Abstract
Activation-induced cytidine deaminase (AID) and apolipoprotein B mRNA-editing catalytic polypeptide 3 (A3) family are cytidine deaminases that play critical roles in B-cell maturation, antiviral immunity and carcinogenesis. Adenoids and palatine tonsils are secondary lymphoid immune organs, in which AID and A3s are thought to have several physiological or pathological roles. However, the expression of AID or A3s in these organs has not been investigated. Therefore, we investigated the expression profiles of AID and A3s, using 67 samples of adenoids and palatine tonsils from patients, with reverse transcription quantitative polymerase chain reaction (RT-qPCR) and immunohistochemical analyses. AID and A3s expression levels in the adenoids and the palatine tonsils of the same individual significantly correlated with each other. Of note, AID expression level in the adenoids negatively correlated with the age (r = −0.373, P = 0.003). The younger group with adenoid vegetation and tonsillar hypertrophy showed more abundant AID expression than the older group with recurrent tonsillitis and peritonsillar abscesses (P = 0.026). Moreover, immunohistochemical analysis revealed the distribution of AID and A3s in the epithelial cells as well as germinal centres. The localisation of AID expression and its relation to age may contribute to adenoid vegetation and inflammation.
Similar content being viewed by others
Introduction
Apolipoprotein B mRNA-editing catalytic polypeptide (APOBEC) enzymes are a family of cytidine deaminases that convert cytosine in DNA and/or RNA into uracil. In humans, the APOBEC family is composed of at least 11 members, including activation-induced cytidine deaminase (AID) and APOBEC1, 2, 3 A, 3B, 3 C, 3D, 3 F, 3 G, 3 H and 41,2. These proteins, particularly AID and APOBEC3s (A3s), are important for both innate and adaptive immune responses. AID is expressed in B cells and is essential for antibody diversification, including somatic hypermutation and class switch recombinations that occur in germinal centres (GCs)3,4. Furthermore, Epstein-Barr virus infected B-cells induces expression of AID5. AID is also found in non-B cells and is induced by the stimulation of inflammatory cytokines that are involved in some viral infections such as hepatitis C virus and hepatitis B virus in hepatocytes6,7,8,9,10. Further, aberrant expression of AID leads to the accumulation of genetic changes and carcinogenesis, including oral squamous cell carcinoma, B-cell lymphoma, gastric cancer and skin cancer11,12,13,14,15. Similarly, A3s act as antiretroviral factors, by introducing frequent C-to-U conversion in newly generated viral DNA16,17. A3 enzymes localise to the cytoplasm and/or nucleus, enabling the protection of both compartments by the restriction of nuclear or cytoplasmic replicating elements. Eventually they show antiviral effects on DNA viruses such as hepatitis B18,19,20,21.
Adenoids and palatine tonsils are lymphoid tissues located in the pharynx that play an important role in host defence against pathogens invading the upper respiratory tract22. In GCs, B cells differentiate into plasma and memory B cells that secrete high-affinity antibodies and endow individuals with immunological memory23. Almost all persons have adenoid vegetation and hypertrophic palatine tonsils during childhood. The size of the adenoids and palatine tonsils decreases gradually and spontaneously with age; hence, these tissues are rudimentary in most adults24. Adenoid vegetation or hypertrophic palatine tonsils often cause clinical symptoms such as nasal obstruction, snoring and obstructive sleep apnoea. Complications of adenoid vegetation, hypertrophic palatine tonsils, or recurrent tonsillitis frequently force patients to undergo either tonsillectomy or adenotonsillectomy. However, the pathophysiology of these diseases remains largely unknown.
To elucidate the pathogenic role of AID or A3s expression on adenoid vegetation, tonsillar hypertrophy, or tonsillitis, we examined their expression profile and distribution in surgically treated adenotonsillar organs with various pathological backgrounds.
Results
Patient characteristics
The characteristics of all patients for RT-qPCR analysis are shown in Table 1. The mean age of patients was 18 years (range, 2–89 years). All 14 patients who underwent adenotonsillectomy were <16 years old and suffered obstructive sleep apnoea because of adenoid vegetation, although 29 patients (91%) <16 years old, with either adenoid vegetation or tonsillar hypertrophy, underwent adenoidectomy or tonsillectomy. The 35 patients ≥16 years old underwent tonsillectomy. Among them, 18 patients (51%) received tonsillectomy because of recurrent tonsillitis, 3 patients (9%) for repeated peritonsillar abscess and 9 patients (26%) for focal tonsils.
We performed immunohistochemical analysis on 15 adenoid specimens and 14 palatine tonsil specimens from the same 15 individuals. The mean age of the 15 patients was 32 years (range, 5–19 years), including nine patients aged ≥16 years (range, 26–89 years) and six patients aged <16 years (range, 5–13 years).
Expression level of AID/A3s in the adenoid and palatine tonsils of each individual
Initially, we determined the expression level of AID/A3s in the adenoids and palatine tonsils of each individual using RT-qPCR. We compared the expression level of AID/A3 mRNA in the adenoids with that of the palatine tonsils in each individual (Fig. 1). The level of all AID/A3s in the adenoids, except for A3F, significantly correlated with those in the palatine tonsils. The levels of AID and A3F were higher in the adenoids than that in the palatine tonsils, whereas the levels of A3A, A3B, A3G and A3H were higher in the palatine tonsils (Fig. 2).
We further examined the correlation between the levels of AID/A3 mRNA in the adenoids and palatine tonsils. In the adenoids, there was no correlation between the levels of AID and any of the A3s (Table 2), whereas the levels of A3s were largely correlated with each other (Table 2). By contrast, in the palatine tonsils, AID levels significantly correlated with A3s (Table 3). In the adenoids, A3s levels moderately correlated with each other.
These results indicate that the expression level of AID/A3s in the adenoids correlated with that in the palatine tonsils for each individual, and that the adenoids and the palatine tonsils have distinct expression profiles for AID/A3s.
Association between age and expression level of AID/A3s in the adenoids and palatine tonsils
Next, we examined the association between AID/A3s expression levels and age. In the adenoids, even though the regression coefficient suggests a relatively weak relationship, AID negatively correlated with age, whereas A3A, A3F, A3G and A3H had significant and positive correlations with age (Fig. 3). In the palatine tonsils, A3G and A3H, but not AID, significantly correlated with the age at operation (Fig. 3). This indicated that A3s expression levels remained stable or increased with age, whereas AID expression levels in the adenoids decreased with age.
Moreover, we investigated associations between AID/A3s expression levels and pathophysiology of the adenoids and the palatine tonsils. Figure 4 shows a comparison of AID expression levels between two groups in these tissues. In the adenoids, AID expression level of adenoid vegetation and tonsillar hypertrophy in patients <16 years old (a) was higher than that observed in patients with recurrent tonsillitis and peritonsillar abscesses who were ≥16 years old (b) (Fig. 4A). In the palatine tonsils, there was no statistically significant difference in AID expression between the two groups (Fig. 4B). Moreover, there was no statistically significant difference in the expression of any A3s between the two groups in the adenoids and the palatine tonsils (see Supplementary Fig. S1). This suggested that the age-related expression of AID, and reason for the operation, affected the volume of tissues in the adenoids but not in the palatine tonsils.
Comparison of AID expression levels in the adenoids and palatine tonsils of two groups. (A) Adenoid vegetation and tonsillar hypertrophy; <16 years old, n = 29 and (B) Recurrent tonsillitis and repeated peritonsillar abscess; ≥16 years old, n = 21. n, number of patients; P, P-value; *P < 0.05, as determined by Mann–Whitney U test.
Subcellular localisation of AID/A3s in the adenoids and palatine tonsils
We performed immunohistochemical analysis to assess the subcellular localisation of AID/A3s in the adenoids and the palatine tonsils.
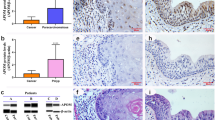
AID expression was detected in the dark zone of GCs in both the adenoids and palatine tonsils, as reported previously25,26,27,28,29 (Fig. 5a,c,q and s). Unexpectedly, AID expression in the epithelial cells was comparable with that in the GCs (Fig. 5r and t). The mean total expression scores were 3.37 for the epithelium of the adenoids, 3.26 for the epithelium, and 2.54 for the follicular GC in the tonsils (Table 3).
Subcellular localisation of AID and A3s in adenoids and palatine tonsils. Representative images of immunohistochemical analyses of AID/A3s. An adenoid (a,b,d,f,i,j,m,n,q and r) and a palatine tonsil (c,d,g,h,k,l,o,p,s and t) from a 5-year-old boy, who suffered from sleep apnoea and underwent adenotonsillectomy, were analysed. The specimens were stained using antibodies against AID (a,c and q–t), A3A (b and d), A3B (e and g), A3C (f and h), A3D (i and k), A3F (j and l), A3G (m and o) and A3H (n and p). The inset figures indicate epithelium (left) and GC (right). Original magnifications: 200 × (a–p), 40 × (q and s), 400 × (r and t) and 600 × (inset figures). Scale bar: 100 μm (a–p), 500 μm (q and s) and 50 μm (r and t).
For A3s, the epithelial cells express A3A, A3B, A3C, A3F, A3G and A3H, whereas the GCs express A3B, A3C, A3F, A3G and A3H (Table 3 and Fig. 5b and d–p). Of note, A3G was found in tangible body macrophages (Fig. 5m and o). Crypts expressed A3A, A3B, A3C, A3F, A3G and A3H, while infiltrated haematopoietic cells expressed A3A. A3D was poorly detected in the epithelium, GCs, or crypts but was detected in vessels.
The subcellular localisation of AID/A3s is summarised in Table 3. All were expressed in both the nucleus and cytoplasm although AID, A3B, A3C, A3D, A3G, A3F and A3H were abundant in the cytoplasm and A3A was rich in the nucleus (Table 3 and Fig. 5). A3B was expressed abundantly in the cytoplasm of the epithelium and in the nucleus of the GC.
Discussion
In this study, we examined the expression and distribution of APOBEC enzymes, particularly AID and A3s, in the adenotonsillar tissues. AID/A3s expression were weakly to moderately correlated with each other within a single subject (Fig. 1). Their expression profile differed between the adenoids and palatine tonsils (Fig. 2). AID was expressed independently from A3s in the adenoids; however, AID/A3s were correlated with each other in the adenoids and the palatine tonsils (Table 2).
Furthermore, there was a negative correlation between age and AID/A3 expression in the adenoids and a positive correlation between age and AID/A3s in the palatine tonsils, respectively (Fig. 3). There was no statistically significant difference between age and AID in the palatine tonsils or A3s in the adenoids and palatine tonsils, but there was statistically significant difference between age and AID in the adenoids (Fig. 3). Immunohistochemical analysis revealed abundant AID expression in the epithelium, comparable with that observed in the GCs (Fig. 5). These results revealed a comprehensive profile of AID/A3s expression in adenotonsillar tissues. However, the samples analysed in this study were all obtained from the patients. The etiologies differed between the younger and the older patients who underwent surgery. Generally, the expression of AID and A3s is affected by the transcriptional factors such as NF-κB that acts downstream of inflammatory cytokines such as TNFα7,8,9,10. Thus, the degree of inflammation and infection, as well as the age of the patients at operation, might have affected the results obtained in this study.
AID is predominantly cytoplasmic and shuttles between the nucleus and cytoplasm30, whereas A3A, A3C and A3H are found in both the nucleus and cytoplasm16,18,24; this was not consistent with our results. In contrast, previous reports indicate that A3B localises in the nucleus, whereas A3D, A3F and A3G are found in the cytoplasm16,18,24, which is not consistent with our data that showed that A3s localised in both the nucleus and cytoplasm (Table 3 and Fig. 5). This discrepancy could be attributed to the different cellular contexts among murine fibroblasts, adenotonsillar epithelial or immune cells, HeLa and 293 T cells used in their studies. Further studies are necessary to identify undetermined factors that regulate the subcellular localisation of A3s in adenotonsillar tissues.
Mattila et al.31 reported that the proportion of B cells producing immunoglobulin decreases with ageing in adenotonsillar tissues, particularly in adenoids. Age-related decreases coincide with decreased B-cell population or production of immunoglobulin. Because AID is a master gene for B-cell maturation3,4, the inverse correlation between AID expression in adenoid vegetation and ageing (Figs 3 and 4A) may reflect decreased maturation of B cells with ageing.
Onal et al.32 reported that the apoptosis of lymphocytes in the GC of tonsillar hypertrophy increased compared with chronic tonsillitis. AID expression in tonsillar hypertrophy at a younger age was not higher than that in repeated tonsillitis (Fig. 4B), which may reflect the increased apoptosis of B cells in GC. Further studies are necessary to clarify the role of AID in adenoid vegetation and tonsillar hypertrophy.
Aberrant AID or A3s expression is associated with inflammation. AID is ectopically induced in gastric epithelium by Helicobacter pylori infection via NF-κB10 or inflammatory cytokines, such as TNFα or IL-1β9,10,33. Further, A3s are induced by interferons and lipopolysaccharides8,21,34. We found abundant AID and A3s expression in the epithelial cells of the adenoids and the palatine tonsils, compared with that in GCs (Fig. 5a,c,r and t). A3 expression positively correlated with age in the palatine tonsils (Table 3) although there was no statistically significant difference between the palatine tonsils with hypertrophy and those with repeated inflammation (Supplementary Fig. S1). Therefore, it is intriguing to speculate that repeated inflammation by repetitive infection, whether clinical or subclinical, induces aberrant AID or A3s expression.
In conclusion, we describe the unique distribution of AID and A3s and their association with age as well as surgical rationale for adenotonsillar tissues. Further investigation is required to reveal the physiological or pathological consequences of AID and A3s expression in adenotonsillar tissues.
Methods
Organs
Adenoid and palatine tonsil samples were collected from 67 patients who underwent adenotonsillectomy or tonsillectomy between October 2012 and March 2014 in the Division of Otolaryngology and Head and Neck Surgery at the Kanazawa University Hospital in Kanazawa and five other branch hospitals. The research protocol was approved by the ethics committees of the Kanazawa University Hospital and the five other participating hospitals. All experiments were carried out in accordance with the relevant guidelines and regulations. Informed consent was obtained from all patients and guardians prior to enrolment. Adenoids from patients who underwent tonsillectomy were collected by biopsy. None of the patients suffered any acute inflammation.
RNA extraction and RT-qPCR
RNA was extracted from adenoid and palatine tonsil tissues using the RNeasy Plus Mini Kit (QIAGEN, Tokyo, Japan) according to the manufacturer’s instructions. Total RNA (5 μg) was reverse transcribed using the SuperScript III First-Strand Synthesis System for RT reactions (Thermo Fisher Scientific, MA, USA). cDNA was amplified using specific primers for A3A, A3B, A3C, A3D, A3F, A3G, A3H, AID and hypoxanthine-guanine phosphoribosyltransferase as described previously7,21.
Statistical analysis for RT-qPCR
AID/A3s expression levels are shown as median values. AID/A3s expression levels between adenoids and palatine tonsils were compared using the Mann–Whitney U test and the Spearman rank correlation coefficient. The association between expression levels of AID/A3s and age was analysed using the Spearman rank correlation coefficient. Moreover, we divided patients into two groups and compared the expression levels of AID/A3s in the adenoids and palatine tonsils between both groups using the Mann–Whitney U test: (a) adenoid vegetation and tonsillar hypertrophy, age < 16 years, (b) recurrent tonsillitis and repeated peritonsillar abscess, age ≥ 16 years.
Immunohistochemical analysis
The expression of AID/A3s was immunohistochemically examined in 15 adenoids and 14 palatine tonsils among 67 patients for RT-qPCR analysis. These 15 adenoids and 14 palatine tonsils were obtained from the same individuals. Consecutive 3-μm sections were cut from formalin-fixed, paraffin-embedded blocks of adenoids and palatine tonsils.
To detect AID, monoclonal antibody clone EK2 5G9 was used (1:5000, Cell Signaling Technology Inc., Boston, USA). Immunohistochemical staining was performed using the Vectastain Elite ABC kit (Vector Laboratories Inc., Burlingame, USA)11. To determine A3s expression, tissue sections were incubated with monoclonal antibodies against A3s. After washing with PBS, the sections were exposed to Envision+ secondary antibody (Dako, Glostrup, Denmark) and counterstained with hematoxylin. Antibodies for A3A, A3G and A3H are described previously21,35. Anti-A3C antibody clone 10591-1-AP (1:50, Proteintech, Chicago, USA), anti-A3B antibody clone ab184990 (1:100, Abcam, Cambridge, UK), anti-A3D antibody clone HPA055116 (1:200, Sigma Aldrich, St. Louis, USA), and anti-A3F antibody clone H00200316-A01 (1:50, abnova, Taipei, Taiwan) were used.
We examined cross-reactivity between anti-AID and A3 antibodies described above by Western blotting. (Supplementary Methods and Supplementary Table S1). The results showed no cross-reactivity between each anti-AID and A3 antibodies except for anti-A3A and anti-A3F antibodies. The anti-A3A antibody mainly reacted with A3A protein and reacts very faintly with A3D protein, which is oeverexpressed in 293FT cells. In contrast, the anti-A3F antibody mainly reacted A3F protein and reacts very faintly with overexpressed A3C protein (Supplementary Fig. S2).
Evaluation of the specimens and statistical analysis
Stained sections were evaluated by two authors (NS and SK), who were independently blinded to the clinical data. The subcellular localisation of immunoreactive cells was separately evaluated for the nucleus and cytoplasm. The adenoid specimens were only evaluated with a part of epithelium because the specimens were tiny and only a few samples included follicles. The palatine tonsil specimens were separately evaluated with a part of the epithelium and GC. The average frequency of immunoreactive cells was calculated after counting the number of immunoreactive cells and the total number of epithelial cells or lymphocytes at three different high-powered fields (400×). The average percentage of immunoreactive cells in the nucleus and cytoplasm was defined as the N expression score and C expression score, respectively. Further, we calculated the total expression score. These three scores were used for statistical analysis. The average frequency of immunoreactive cells was classified into three groups to determine localisation of nucleus and cytoplasm: expression score 0, <10% immunoreactive cells; expression score 1, 10%–50% immunoreactive cells; expression score 2, >50% immunoreactive cells36. We evaluated the number of cells expressing AID/A3s to determine the expression level of these enzymes.
References
Harris, R. S. & Liddament, M. T. Retroviral restriction by APOBEC proteins. Nat. Rev. Immunol. 4, 868–877 (2004).
Goila-Gaur, R. & Strebel, K. HIV-1 Vif, APOBEC, and intrinsic immunity. Retrovirology 5, 51 (2008).
Muramatsu, M. et al. Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J. Biol. Chem. 274, 18470–18476 (1999).
Muramatsu, M. et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102, 553–563 (2000).
Kalchschmidt, J. S. et al. Epstein-Barr virus nuclear protein EBNA3C directly induces expression of AID and somatic mutations in B cells. J. Exp. Med. 213, 921–928 (2016).
Machida, K. et al. Hepatitis C virus induces a mutator phenotype: enhanced mutations of immunoglobulin and protooncogenes. Proc. Natl. Acad. Sci. USA 101, 4262–4267 (2004).
Liang, G. et al. TGF-β suppression of HBV RNA through AID-dependent recruitment of an RNA exosome complex. PLoS Pathog. 11, e1004780 (2015).
Kou, T. et al. Expression of activation-induced cytidine deaminase in human hepatocytes during hepatocarcinogenesis. Int. J. Cancer. 120, 469–476 (2007).
Endo, Y. et al. Expression of activation-induced cytidine deaminase in human hepatocytes via NF-kappaB signaling. Oncogene 26, 5587–5595 (2007).
Watashi, K. et al. Interleukin-1 and tumor necrosis factor-α trigger restriction of hepatitis B virus infection via a cytidine deaminase activation-induced cytidine deaminase (AID). J. Biol. Chem. 288, 31715–31727 (2013).
Nakanishi, Y. et al. Role of activation-induced cytidine deaminase in the development of oral squamous cell carcinoma. PLoS One 25, e62066 (2013).
Morisawa, T. et al. Organ-specific profiles of genetic changes in cancers caused by activation-induced cytidine deaminase expression. Int. J. Cancer 123, 2735–2740 (2008).
Nonaka, T. et al. Involvement of activation-induced cytidine deaminase in skin cancer development. J. Clin. Invest. 126, 1367–1382 (2016).
Greeve, J. et al. Expression of activation-induced cytidine deaminase in human B-cell non-Hodgkin lymphomas. Blood 101, 3574–3580 (2003).
Burns, M. B., Temiz, N. A. & Harris, R. S. Evidence for APOBEC3B mutagenesis in multiple human cancers. Nat. Genet. 45, 977–983 (2013).
Delebecque, F. et al. Restriction of foamy viruses by APOBEC cytidine deaminases. J. Virol. 80605–80614 (2006).
Knisvacher, B. A., Gerner, D. & Levaon, E. Y. DNA editing by APOBECs: a genomic preserver and transformer. Trends Genet. 32, 16–28 (2016).
Kinomoto, M. et al. All APOBEC3 family proteins differentially inhibit LINE-1 retrotransposition. Nucleic Acids Res. 35, 2955–2964 (2007).
Lackey, L. et al. APOBEC3B and AID have similar nuclear import mechanisms. J. Mol. Biol. 419, 301–314 (2012).
Vieira, V. C. & Soares, M. A. The role of cytidine deaminases on innate immune responses against human viral infections. Biomed. Res. Int. 2013, 683095 (2013).
Kitamura, K. et al. Uracil DNA glycosylase counteracts APOBEC3G-induced hypermutation of hepatitis B viral genomes: excision repair of covalently closed circular DNA. PLoS Pathog. 9, e1003361 (2013).
van Kempen, M. J., Rijkers, G. T. & Van Cauwenberge, P. B. The immune response in adenoids and tonsils. Int. Arch. Allergy Immunol. 122, 8–19 (2000).
De Silva, N. S. & Klein, U. Dynamics of B cells in germinal centres. Nat. Rev. Immunol. 15, 137–148 (2015).
Mattila, P. S. & Tarkkanen, J. Age-associated changes in the cellular composition of the human adenoid. Scand. J. Immuno. 45, 423–427 (1997).
Moldenhauer, G. et al. AID expression identifies interfollicular large B cells as putative precursors of mature B-cell malignancies. Blood 107, 2470–2473 (2006).
Cattoretti, G. et al. Nuclear and cytoplasmic AID in extrafollicular and germinal center B cells. Blood 107, 3967–3975 (2006).
Willenbrock, K., Jungnickel, B., Hansmann, M. L. & Küppers, R. Human splenic marginal zone B cells lack expression of activation-induced cytidine deaminase. Eur. J. Immunol. 35, 3002–3007 (2005).
Greiner, A. et al. Differential expression of activation-induced cytidine deaminase (AID) in nodular lymphocyte-predominant and classical Hodgkin lymphoma. J. Pathol. 205, 541–547 (2005).
Ito, S. et al. Activation-induced cytidine deamenase shuttles between nucleus and cytoplasm like apolipoprotein B mRNA editing catalytic polypeptide 1. Proc. Natl. Acad. Sci. USA 101, 1975–1980 (2004).
Muckenfuss, H. et al. APOBEC3 proteins inhibit human LINE-1 retrotransposition. J. Biol. Chem. 281, 22161–22172 (2006).
Önal, M. et al. Apoptosis in chronic tonsillitis and tonsillar hypertrophy. Int. J. Pediatr. Otorhinolaryngol. 79, 191–195 (2015).
Matsumoto, Y. et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat. Med. 13, 470–476 (2007).
Wang, Z. et al. APOBEC3 deaminases induce hypermutation in human papillomavirus 16 DNA upon beta interferon stimulation. J. Virol. 88, 1308–1317 (2014).
Mehta, H. V., Jones, P. H., Weiss, J. P. & Okeoma, C. M. IFN-αand lipopolysaccharide upregulate APOBEC3 mRNA through different signaling pathways. J. Immunol. 189, 4088–4103 (2012).
Kondo, S. et al. APOBEC3A associates with human papillomavirus genome integration in oropharyngeal cancers. Oncogene 36, 1687–1697 (2017).
Horikawa, T. et al. Induction of c-Met proto-oncogene by Epstein-Barr virus latent membrane protein-1 and the correlation with cervical lymph node metastasis of nasopharyngeal carcinoma. Am. J. Pathol. 59, 27–33 (2001).
Acknowledgements
This study was supported by research grants from the Ministry of Education, Science, Sports, Culture and Technology of Japan (A24689064 for SK and B23390396 for TY). The authors would like to thank Dr. Y. Maruyama, T. Yamamoto, T. Tsukatani, M. Sakumoto and N. Uramoto for providing samples. The authors have no funding, financial relationships or conflicts of interest to disclose. The authors would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Contributions
N.S., S.K., M.K., M.M.K., H.S., M.H., T.U., E.K. and M.K. designed the research, performed the experiments and analysed the data. N.S., S.K., K.W., M.M. and T.Y. wrote the manuscript; N.W., M.K., M.M.K., Y.N., K.E., T.I., K.I., H.S., M.H., T.U. and K.K. discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seishima, N., Kondo, S., Wakae, K. et al. Expression and subcellular localisation of AID and APOBEC3 in adenoid and palatine tonsils. Sci Rep 8, 918 (2018). https://doi.org/10.1038/s41598-017-18732-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-18732-w
This article is cited by
-
Mutational spectrum of SARS-CoV-2 during the global pandemic
Experimental & Molecular Medicine (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.