisphenol A.
Evaluation of E-cadherin, vimentin, ERα, and AR expression in the DLP
The expression of AR, ERα, E-cadherin, and vimentin was immunohistochemically analyzed to verify further the BPA-induced signaling pathway related to EMT observed in a microarray analysis. The results showed that E-cadherin and vimentin were mainly expressed in epithelial and stromal cells, respectively. The expression of E-cadherin decreased and the expression of vimentin increased gradually with the increase in dose compared with the control group (P < 0.05, P < 0.01). ERα was mainly expressed in the nucleus. AR was expressed in both the nucleus and the cytoplasm, but the expression was less in the latter. As the dose increased, BPA upregulated the expression of both ERα and AR incrementally (P < 0.05, P < 0.01) (Table 3 and Fig. 6).
Discussion
The United States Environmental Protection Agency considers 50 μg/(kg.day) BPA as a relatively safe dose12. In this study, less than 50 μg/(kgday) BPA did have the trend of promoting prostate hyperplasia in elderly rats, which was similar to low-dose effects of BPA on a rat model of BPH4. Studies have suggested that the injection of 10 μg/(kg.day) BPA in SD rats was equivalent to the BPA detected in serum on environmental exposure13. BPA is taken directly into the blood circulation by injection; however, BPA passes through the first-pass effect in oral administration. Therefore, the actual BPA dose given by injection was much higher than that given through the mouth14,15. This study showed that 90 μg/(kg.day) BPA could promote the hyperplasia of prostate, which was consistent with the report that the injection of 10 μg/(kg.day) BPA in SD rats increased the incidence of prostate epithelial tumor13. Fig. 7.
Immunohistochemical analysis of dorsolateral prostate E-cadherin, Vimtein, ERα and AR expression in aged rats. The expression of vimentin, ERα and AR increased, and the expression of E-cadherin decreased in BPA-treated groups. (a–p) Representative sections of comparable regions are shown for vehicle control rats (a,e,i,m), and animals exposed to BPA (10 μg/kg/day) (b,f,j,n), BPA (30 μg /kg/day) (c,g,k,o), and BPA (90 μg/kg/day) (d,h,l,p) (scale bar: 50 μm, ×400). BPA: bisphenol A; AR: androgen receptor; ERα:estrogen receptor-α.
The proliferation in the DLP was more obvious than that in the VP in transgenic mice because the DLP in mice the mouse was homologous with the peripheral zone in human prostate and BPH originated in the transitional and peripheral zones in humans16. After 3-month administration, the DLP was more sensitive to low-dose BPA compared with the VP, especially in the group at 90 μg/kg dose, which also suggested that the DLP in rats might be homologous with the peripheral or transitional zone in human prostate.
Proliferation marker, PCNA, is a proliferating cell nuclear antigen, which is related to DNA repair and cell proliferation. The increase of PCNA expression indicated that the cells were in the proliferation state. In aged rats, serum E2 increased with decreasing levels of serum T, and the E2/T ratio increased with the increase in the BPA dose. Low-dose E2 could promote prostate proliferation through the direct stimulation of prostate stromal cells or the regulation of epithelial cells17. BPA is considered to have weak estrogenic activity, approximately 1000–10,000 times less than that of E2 18, and it has the same effects on prostate as E2. Testosterone (T) can be converted into estrogen by aromatase. Aromatase mRNA expression was found to increase on exposure to BPA, suggesting that BPA decreased T levels on the way of transformation19. The study found that 10 μg/kg BPA did not reduce the T level, probably because the exposure level was too low to start the anti-androgenic activity. Estrogen and androgen balance plays an important role in maintaining physiological characteristics and reproductive functions in males. With the increasing age, the androgen level decreases and the estrogen level increases correspondingly. The imbalance of the estrogen/androgen ratio is also considered as one mechanism of BPH20. The E2/T ratio promotes synergistically prostate hyperplasia between a certain range.
Insulin and insulin-like growth factors promote the proliferation of prostate epithelial cells. It was reported that BPA could combine with ERα or G protein–coupled estrogen receptor (GPER) to increase insulin content and secretion after exposure to low-dose BPA in vitro 21,22; 10 nM BPA was reported to promote the synthesis of insulin by simulating the effect of estrogen on ERβ and inhibiting the KATP 23. In this study, the insulin level just tended to increase in the relatively high-dose BPA group, which was not consistent with the results of in vitro studies, suggesting the complexity of BPA in vivo. The insulin content of adult mice increased after 1-week administration of low-dose BPA. However, the content decreased after continuous administration for 5 weeks. This might be the result of BPA stimulating insulin synthesis by acting on estrogen receptors and inhibiting insulin by downregulating glucose transporter-2 (Glut-2) at the same time, but the promoting effect was the main advantage in a short time24.
E-cadherin is the marker protein of epithelial cells mainly distributed in the cytoplasm. E-cadherin belongs to the calcium family. It is a Ca2+-dependent transmembrane protein that plays an important role in cell attachment. Vimentin, one of the intermediate filaments, is the marker protein of mesenchymal cells mainly distributed in interstitial cells, participating in the composition of cytoskeleton. This study found that 10 μg/(kgday) BPA significantly upregulated the expression of vimentin and tended to downregulate the expression of E-cadherin. Also, the upregulation of some regulatory factors in charge with EMT genes was visible, including Snail, Twist, Wnt, transforming growth factor beta 1 (TGF-β1), ERα, and so on, most of which have been reported to be related to the formation and metastasis of tumors.
Snail belongs to the superfamily of zinc finger transcription factor, and E-box is the E-cadherin proximal promoter. The combination of Snail and E-box inhibits the expression of E-cadherin to start EMT. Snail can also inhibit the transcription of epithelial cell marker cytokeratin-8 (Krt-8) directly25,26. Moreover, 10−5 M BPA regulated the stability of Snail by the protein kinase B/glycogen synthase kinase-3β (Akt/GSK-3β) signaling pathway and significantly upregulated the expression of Twist, inducing the occurrence of EMT in colorectal cancer cells27. TGF-β1 plays a vital role in EMT, and it can directly activate Smad3, thus stimulating the expression of Snail. TGF-β1 can also combine with the TGF-β1 receptor, evoking the phosphorylation of Smad2 and Smad3 and mixing with Smad4 to form a complex. The complex enters the nucleus to activate the EMT gene28,29. In addition to the aforementioned genes, the expression of ERα was also upregulated in the BPA group compared with the control group. The expression of snail and vimentin increased three times, while E-cadherin expression was almost halved after 48-h exposure of BPA in ovarian cancer cells. However, Snail and vimentin were rarely expressed, and E-cadherin expression was not significantly different between the control and BPA groups after administering ER antagonists30. BPA could increase the Snail expression and induce the occurrence of EMT through the ER-dependent signaling pathway.
Cconsistent with the results of gene chip, the expression of E-cadherin protein decreased and the expression of vimentin protein increased, which verified the occurrence of EMT at the protein level. Estrogen needs to be combined with the receptor to exert an effect. Estrogen receptor belongs to the nuclear receptor family, mainly including ERα and estrogen receptor beta (ERβ), in which ERα has the role of promoting the proliferation of prostate cells. BPA can compete with E2 in binding to ERα due to its similar structure to E2, and then combines with specific DNA sequences to promote target genes after entering the nucleus31. The combination of BPA and ERα leads to the augmentation of ERα expression, correspondingly promoting proliferation. The prostate is an androgen-dependent organ, and AR plays a pivotal role in regulating function, growth, and differentiation of the prostate gland. In this study, the expression of AR gradually increased with the increase in the BPA dose. AR, as a transcription factor, is activated by the ligand, which can combine with a specific androgen response element to stimulate the transcription, but BPA can interrupt this transcription pathway by binding to AR to exert an anti-androgenic effect32. Compared with the anti-androgenic activity, BPA showed a stronger estrogenic activity, with a lower affinity for AR than for ER30. AR has also been reported to mediate the EMT process by regulating the expression of zinc finger E-box-binding protein 2 in androgen-dependent cells33.
Conclusion
Taken together, it was concluded that environmentally relevant BPA levels could aggravate BPH in aged rats, and the effect was enhanced with the increase in dose, which is not completely consistent with the effects of BPA in a rat model of BPH in previous studies. Moreover, the DLP was more sensitive to 90 μg/kg BPA. For the DLP, BPA increased the estrogen-to-androgen ratio and upregulated ERα and AR expression, so as to further induce the occurrence of EMT. However, which pathways are involved in EMT needs further confirmation.
Material and Methods
Animals and housing
Male Sprague–Dawley (SD) rats (5–7 weeks old, weighing 200–220 g) were purchased from Sino-British SIPPR/BK Laboratory Animal Co., Ltd., Shanghai, China. The animals were housed on sawdust bedding in standard polypropylene cages till the age of 1.5 years. Drinking water and pellet diet (Shanghai Shilin Science & Tech Co., Ltd, China) were available ad libitum in glass bottles. The rooms were maintained at a temperature of 20–26 °C and 40–70% humidity under a 12-h:12-h light/dark cycle. All animal procedures were approved by the Animal Care and Use Committee of Shanghai Institute of Planned Parenthood Research and conformed to the Guide for the Care and Use of Laboratory Animals.
Reagents
BPA (Lot No. 239658, purity, ≥99.5%) was purchased from Sigma–Aldrich Chemical Company (USA). BPA was solubilized in 0.5% sodium carboxymethyl cellulose (Lot No. 20140520; Sinopharm Chemical Reagent Co., Ltd, Shanghai) solution and stored at room temperature.
Animal treatment
The rats were randomly divided into four groups (n = 8) according to body weights after acclimatization. It has been shown that low doses of BPA may induce the proliferation of ventral prostate (VP) in adult rats9. Therefore, 10–90 μg/kg and 3 months were selected as the exposure dose and duration, respectively. The animals were treated with BPA (10, 30, and 90 μg/kg, intragastrically, daily) or vehicle for 3 months, and weighed once a week. All animals were anesthetized with pentobarbital sodium and sacrificed on the day subsequent to last treatment. The DLP were dissected, weighed, and divided into three parts. One part was fixed immediately in 10% formalin, and the other two parts were preserved in frozen liquid nitrogen for the follow-up study.
Histology
After fixing in formalin for 48 h, DLP tissues were embedded in paraffin, sectioned at 4 μm, and prepared for routine hematoxylin and eosin (H&E) staining. Histological changes were observed under an optical microscope (Nikon Eclipse 50i, Japan), and the height of DLP epithelium was determined using the Nikon NIS-Elements BR 3.1 software (Japan). A total of 20 epithelium samples from each animal and 160 epithelium samples from each group were selected for analysis.
Hormone level detection
The blood samples were collected and centrifuged for 15 min (3000 rpm, 4 °C) to collect serum. Then, the serum was instantly stored at −80 °C for concentration measurement. Serum estradiol (E2), T, and insulin levels were assayed according to the instruction in the corresponding enzyme-linked immunosorbent assay kits (NovaTeinBio, Inc, Cambridge, USA and detected by an enzyme reader (Zenyth 200 st, Austria).
Evaluation of EMT gene expression by a microarray analysis
Total RNA was extracted from four samples in control and 10.0 μg/kg BPA groups using an RNeasy Microarray Tissue Mini Kit (SABiosciences, Qiagen, Maryland 21703, USA), including the optional on-column DNase digestion step described in the handbook. The concentration and purity of RNA were determined by ultraviolet spectrophotometry and denaturing gel electrophoresis. Then, amplification, array hybridization, washing, and scanning were carried out according to the manufacturer protocol. Data were analyzed by using the ΔΔCT method and microarray data analysis software.
Evaluation of PCNA, androgen receptor, estrogen receptor subtypes, E-cadherin, and vimentin expression by an immunocytochemical analysis
The expression of androgen receptor (AR), estrogen receptor subtypes (ERα), E-cadherin, and vimentin were detected by the streptavidin–peroxidase method in accordance with the 1:100 (PCNA: 1:50) dilution of the first antibody.
The paraffin-fixed sections were deparaffinized with xylene and rehydrated with gradient ethanol. Then, the sections were immersed in 0.01 M sodium citrate buffer (pH 6.0) and heated to boiling in a microwave for 20 min for retrieval, and endogenous peroxidase was quenched with oxidase blocking solution (Reagent A) for 10 min at room temperature. The sections were incubated in normal nonimmune serum (Reagent B) for 10 min at room temperature to block nonspecific binding. The sections were covered with primary antibodies (ERα was purchased from Proteintech Group, Inc, Rosemont, IL 60018, USA; PCNA was purchased from Santa Cruz Biotechnology, Inc; other antibodies were purchased from Wuhan Boster Bio-engineering Company, Wuhan, China) in wet boxes at 4 °C overnight (PCNA: RT I h), but the first antibody was substituted with phosphate-buffered saline in the negative control group. Corresponding secondary antibodies (Reagent C) and Streptomyces antibiotic peroxidase solution (Reagent D) were added successively at room temperature for 10 min, followed by staining with a 3,3′-diaminobenzidine kit. The sections were treated with hematoxylin, dehydrated, washed, mounted, and observed under an optical microscope (Nikon Eclipse 50i, Japan). Finally, the mean optical density values were analyzed using the Image-Pro Plus 6.0 software.
Statistical analysis
Data were analyzed using SPSS version 17.0 (SPSS, IL, USA) and presented as mean ± standard deviation. Statistical comparisons were performed by one-factor analysis of variance. If statistically significant, the differences between control and treatment groups were tested using the least-squares means test.
Ethical standards
All animals in this study were treated humanely according to the Guide for the Care and Use of Laboratory Animals of Shanghai Institute of Planned Parenthood Research Animal Care and Use Committee.
References
Liao, L. M. & Schaefer, W. Cross-sectional and longitudinal studies on interaction between bladder compliance and outflow obstruction in men with benign prostatic hyperplasia. Asian J Androl. 9, 51–56 (2007).
Xia, S. J., Xu, X. X., Teng, J. B., Xu, C. X. & Tang, X. D. Characteristic pattern of human prostatic growth with age. Asian J Androl. 4, 269–271 (2002).
Roehrborn, C. G. Pathology of benign prostatic hyperplasia. Int J Impot Res. 20, S11–S18 (2008).
Wu, J. H. et al. Oral exposure to low-dose bisphenol A aggravates testosterone-induced benign hyperplasia prostate in rats. Toxicol Ind Health. 27, 810–819 (2011).
Liu, X.Y. et al. The study of the relation between estrogen or androgen and mechanism of benignprostatic hyperplasia [D]. Fudan University, 2008.
Guenther, K. et al. Endocrine disrupting nonylphenols are ubiquitous in food. Environ Sci Technol. 36, 1676–1680 (2002).
Takahashi, O. & Oishi, S. Testicular toxicity of dietary 2,2-bis(4-hydroxyphenyl)propane (bisphenol A) in F344 rats. Arch Toxicol. 75, 42–51 (2001).
Herath, C. B. et al. Adverse effects of environmental toxicants, octylphenol and bisphenol A, on male reproductive functions in pubertal rats. Endocrine. 25, 163–172 (2004).
Wu, J. H. et al. Oral administration of low-dose bisphenol A promotes proliferation of ventral prostate and upregulates prostaglandin D2 synthase expression in adult rats. Toxicol Ind Health 21, 1848–1858 (2016).
Xu, J. S., Lamouille & Derynck, R. TGF-β-induced epithelial to mesenchymal transition. Cell res. 19, 156–172 (2009).
Alonso-Magdalena, P. et al. A role for epithelial-mesenchymal transition in the etiology of benign prostatic hyperplasia. Proc Natl Acad Sci USA 106, 2859–2863 (2009).
Colborn, T., Vom-Saal, F. S. & Soto, A. M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 101, 378–384 (1993).
Bouskine, A. et al. Low doses of bisphenol A promote human seminoma cell proliferation by activating PKA and PKG via a membrane G-protein-coupled estrogen receptor. Environ Health Perspect. 117, 1053–1058 (2009).
Dekant, W. & Volkel, W. Human exposure to bisphenol A by biomonitoring: methods, results and assessment of environmental exposures. Toxicol Appl Pharmacol. 228, 114–134 (2008).
Matsumoto, J., Yokota, H. & Yuasa, A. Developmental increases in rat hepatic microsomal UDP-glucuronosyltransferase activities toward xenoestrogens and decreases during pregnancy. Environ Health Perspect. 110, 193–196 (2002).
Konno-Takahashi, N. et al. Engineered FGF-2 expression induces glandular epithelial hyperplasia in the murine prostatic dorsal lobe. Eur Urol. 46, 126–132 (2004).
Richter, C. A., Taylor, J. A., Ruhlen, R. L., Welshons, W. V. & Vom-Saal, F. S. Estradiol and bisphenol A stimulate androgen receptor and estrogen receptor gene expression in fetal mouse prostate mesenchyme cells[J]. Environ health persp. 115, 902–908 (2007).
Vom Saal, F. S. & Hughes, C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ health persp. 113, 926–933 (2005).
Torres, J. M., Gomez-Capilla, J. A., Ruiz, E. & Ortega, E. Semiquantitative RT-PCR method coupled to capillary electrophoresis to study 5alpha-reductase mRNA isozymes in rat ventral prostate in different androgen status. Mol Cell Biochem. 250, 125–130 (2003).
Lee, C. H., Akin-Olugbade, O. & Kirschenbaum, A. Overview of prostateanatomy, histology,and pathology. Endocrinol Metab Clin North Am. 40, 565–575 (2011).
Makaji, E. et al. Effect of enviromental contaminants on Beta cell function. Int J Toxicol. 30, 1243–1250 (2011).
Alonso-Magdalena, P., Morimoto, S., Ripoll, C., Fuentes, E. & Nadal, A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ health persp. 114, 106–112 (2006).
Soriano, S. et al. Rapid insulinotropic action of low doses of bisphenol-A on mouse and human islets of Langerhans: role of estrogen receptor beta. Plos One. 7, e31109 (2012).
Pan Y. The effect of low dose Bisphenol A on insulin synthesis and insulin secretion.[D]. Nanjing Medical University, 2014.
Kudo-Saito, C., Shirako, H., Takeuchi, T. & Kawakami, Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 15, 195–206 (2009).
Christiansen, J. J. & Rajasekaran, A. K. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 66, 8319–8326 (2006).
Chen, Z. J. et al. Bisphenol A modulates colorectal cancer protein profile and promotes the metastasis via induction of epithelial to mesenchymal transitions. Arch Toxicol. 89, 1371–1381 (2015).
Liu, Y. N. et al. Critical and reciprocal regulation of KLF4 and SLUG in transforming growth factor beta-initiated prostate cancer epithelial-mesenchymal transition. Mol Cell Biol. 32, 941–953 (2012).
Kim, Y. S. et al. Bisphenol A and nonylphenol have the potential to stimulate the migration of ovarian cancer cells by inducing epithelial-mesenchymal transition via an estrogen receptor dependent pathway. Chem Res Toxicol. 28, 662–671 (2015).
Weng, Y. I. et al. Epigenetic influences of low-dose bisphenol A in primary human breast epithelial cells. Toxicol Appl Pharm. 248, 111–121 (2010).
Wetherill, Y. B. et al. Xenoestrogen action in prostate cancer: pleiotropic effects dependent on androgen receptor status. Cancer Res. 65, 54–65 (2005).
Luccio-Camelo, D. C. & Prins, G. S. Disruption of androgen receptor signaling in males by environmental chemicals. J Steroid Biochem Mol Bio. 127, 74–82 (2011).
Jacob, S. et al. Androgen receptor as a regulator of ZEB2 expression and its implications in epithelia-to-mesenchymal transition in prostate cancer. Endocr Relat Cancer. 21, 473–486 (2014).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 21007041); the Shanghai Public Service Platform of Research & Development (No. 13DZ2291300); and the Talents Development Foundation of Shanghai Municipality (No. 201372).
Author information
Authors and Affiliations
Contributions
D.-Y.H. and J.-H.W. conceived of and designed experiments. D.-Y.H., C.-C.Z., Q.P., X.S., and L.L. executed experiments. D.-Y.H. and S.-S.W. performed a data analysis. D.-Y.H. wrote the manuscript. J.-H.W. and Z.-Y.S. theoretical directed the study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, DY., Zheng, CC., Pan, Q. et al. Oral exposure of low-dose bisphenol A promotes proliferation of dorsolateral prostate and induces epithelial–mesenchymal transition in aged rats. Sci Rep 8, 490 (2018). https://doi.org/10.1038/s41598-017-18869-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-18869-8
This article is cited by
-
Long exposure to a mixture of endocrine disruptors prediposes the ventral prostate of rats to preneoplastic lesions
Environmental Science and Pollution Research (2023)
-
A comprehensive review on the carcinogenic potential of bisphenol A: clues and evidence
Environmental Science and Pollution Research (2021)
-
TBC1D20 Is Essential for Mouse Blood–Testis Barrier Integrity Through Maintaining the Epithelial Phenotype and Modulating the Maturation of Sertoli Cells
Reproductive Sciences (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.