Abstract
Despite controlling a diverse array of regulatory processes in plants, UV-B wavelengths (280–315 nm) are attenuated by common greenhouse materials such as glass and polycarbonate and are therefore depleted in many commercial growing environments. In this study, we analysed the architecture, pigment accumulation and antioxidant capacity of coriander (Coriandrum sativum, also known as cilantro) plants grown with and without supplementary UV-B (1.5 µmol m−2 s−1). We demonstrate that UV-B limits stem elongation responses to neighbour proximity perception (shade avoidance), promoting a more compact plant architecture. In addition, UV-B increased leaf quercetin content and total antioxidant capacity. Arabidopsis thaliana mutants deficient in flavonoid biosynthesis were not impaired in shade avoidance inhibition, suggesting that UV-B-induced flavonoid synthesis is not a component of this response. Our results indicate that UV-B supplementation may provide a method to manipulate the architecture, flavour and nutritional content of potted herbs whilst reducing the deleterious impacts of dense planting on product quality.
Similar content being viewed by others
Introduction
UV-B comprises less than 1% of sunlight, yet regulates a wide range of physiological and developmental processes in plants. These include the production of protective sunscreen compounds, plant growth, photosynthesis, stomatal regulation and defence against pests and pathogens1. In Arabidopsis, photomorphogenic UV-B signals are perceived by the photoreceptor UV RESISTANCE LOCUS 8 (UVR8)2. Upon UV-B absorption, UVR8 dimers monomerise and interact with the E3 ubiquitin ligase CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) to initiate the transcriptional regulation of hundreds of genes3. The most striking phenotype displayed by UV-B-treated plants is a reduction in plant size, resulting from reduced leaf expansion and an inhibition of stem elongation4,5. This is most apparent in environmental conditions whereby stem elongation is promoted, such as exposure to low red to far red ratio light (low R:FR)5, and high temperature6. Low R:FR is a component of vegetational shade and a signal of neighbour proximity prior to shading7. Plant perception of reduced R:FR by phytochrome photoreceptors leads to a rapid elongation of stems, as plants attempt to overtop neighbours. This response is largely driven by an increase in auxin synthesis, via low R:FR-mediated stabilisation and activation of PHYTOCHROME INTERACTING FACTOR (PIF) transcription factors7. UV-B perceived by UVR8 decreases auxin activity by reducing PIF4/5 abundance and activity. This is achieved by promoting PIF degradation5, increasing levels of ELONGATED HYPOCOTYL 5 (HY5) transcription factor (which competes with PIF4 for target promoters)8 and increasing levels of growth-suppressing DELLA proteins which bind to PIFs, inhibiting their function and promoting their degradation5,9,10.
Control of stem length is a key objective in horticulture, where the aesthetic quality of plants is paramount to commercial success. Excessive stem growth can reduce both the market value and shelf life of produce. This problem is exacerbated by dense planting which can initiate shade avoidance responses7. The molecular processes controlling light-regulated stem elongation are well characterised in Arabidopsis, but our understanding of photomorphogenesis in culinary herbs is more limited. Recent studies have, however, shown that mixed narrow bandwidth LED treatments of red, blue, green and yellow light can be used to manipulate the plant architecture and antioxidant content of sweet basil and coriander11,12. Manipulation of UV-B signalling in commercial growing environments is an emerging area of research13,14,15,16. One study reports that lettuce pre-treated with UV-B outperformed control plants, post transplantation to field17. UV-B supplementation has also been shown to increase the leaf area, dry biomass and antioxidant capacity of sweet basil, thus providing a potential mechanism to adjust the harvest yield and nutritional content of horticultural produce18. In coriander, high dose narrowband UV-B has been shown to induce chromosomal aberrations, and decrease pigment and carbohydrate content19. The impact of lower dose (non-stressful) levels of UV-B on coriander growth and development remains poorly studied.
Here, we analysed the effects of low dose UV-B on coriander architecture, pigment and antioxidant content, in backgrounds of high and low R:FR, to simulate well-spaced and dense planting, respectively. We sought to determine whether coriander displays similar UV-B-mediated photomorphogenic responses to Arabidopsis1,5 to establish whether UV-B supplementation could, in principle, be incorporated into commercial lighting regimes to improve the quality of potted herbs.
Materials and Methods
Plant material
Coriandrum sativum cv. Slow Bolt and cv. Cruiser were provided by Vitacress Herbs Ltd. Arabidopsis thaliana tt4 and tt7 mutants in the Col-0 background20 were used for comparison in flavonol glycoside detection experiments and hypocotyl elongation assays.
Growth conditions
Coriandrum sativum seeds were scarified to break dormancy and synchronise germination. Fruit were manually split into two mericarps through gentle abrasion with a mortar and pestle and soaked in H2O for 48 h. Seeds were germinated on damp tissue in controlled growth cabinets (Microclima 1600E, Snijder Scientific) in white light (WL) (cool white fluorescent tubes 400–700 nm, Photosynthetically Active Radiation (PAR) = 70 μmolm−2s−1 measured at the soil surface, R:FR = 5) in 12 h photoperiods, 20 °C and 70% relative humidity. After 3 days in these conditions, germinated seeds were selected for potting on to a 3:1 mixture of compost (Levingtons F2) and silver sand to a depth of 10 mm. Subsequently, plants were moved to treatment conditions (12 h light:12 h dark cycles, 20 °C and 70% relative humidity). R:FR ratios were set at 5 for high R:FR and 0.05 for low R:FR using supplementary Far Red LEDs (+FR). Supplementary UV-B at 1.5 μmolm−2s−1 (0.6 W m−2) was provided with Philips TL100W/01 narrow band UV-B bulbs. Polycarbonate filters (6 mm thickness) were used to attenuate UV-B for plants grown in -UV-B conditions. See Figure S1a for light spectra. Arabidopsis seeds were surface sterilised with 70% v/v EtOH and 20% v/v Sodium Hypochlorite treatments. After three days stratification in darkness at 4 °C, seeds were germinated following similar protocols to coriander.
For glasshouse experiments, plants were exposed to ambient PAR levels which ranged from 60 to 800 µmolm−2s−1 throughout the experiment (Figure S1b). A minimum PAR of 165 µmolm−2s−1 and 16 h photoperiods were maintained using supplementary fluorescent lamps (Plug and Grow compact 200 W). UV-B supplementation was provided using Philips TL100W/01 narrow band UV-B bulbs. Temperature was maintained at 18 °C.
Image analysis
Morphological data from seedlings and mature plants was extracted from images using FIJI21. Hypocotyls were measured from the shoot apical meristem to the shoot-root junction. Petiole lengths were measured from the shoot apex to the base of the leaf blade. Visible leaf area was measured by counting pixels from binarized images of flattened leaf blades.
Timelapse imaging of elongating hypocotyls
For Time-Lapse Infra Red (IR) photography, a custom built 8 × 8 array of 880 nm IR LEDs were controlled with a 24 h timer. Timelapse images were captured with a Nikon D80 DSLR camera with its IR blocking filter removed, a SIGMA 105 mm macro lens and an IR pass filter (>850 nm) (Zomei, Jiangsu, China) operated with Digicamcontrol software. Images were taken at 60 min intervals. Timelapse start and duration was as specified in the experiment. Hypocotyl lengths from individual images and time lapse image stacks were manually measured from images using FIJI.
Chlorophyll abundance
Leaf chlorophyll content was determined by the Witham et al. method22. 100 mg tissue from leaf 2 of 28-day-old plants was snap frozen at −80 °C and homogenised using stainless steel beads and a TissueLyser (Qiagen). Chlorophyll from homogenised tissue was extracted in 80% (v/v) acetone. Absorbances were recorded at 663, 645 and 652 nm with 80% acetone used as a blank.
Total antioxidant activity
Total antioxidant capacity was recorded using a commercially available kit (Total Antioxidant Capacity Assay kit, Sigma-MAK187). 100 mg of leaf tissue from leaf 3 was frozen at −80 °C and homogenised using stainless steel beads and a TissueLyser (Qiagen). Samples were extracted in 1 ml of ice cold 1 X Phosphate Buffered Saline (PBS) and the supernatant was diluted 1:100 to bring values within range of kit standards. Samples were assayed according to the manufacturer’s protocol, by comparing the absorbances of diluted extracts at 570 nm with Trolox standards. Values were then normalised to tissue fresh weight.
Detection of flavonol glycosides by thin layer chromatography
Flavonol glycoside extraction and thin layer chromatography were carried out as described previously23. 100 mg of leaf tissue was homogenised and extracted in 0.4 ml 80% (v/v) methanol. Samples were incubated for 15 min at 70 °C then centrifuged for 10 min. Supernatants were vacuum-dried at 65 °C and dried pellets dissolved in 1 μl 80% methanol mg−1 fresh weight.
1 μl of methanolic extracts were spotted onto HPTLC silica gel 60 glass plates (Millipore). Chromatography was performed in a closed glass tank with a mobile phase of ethyl acetate, formic acid, acetic acid and water (100:26:6:12 v/v). After separation, plates were air dried and flavonols detected by spraying 2 ml of of 1% (w/v) 2,3-dibromopropanal (DPBA) in MeOH 3 times with 5 min between sprayings. This was followed by 2 ml of 5% (w/v) PEG 4000 in MeOH 3 times with 5 min between sprayings. After 15 min, the stained HPTLC plate was visualized under UV (365 nm). Flavonol glycoside-DPBA derivatives fluoresce under UV light. Liquid chromatography-Mass Spectrometry (LC-MS) has been used to profile these flavonol glycosides and assign them to different colours23. Here we compared methanolic extracts of coriander against Arabidopsis thaliana flavonoid biosynthesis mutants to gain qualitative information about flavonoid content.
Light Measurements
Data were recorded using Ocean Optics FLAME and USB2000 spectrophotometers and analysed using Ocean Optics Oceanview software and Microsoft Excel.
Statistical Analyses
SPSS v. 23 (IBM) was used to analyse quantitative data.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Results
UV-B inhibits shade avoidance responses in coriander
Coriander seedlings were grown in high (5) and low (0.05) R:FR ratio conditions with and without UV-B supplementation (Figs 1a, S1a). Plants grown in low R:FR ratio (+FR) were significantly elongated when compared to plants grown in white light alone (WL), confirming that coriander displays shade avoidance (Fig. 1b)7. Low dose UV-B did not result in significantly shorter hypocotyls in a background of WL (Fig. 1b). In low R:FR, however, UV-B-mediated inhibition of hypocotyl elongation was observed (Fig. 1b). These data show that low dose UV-B antagonises shade avoidance in coriander, similarly to Arabidopsis5. Interestingly, the magnitude of hypocotyl elongation was cultivar-dependent. Both the ‘Slow Bolt’ and ‘Cruiser’ cultivars displayed enhanced hypocotyl elongation in low R:FR, but absolute lengths for ‘Slow Bolt’ were significantly longer than ‘Cruiser’. UV-B inhibited low R:FR-induced hypocotyl elongation of both cultivars, although the hypocotyl lengths of the ‘Slow Bolt’ cultivar were, again, significantly longer than ‘Cruiser’ (Fig. 1c). Due to its enhanced stem elongation, we selected ‘Slow Bolt’ for further experimentation.
UV-B inhibits hypocotyl elongation in shade-avoiding coriander. (a) Coriander (Slow Bolt) seedlings were germinated in WL for 3 days and then placed into WL, WL + UV-B, WL + FR or WL + FR + UV-B conditions as indicated for a further 10 days. Scale bar = 20 mm (b) Hypocotyl Lengths (mm) as grown in (a) ANOVA (F(3,60) = 13.865 p < 0.001) n = 16. Different red Letters indicate statistically significant differences by Tukey’s post hoc test at p < 0.05. (c) Hypocotyl elongation in different coriander cultivars. Slow Bolt (Blue) and Cruiser (Red) were germinated and grown as in (a). n = 18–21 seedlings. Student’s T-tests were performed to test for differences between cultivars in the indicated conditions. *Indicates significant difference between cultivars at p < 0.05. WL + FR (t(37) = 2.624, p = 0.0126), WL + FR + UV-B (t(38) = 2.541, p = 0.0153). **Indicates significant difference between WL + FR and WL + FR + UV-B in the Cruiser cultivar at p < 0.05 (t(39) = 7.988, p < 0.001).
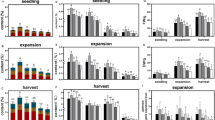
We next examined the effects of low R:FR and UV-B on adult plants. These were grown in the indicated conditions until they were 28 days old, which is similar to the age at which commercially-grown coriander is transported to customers. The morphology of mature plants is more complex than juveniles as coriander produces multiple petioles with variable leaf phenotypes (Fig. 2a). Shade-avoiding Arabidopsis plants have been shown to produce fewer leaves and smaller leaf blades, as resources are diverted towards stem elongation24,25. A complex interaction between low R:FR and UV-B signalling has additionally been reported, with low R:FR promoting leaf expansion in the presence of UV-B5. We therefore quantified total visible leaf blade area in coriander. UV-B did not significantly affect this parameter in a background of WL. Low R:FR, however, significantly reduced total visible leaf blade area. In contrast to hypocotyl elongation data (Fig. 1), UV-B did not antagonise this response (Fig. 2b). Total visible leaf blade area could be correlated with petiole number. We therefore compared petiole number between treatments. UV-B did not significantly affect petiole number in a background of WL (Fig. 2c). Low R:FR significantly reduced petiole number which may contribute to the reduced total visible leaf blade area recorded in these plants. This response was not, however, antagonised by UV-B (Fig. 2c).
UV-B inhibits petiole elongation but does not significantly increase leaf blade area or petiole number in shade-avoiding coriander. (a) 28-day-old plants grown in 12 h photoperiods. Seedlings were germinated in WL for 3 days and then placed into WL, WL + UV-B, WL + FR or WL + FR + UV-B conditions as indicated for a further 25 days. Scale bar = 50 mm. (b) Total Visible Leaf Blade Area, ANOVA (F(3,44) = 5.696, p = 0.002. (c) Petiole number, Median + /− 1 S.D., ANOVA (F(3,44) = 19.319, p < 0.001). (d) Mean Petiole length, ANOVA (F(3,44) = 32.694, p < 0.001 (e) Ratio of Total Visible Leaf Area to Total Petiole Length, ANOVA (F(3,44) = 25.926, p < 0.001. n = 12. Different Letters indicate statistically significant differences by Tukey’s post hoc test at p < 0.05.
In low R:FR, UV-B treated plants appeared more compact than untreated controls, which was reflected in the lengths of the longest petioles. UV-B had no effect on petiole length in a background of WL (Fig. 2d). Low R:FR treatment significantly elongated petioles, consistent with shade avoidance7. As with hypocotyls, UV-B supplementation antagonised petiole elongation in these conditions (Fig. 2d). The ratio of total visible leaf area/total petiole length was used as a measure of plant compactness. UV-B did not significantly affect plant compactness in a background of WL (Fig. 2e). Low R:FR significantly reduced plant compactness, resulting in more spindly plants (Fig. 2a,e). This response was partially attenuated by UV-B supplementation (Fig. 2e).
Our data show that light quality has significant effects on coriander architecture. Low R:FR drives elongation of hypocotyls and petioles, with both responses inhibited by UV-B. In contrast, low R:FR-mediated reductions in total leaf area and petiole number were not significantly attenuated by UV-B. Nevertheless, plants treated with UV-B in a low R:FR background were still more compact than their untreated controls as demonstrated by the ratio of total visible leaf area to total petiole length (Fig. 2e).
The data described above suggest that UV-B supplementation may be a potential method for increasing the compactness (and therefore aesthetic quality) of densely-grown coriander in commercial settings. The harmful effects of UV-B exposure do, however, present health and safety concerns for workers and continuous UV-B illumination is not economically or environmentally desirable. We therefore questioned whether there was an optimum time of day for UV-B-mediated inhibition of shade avoidance. In short (8 h photoperiod) day- grown Arabidopsis, hypocotyl elongation is greatest at the end of the night, with the phase of maximum elongation growth moving in to the day with longer photoperiods26. We applied time lapse infrared imaging to analyse diurnal growth rhythms of coriander grown in 12 h photoperiods. Our data show clear UV-B-mediated inhibition of growth in both white light (Fig. 3a) and low R:FR (Fig. 3b). In all conditions, the period of maximum growth occurred during the light period (Fig. 3c,d). To investigate whether an optimal time of day exists for UV-B-mediated suppression of shade avoidance, we applied a 4 h UV-B treatment at 3 different times of day (1–4 h, 4–8 h, 8–12 h). Our results show that a short (4 h) dose of UV-B is sufficient to significantly inhibit hypocotyl elongation and that the time of day of this treatment has only marginal effects on the magnitude of the response (Fig. 3e). A 4 h UV-B treatment given in the final third of the day inhibited hypocotyl elongation the greatest with a 17% decrease in mean hypocotyl length recorded between treated and control plants. UV-B treatment at the start of the day was the next effective (13% decrease), followed by treatment in the middle of the day (8% decrease). Notably, the 12 h UV-B treatment resulted in the shortest hypocotyls (25% decrease) suggesting that total UV-B dose may be the determining factor in regulating hypocotyl growth inhibition.
Coriander exhibits rhythmic growth but the timing of UV-B supplementation is unimportant for inhibition of shade avoidance. Seedlings were germinated in WL for 3 days then placed into the indicated conditions for timelapse imaging. (a–d) Hourly hypocotyl length and growth rate in WL ± UV-B (a,c) and (WL + FR) ± UV-B (b,d) n = 8 ± 1 S.E.M. (e) Seedlings were germinated in WL for 3 days and then placed into WL + FR and WL + FR supplemented with UV-B at the indicated times for a further 10 days. 0–12 represents a control, whereby UV-B was provided throughout the light period. n = 57–61. ANOVA (F(4,288) = 25.484, p < 0.001) Different Letters indicate statistically significant differences by Tukey’s post hoc test at p < 0.05.
UV-B elevates leaf flavonoid content and antioxidant activity
A key objective for the herb growing industry is the production of visually appealing plants with dark green leaves. Previous reports have found that light quality can impact chlorophyll abundance27 and photosynthetic efficiency28, but in our conditions neither R:FR ratio nor UV-B significantly affected chlorophyll content in mature coriander leaves (Fig. 4a).
UV-B and low R:FR ratio modulate coriander leaf anti-oxidant capacity and quercetin content. Leaf tissue was sampled from 28-day-old plants grown in 12 h photoperiods. Seedlings were germinated in WL for 3 days and then placed into WL, WL + UV-B, WL + FR and WL + FR + UV-B conditions as indicated for a further 25 days. (a) Chlorophyll A and B content in different light conditions. Chlorophyll A, ANOVA (F(3,12) = 2.84, p = 0.083); Chlorophyll B, ANOVA (F(3,12) = 2.84, p = 0.363); Chlorophyll A and B, ANOVA (F(3,12) = 1.918, p = 0.181) n = 4. Means ± 1 S.E.M. (b) Anti-oxidant activity (in Trolox equivalent nmol mg−1 fresh leaf tissue) in UV-B- treated plants (n = 8). Data represent means ± 1 S.E.M. In high R:FR, t(14) = −4.419, p = 0.000584. *statistically significant differences at p < 0.05. (c,d) Leaf flavonol glycoside accumulation as assayed by thin layer chromatography and derivation with DPBA. Flavonol glycoside derivatives are imaged with UV-illumination (365 nm). Fluorescent Colour key: green = kaempferol derivatives; orange = quercetin derivatives; blue = sinapate derivatives and unknown substances; dark red = chlorophyll. (e) Hypocotyl elongation of flavonoid biosynthesis mutants. Plants were grown in long (16 h) photoperiods for 3 days in WL and then 4 days in the indicated light conditions. Two Way ANOVA interaction with genotype and light condition as factors (F(6,166) = 1.480, p = 0.188).
Low dose UV-B has been hypothesised to act as a ‘eustress’ that activates antioxidant defences prior to the onset of oxidative pressure that can be caused by exposure to high ‘distress’ levels of UV-B29. We therefore assayed antioxidant capacity in 28-day-old coriander leaves. WL + UV-B-treated plants displayed significantly greater antioxidant capacity than WL-grown plants (Fig. 4b). Consistent with studies in other species27, low R:FR-treated plants had a reduced antioxidant capacity when compared to WL-grown plants. In a background of low R:FR, UV-B did not elevate leaf antioxidant capacity, suggesting that low dose UV-B is not sufficient to counteract the reduction in antioxidant capacity caused by a low R:FR ratio (Fig. 4b).
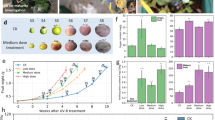
Flavonols are some of the most abundant flavonoids in plants and act as UV-B filters due to their absorption of UV light in the 280–320 nm region. UV-B induces flavonol accumulation30, which impacts on plant flavour31 and potentially increases health benefits32. We assayed coriander leaves for changes in flavonol glycoside content in response to UV-B using thin layer chromatography and derivation with DPBA as described previously23. Arabidopsis mutants, deficient in flavonoid synthesis were included as controls. Under UV-illumination (365 nm), methanolic flavonol glycoside extracts conjugated with DPBA fluoresce as follows: green- kaempferol derivatives; orange- quercetin derivatives; blue- sinapate derivatives and unknown substances; dark red- chlorophyll23. In Arabidopsis, CHALCONE SYNTHASE (encoded by the TT4 gene) catalyses the first step of the flavonoid biosynthesis pathway20 and tt4 mutants clearly lack flavonol glycosides as indicated by the absence of green and orange derivatives when compared to wild-type extracts (Fig. 4c). The FLAVONOID 3′-HYDROXYLASE enzyme (encoded by the TT7 gene) of the flavonoid biosynthesis pathway catalyses the conversion of dihydrokaempferol to dihydroquercetin20. The absence of orange derivatives in tt7 mutant extracts therefore indicates the absence of quercetin derivatives. In both high- and low R:FR-grown coriander, UV-B predominantly induces the accumulation of quercetin (as indicated by orange fluorescent derivatives) (Fig. 4d).
Flavonoids have been shown to modulate auxin responses in plants. In particular, quercetin has been shown to inhibit polar auxin transport in planta 33. UV-B-induced flavonoid production may therefore, in part, contribute to the inhibition of shade avoidance in low R:FR. We therefore analysed hypocotyl elongation in tt4 and tt7 mutants grown in high and low R:FR with and without UV-B supplementation. No statistically significant differences were observed between wild-type and mutant plants, suggesting that UV-B-induced flavonoid production does not contribute to UV-B-mediated inhibition of shade avoidance.
UV-B supplementation inhibits stem elongation in the glasshouse
Laboratory experiments are often constrained by the maximum light levels produced by commercial growth cabinets. To assess the effectiveness of UV-B supplementation as a means for plant architecture manipulation in commercial horticulture, we performed glasshouse experiments with sunlight levels of PAR (Figs 5, S1b). A daily 4 h UV-B treatment (1.5 µmolm−2s−1) given in the middle of the day sucessfuly inhibited hypocotyl elongation in 10-day-old seedlings. Greater hypocotyl elongation was observed when UV-B treatment was applied throughout the photoperiod (16 h) (Fig. 5). When plants were grown for 32 days, 4 h and 16 h UV-B treatments were similarly effective at inhibiting hypocotyl elongation (Figure S2a). Inhibition of petiole elongation was, however, only observed with continuous (16 h) UV-B treatment.
UV-B supplementation inhibits coriander hypocotyl elongation in the glasshouse. Seedlings were grown for 10 days in 16 h photoperiods maintained by supplemental light. Narrow band UV-B was provided for either the entire photoperiod (16 h) or for 4 h at the middle of the day. (a) Hypocotyl lengths of seedlings grown at a density of 4 per 10 cm−2, n = 20, ANOVA (F(2,57) = 23.106, p < 0.001). Different Letters indicate statistically significant differences by Tukey’s post hoc test at p < 0.05. (b) Photograph of representative seedlings as seen in (a).
Discussion
Consistent with studies in Arabidopsis, we found that low dose UV-B (equivalent to a summer afternoon in the UK34) antagonised low R:FR-induced stem elongation in coriander5 (Figs 1, 2). The shade avoiding nature of coriander may contribute to its spindly appearance when grown commercially in dense stands. In a background of WL (PAR = 70 μmolm−2s−1), UV-B did not significantly inhibit hypocotyl elongation. It is, however, possible that UV-B may suppress stem growth in a low PAR environment where blue and red light are simultaneously depleted35. Hypocotyl elongation responses to light quality treatments differed significantly between cultivars (Fig. 1c). The ‘Cruiser’ cultivar is a compact variety and while it does not elongate as much as ‘Slow Bolt’ in response to low R:FR, its elongation is still inhibited by UV-B supplementation. Phytochrome B (phyB) promotes shoot branching by suppressing auxin signalling in stems. Inactivation of phyB in low R:FR therefore reduces branching, establishing apical dominance36. UV-B did not significantly affect coriander branching or leaf blade expansion in our conditions, but increased plant compactness, primarily through inhibition of petiole elongation (Fig. 2). The plant growth hormone auxin is a key regulator of stem elongation, with an established role in driving shade avoidance7. UV-B has previously been reported to suppress auxin biosynthesis and signalling in Arabidopsis shade avoidance, thereby reducing plant stem elongation and promoting a compact phenotype5. The similarities in architectural response to low R:FR and UV-B displayed by Arabidopsis and coriander suggest that similar signalling mechanisms may exist in both species.
UV-B levels vary diurnally in natural environments34. In Arabidopsis, transcriptional responses to UV-B have been shown to be gated by the circadian clock in free running conditions and vary depending on the time of day in light-dark cycles37,38. Coriander displayed maximum growth during the day in 12 hour photoperiods, consistent with Arabidopsis grown in longer photoperiods (Fig. 3a–d). In our experiments, time of day had little impact on UV-B-mediated inhibition of hypocotyl elongation (Fig. 3e). We predicted that UV-B treatment would maximally inhibit elongation growth in the afternoon, when shade is most effective at promoting hypocotyl elongation in diurnal cycles39 and sunflecks are most effective at inhibiting shade avoidance within canopies40. Our data may suggest that, unlike many reported UV-B responses, UV-B-mediated hypocotyl growth inhibition is not gated by the circadian clock. Data may also reflect species-specific differences in circadian regulation or photomorphogenesis between Arabidopsis and coriander. Alternatively, circadian gating of UV-B-mediated hypocotyl growth inhibition may negate opposing rhythms of UV-B responsiveness, thereby ensuring uniform UV-B-mediated growth inhibition throughout the day and preserving rhythmic growth26.
Low R:FR ratio can reduce leaf chlorophyll content27. A similar response has been observed in coriander in response to high dose narrowband UV-B and likely results from damage to the photosynthetic apparatus19. Given the documented repair of the Earth’s ozone layer41, greater research effort is now being focussed on the regulatory effects of low-dose, non-harmful UV-B radiation, which has been implicated in improving photosynthetic efficiency28. We found no effect of R:FR or UV-B on leaf chlorophyll content (Fig. 4a), suggesting that coriander chlorophyll accumulation may not be as sensitive to light quality as in other species.
Following UV-B perception, plants activate antioxidant defences to alleviate oxidative pressure and minimise DNA damage via a UVR8-COP1-HY5 signalling pathway1,2,3. This includes increased synthesis of flavonoids, including the ‘effective antioxidant’ quercetin42. We therefore assayed leaf tissue for total antioxidant capacity after low dose UV-B irradiation (Fig. 4b). Consistent with other studies43, low dose UV-B significantly increased antioxidant capacity in plants grown in white light. The same UV-B treatment given to coriander grown in low R:FR was, however, ineffective. We found that plants grown in low R:FR had reduced antioxidant capacity compared to white light- grown controls, consistent with findings in other species27. It is possible that this represents the diversion of resources from defence towards elongation44. Alternatively, as low R:FR ratio is a signal of impending shade, plants may minimise photoprotective mechanisms in these conditions. It is intriguing that UV-B, a component of direct sunlight, did not significantly elevate antioxidant capacity in low R:FR ratio and suggests that low R:FR ratio may block UV-B-mediated activation of antioxidant defences. However, in natural environments, plants would only perceive sunlight levels of UV-B in combination with very low R:FR when emerging from a canopy.
Due to their absorption of UV light in the 280–320 nm region and the hypersensitivity of flavonoid biosynthesis mutants to UV-B radiation45, flavonoids are regarded as sunscreening compounds in Arabidopsis. UV-B induces their accumulation30 and CHALCONE SYNTHASE, an enzyme that catalyses the conversion of coumaroyl-CoA and malonyl-CoA in the flavonoid biosynthesis pathway20,46, is a key marker for plant UV-B signalling47. Our analysis of flavonoid levels suggested that UV-B mainly induces the accumulation of quercetin in coriander (Fig. 4c). An increase in quercetin levels following UV-B treatment has previously been reported in unrelated taxa such as petunia48, Brassica napus 49 and apple50. Like other flavonoids, quercetin has UV-B absorptive properties, but it also acts as an antioxidant, which is likely due to multiple hydroxy groups in the A, B and C aromatic rings51. Intriguingly, in a background of low R:FR, we observed a clear increase in quercetin but no significant increase in total antioxidant capacity (Fig. 4b,c). It is possible that this reflects low R:FR-mediated supression of antioxidant activity, as described above. Despite conveying potential health benefits, quercetin, like other flavonoids, is associated with bitter flavours52. Growers will therefore need to consider the potential flavour alterations caused by UV-B supplementation alongside aesthetic improvements.
In addition to their antioxidant properties, flavonoids have been shown to negatively regulate auxin transport, affecting shoot branching, lateral root formation and root gravitropism33. In Arabidopsis, low R:FR-induced auxin biosynthesis and transport are central to shade avoidance53. Low R:FR increases expression and re-localisation of the auxin efflux regulator PIN-FORMED 3 (PIN3) to direct auxin to the hypocotyl epidermis, promoting cell elongation54. We therefore speculated that the flavonoid deficient tt4 and tt7 mutants may display impaired UV-B-mediated inhibition of shade avoidance. These mutants behaved similarly to wild-type controls, suggesting that UV-B-mediated flavonoid production is not a key component of shade avoidance inhibition.
Collectively, our results suggest that the inclusion of UV-B into commercial growth regimes may give appreciable benefits to coriander architecture and phytonutrient content. Although a daily 4 h UV-B treatment was sufficient to elicit a significant suppression of hypocotyl elongation in both growth cabinet and glasshouse settings (Figs 3e, 5, S2), our experiments indicate that timing of the UV-B dose is relatively unimportant (Fig. 3e). An additional consideration in a glasshouse setting is that different photon irradiances of UV-B may need to be applied to maintain the UV-B:PAR ratio, as light quantity changes throughout the day. Future applications are likely to utilise advancements in UV-B LED technology55, although a more cost-effective approach may involve the construction of greenhouses from materials with higher UV-B transmission56. It will be of interest to see if UV-B transparent materials can improve the architecture, flavour and phytonutrient content of glasshouse- grown coriander in an energy- and cost-effective manner.
References
Jenkins, G. I. Photomorphogenic responses to ultraviolet-B light. Plant Cell Environ. https://doi.org/10.1111/pce.12934 (2017).
Rizzini, L. et al. Perception of UV-B by the Arabidopsis UVR8 protein. Science 332, 103–106 (2011).
Ulm, R. et al. Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc. Natl. Acad. Sci. USA 101, 1397–1402 (2004).
Hectors, K., Prinsen, E., De Coen, W., Jansen, M. A. K. & Guisez, Y. Arabidopsis thaliana plants acclimated to low dose rates of ultraviolet B radiation show specific changes in morphology and gene expression in the absence of stress symptoms. New Phytol. 175, 255–270 (2007).
Hayes, S., Velanis, C. N., Jenkins, G. I. & Franklin, K. A. UV-B detected by the UVR8 photoreceptor antagonizes auxin signaling and plant shade avoidance. Proc. Natl. Acad. Sci. USA 111, 11894–11899 (2014).
Hayes, S. et al. UV-B perceived by the UVR8 photoreceptor inhibits plant thermomorphogenesis. Curr. Biol. 27, 120–127 (2017).
Fraser, D. P., Hayes, S. & Franklin, K. A. Photoreceptor crosstalk in shade avoidance. Curr. Opin. Plant Biol. 33, 1–7 (2016).
Toledo-Ortiz, G. et al. The HY5-PIF regulatory module coordinates light and temperature control of photosynthetic gene transcription. PLoS Genet. 10, e1004416 (2014).
de Lucas, M. et al. A molecular framework for light and gibberellin control of cell elongation. Nature 451, 480–484 (2008).
Li, K. et al. DELLA-mediated PIF degradation contributes to coordination of light and gibberellin signalling in Arabidopsis. Nat. Commun. 7, e11868 (2016).
Carvalho, S. D., Schwieterman, M. L., Abrahan, C. E., Colquhoun, T. A. & Folta, K. M. Light quality-dependent changes in morphology, antioxidant capacity and volatile production in sweet basil (Ocimum basilicum). Front Plant Sci. 7, 1328 (2016).
Naznin, M. T., Lefsrud, M., Gravel, V. & Hao, X. Different ratios of red and blue LED light effects on coriander productivity and antioxidant properties. Acta Hortic. 1134, 223–230 (2016).
Wargent, J. J. & Jordan, B. R. From ozone depletion to agriculture: understanding the role of UV radiation in sustainable crop production. New Phytol. 197, 1058–1076 (2013).
Wargent, J. J. UV LEDs in horticulture: from biology to application. Acta Hortic. 1134, 25–32 (2016).
Thomas, T. T. D. & Puthur, J. T. UV radiation priming: A means of amplifying the inherent potential for abiotic stress tolerance in crop plants. Environ. Exp. Bot. 138, 57–66 (2017).
Dou, H., Niu, G., Gu, M. & Masabna, J.G. Effects of Light Quality on Growth and Phytonutrient Accumulation of Herbs under Controlled Environments. Horticulturae, https://doi.org/10.3390/horticulturae3020036 (2017).
Wargent, J. J., Elfadly, E. M., Moore, J. P. & Paul, N. D. Increased exposure to UV-B radiation during early development leads to enhanced photoprotection and improved long-term performance in Lactuca sativa. Plant Cell Environ. 34, 1401–1413 (2011).
Sakalauskaitė, J. et al. The effects of different UV-B radiation intensities on morphological and biochemical characteristics in Ocimum basilicum L. J. Sci. Food Agric. 93, 1266–1271 (2013).
Kumar, G. & Pandey, A. Effect of UV-B Radiation on Chromosomal Organisation and Biochemical Constituents of Coriandrum sativum L. Jordan J. Biol. Sci. 10, 85–93 (2017).
Shirley, B. W. et al. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 8, 659–671 (1995).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Method. 9, 676–682 (2012).
Witham, F. H., Blaydes, D. F. & Devlin, R. M. Experiments in Plant Physiology (Van Nostrand Reinhold, 1971).
Stracke, R. et al. Analysis of PRODUCTION OF FLAVONOL GLYCOSIDES-dependent flavonol glycoside accumulation in Arabidopsis thaliana plants reveals MYB11-, MYB12- and MYB111-independent flavonol glycoside accumulation. New Phytol. 188, 985–1000 (2010).
Nagatani, A., Chor, J. & Furuya, M. Phytochrome B is not detectable in the hy3 mutant of Arabidopsis, which is deficient in responding to end-of-day far-red light treatments. Plant Cell Physiol. 32, 1119–1122 (1991).
Finlayson, S. A., Krishnareddy, S. R., Kebrom, T. H. & Casal, J. J. Phytochrome Regulation of Branching in Arabidopsis. Plant Physiol. 152, 1914–1927 (2010).
Nozue, K. et al. Rhythmic growth explained by coincidence between internal and external cues. Nature 448, 358–361 (2007).
Bartoli, C. G., Tambussi, E. A., Diego, F. & Foyer, C. H. Control of ascorbic acid synthesis and accumulation and glutathione by the incident light red/far red ratio in Phaseolus vulgaris leaves. FEBS Lett. 583, 118–122 (2009).
Davey, M. P. et al. The UV-B photoreceptor UVR8 promotes photosynthetic efficiency in Arabidopsis thaliana exposed to elevated levels of UV-B. Photosyn. Res. 114, 121–131 (2012).
Hideg, É., Jansen, M. A. & Strid, A. UV-B exposure, ROS, and stress: inseparable companions or loosely linked associates? Trends Plant Sci. 18, 107–115 (2013).
Lois, R. Accumulation of UV-Absorbing Flavonoids Induced by UV-B Radiation in Arabidopsis thaliana L.: I. Mechanisms of UV-Resistance in Arabidopsis. Planta 194, 498–503 (1994).
Roland, W. S. U. et al. Bitter Taste Receptor Activation by Flavonoids and Isoflavonoids: Modeled Structural Requirements for Activation of hTAS2R14 and hTAS2R39. J. Agric. Food Chem. 61, 10454–10466 (2013).
Yao, L. H. et al. Flavonoids in foods and their health benefits. Plant Foods Hum. Nutr. 59, 113–122 (2004).
Peer, W. A. & Murphy, A. S. Flavonoids and auxin transport: modulators or regulators? Trends Plant Sci. 12, 556–563 (2007).
Findlay, K. M. W. & Jenkins, G. I. Regulation of UVR8 photoreceptor dimer/monomer photo-equilibrium in Arabidopsis plants grown under photoperiodic conditions. Plant Cell Environ. 39, 1706–1714 (2016).
de Wit, M. et al. Integration of Phytochrome and Cryptochrome Signals Determines Plant Growth during Competition for Light. Curr. Biol. 26, 3280–3287 (2016).
Reddy, S. K. & Finlayson, S. A. Phytochrome B promotes branching in Arabidopsis by suppressing auxin signalling. Plant Phys. 164, 1542–1550 (2014).
Fehér, B. et al. Functional interaction of the circadian clock and UV RESISTANCE LOCUS 8 controlled UV-B signaling pathways in Arabidopsis thaliana. Plant J. 67, 37–48 (2011).
Takeuchi, T., Newton, L., Burkhardt, A., Mason, S. & Farré, E. M. Light and the circadian clock mediate time-specific changes in sensitivity to UV-B stress under light/dark cycles. J. Ex. Bot. 65, 6003–6012 (2014).
Sellaro, R., Pacín, M. & Casal, J. J. Growth responses to shade show diurnal dependence in Arabidopsis: Role of hormone, clock and light signaling. Mol. Plant 5, 619–28 (2012).
Sellaro, R., Yanovsky, M. J. & Casal, J. J. Repression of shade-avoidance reactions by sunfleck-induction of HY5 expression in Arabidopsis. Plant J. 68, 919–928 (2011).
Solomon, S. et al. Emergence of healing in the Antarctic ozone layer. Science 353, 269–274 (2016).
Agati, G. & Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 186, 786–793 (2010).
Csepregi, K. et al. Developmental age and UV-B exposure co-determine antioxidant capacity and flavonol accumulation in Arabidopsis leaves. Environ. Exp. Bot. 140, 19–25 (2017).
Ballaré, C.L. & Pierik, R. The shade-avoidance syndrome: multiple signals and ecological consequences. Plant Cell Environ. https://doi.org/10.1111/pce.12914 (2017).
Li, J., Ou-Lee, T. M., Raba, R., Amundson, R. G. & Last, R. L. Arabidopsis flavonoid mutants are hypersensitive to UV-B Irradiation. Plant Cell 5, 171–179 (1993).
Koes, R. E., Quottrochhio, F. & Mol, J. N. M. The Flavonoid Biosynthetic Pathway in Plants: Function and Evolution. BioEssays 16, 123–132 (1994).
Fuglevand, G., Jackson, J. A. & Jenkins, G. I. UV-B, UV-A, and Blue Light Signal Transduction Pathways Interact Synergistically to Regulate Chalcone Synthase Gene Expression in Arabidopsis. Plant Cell 8, 2347–2357 (1996).
Ryan, K. G. et al. UV-B Radiation Induced Increase in Quercetin: Kaempferol Ratio in Wild-Type and Transgenic Lines of Petunia. Photochem. Photobiol. 68, 323–330 (1998).
Wilson, K. E., Wilson, M. I. & Greenbergt, B. M. Identification of the Flavonoid Glycosides that Accumulate in Brassica napus L. cv. Topas Specifically in Response to Ultraviolet-B Radiation. Photochem. Photobiol. 67, 547–553 (1998).
Solovchenko, A. & Schmitz-Eiberger, M. Significance of skin flavonoids for UV-B-protection in apple fruits. J. Exp. Bot. 54, 1977–1984 (2003).
Rice-Evans, C. A., Miller, N. J. & Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 20, 933–956 (1996).
Drewnowski, A. & Gomez-Carneros, C. Bitter taste, phyonutrients, and the consumer: a review. Am. J. Clin. Nutr. 72, 1424–1435 (2000).
Tao, Y. et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133, 164–176 (2008).
Keuskamp, D. H., Pollmann, S., Voesenek, L. A. C. J., Peeters, A. J. M. & Pierik, R. Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc. Natl. Acad. Sci USA 107, 22740–22744 (2010).
Wargent, J. J., Taylor, A. & Paul, N. D. UV supplementation for growth regulation and disease control. Acta Hortic. 711, 333–338 (2006).
Paul, N. D., Jacobson, R. J., Taylor, A., Wargent, J. J. & Moore, J. P. The Use of Wavelength-selective Plastic Cladding Materials in Horticulture: Understanding of Crop and Fungal Responses Through the Assessment of Biological Spectral Weighting Functions. Photochem. Photobiol. 81, 1052–1060 (2005).
Acknowledgements
This work was funded by a BBSRC iCASE studentship to KF and Vitacress Herbs.
Author information
Authors and Affiliations
Contributions
All authors designed experiments. D.P.F., A.S. and T.F. performed experiments. D.P.F., A.S., T.F., A.N.D. and K.A.F. analysed data. D.P.F. and K.A.F. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fraser, D.P., Sharma, A., Fletcher, T. et al. UV-B antagonises shade avoidance and increases levels of the flavonoid quercetin in coriander (Coriandrum sativum). Sci Rep 7, 17758 (2017). https://doi.org/10.1038/s41598-017-18073-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-18073-8
This article is cited by
-
The effect of Moringa oleifera crude extract on liver cell line, HepG2
BMC Complementary Medicine and Therapies (2023)
-
Interactive effects of changes in UV radiation and climate on terrestrial ecosystems, biogeochemical cycles, and feedbacks to the climate system
Photochemical & Photobiological Sciences (2023)
-
Response of the moss Physcomitrium patens to satellite-associated cucumber mosaic virus infection on the level of salicylic acid, quercetin and indole-3-acetic acid
European Journal of Plant Pathology (2022)
-
Flavored Food Additives on the Leaves of Piper betle L.: A Human Health Perspective
Applied Biochemistry and Biotechnology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.