Abstract
We evaluated the in-vitro effect of potassium on CD4+ T cells and the role of urinary potassium as a potential biomarker of disease activity in patients with ulcerative colitis (UC). This prospective observational cohort study included healthy controls (n = 18) and UC patients [n = 30, median age: 40 (IQR: 28–46) years, 17 males)] with active disease(assessed by Mayo score) from September 2015–May 2016. Twenty-four hours urinary potassium along with fecal calprotectin (FCP) were estimated in UC patients (at baseline and follow-up after 3–6 months) and controls. In healthy volunteers, we also assessed the effect of potassium on CD4+ T cells differentiated in the presence of Th17 polarizing condition. UC patients had significantly higher FCP (368.2 ± 443.04 vs 12.44 ± 27.51, p < 0.001) and significantly lower urinary potassium (26.6 ± 16.9 vs 46.89 ± 35.91, p = 0.01) levels than controls. At follow-up, a significant increase in urinary potassium among patients who had clinical response [n = 22, 21.4 (14.4–39.7) to 36.5 (20.5–61.6), p = 0.04] and remission [n = 12, 18.7 (9.1–34.3) to 36.5 (23.4–70.5), p = 0.05] was accompanied with a parallel decline in FCP. On in-vitro analysis, potassium under Th17 polarizing conditions significantly inhibited IL-17 and interferon-\(\gamma \) expression while favoring the induction of FoxP3+ T cells. Therefore, urinary potassium levels are inversely associated with disease activity in UC with in-vitro data supporting an immune-tolerant role of potassium.
Similar content being viewed by others
Introduction
The disease course of ulcerative colitis (UC) is characterized by concurrent periods of relapses and remissions and these relapses often occur in an unpredictable manner1. The goals of IBD therapy have not just expanded from clinical remission to mucosal healing but also improvement in quality of life, reduction in surgeries and hospitalizations, and regular assessment of disease activity are considered important to accomplish this goal. The assessment of disease activity at index presentation is usually done by endoscopic examination. However, assessment at follow up becomes difficult as repetitive semi invasive and costly investigations such as endoscopy are unacceptable especially to the patients.
In the past decades, various laboratory markers based on inflammation and acute phase response have been studied as objective parameters to assess disease activity and avoid invasive endoscopic procedures2,3. Biomarkers which have been used can be divided into serological and fecal categories. Of the serological markers, C-reactive protein (CRP) is the most widely studied parameter, but is limited by being a non-specific marker of inflammation4,5. Potentially, fecal markers have the advantage of possessing higher specificity for gastrointestinal diseases and include labeled leucocyte scintigraphy6, fecal calprotectin7, lactoferrin and neoptrin. However, their use in clinical practice is limited by the cost especially in a resource intensive setting like that in developing countries.
Apart from the genetic factors, environment plays a major role in regulating the balance between the immune tolerance and inflammation in the gut. One such factor is salt, which due to its higher consumption in the western diet, has recently emerged to play a game changing role in the gut immune response. High sodium intake and excretion (a reflection of sodium intake) has been found to be associated with increased inflammation, higher cardiovascular risks, cancer and renal inflammation in chronic kidney disease patients8,9. Recent murine studies have also shown that excess salt (sodium chloride) via a p38/MAPK-NFAT5-SGK1 signaling induces a highly pathogenic and stable Th17 phenotype10. High NaCl also inhibits IL-10 secretion11 and suppressive function of Foxp3+ regulatory T cells thereby promoting the proinflammatory response in the gut12. Moreover, high salt exacerbates the immune response by activating the pro-inflammatory M1 macrophages13 while alternatively reducing the activation of anti-inflammatory M2 macrophage14. While there is considerable expansion of knowledge for sodium dependent immune responses, the literature on the association of potassium with inflammatory responses is limited. A recent study found an inverse relationship between dietary potassium intake and risk of Crohn’s disease and UC. This suggests that potassium may be associated with attenuated gut inflammatory responses.
Therefore, the goal of this study was to elucidate the role of potassium as an anti-inflammatory marker in patients with ulcerative colitis. We investigated alterations in urinary potassium levels in patients with UC having active disease and subsequently on follow up after achieving clinical response. Finally, to support our clinical findings, we explored the in vitro effect of potassium on effector CD4+ T cells in the presence of Th17 inducing inflammatory environment.
Materials and Methods
Study design
A prospective observational cohort study was conducted at the inflammatory bowel disease (IBD) clinic, All India Institute of Medical Sciences (AIIMS) from September 2015 to May 2016. Eighteen healthy volunteers and thirty Ulcerative colitis patients with mild to severe disease activity as assessed by Mayo score were included in this study. Written, informed consent was taken from patient and control populations, before taking urine, fecal and blood samples. Patients with age <18 years or >75 years, pregnant women, patients with history of diabetes mellitus, chronic kidney disease, hypertension, coronary artery disease, patients on drugs which might influence the urinary potassium viz. diuretics, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs) were excluded from the study. The study protocol was approved by institutional ethics committee (IESC/T-215/05.05.15) of AIIMS. All experiments were performed in accordance with relevant guidelines and regulations.
Clinical information and sample collection
Clinical information was collected incorporating all baseline characteristics as well as treatment details. Data was collected for patient demographics, disease duration, type of disease and severity, treatment given and outcome. Montreal classification15 was used to determine the disease extent while the disease activity in UC patients were characterized using Mayo score16.
Serum samples from both UC patient and controls were collected for determining C-reactive protein while 24 hours urinary sample was collected for estimating urinary potassium excretion. On the same day, fecal samples were collected for measuring fecal calprotectin (FC) levels. CRP, fecal calprotectin and 24 hour urinary potassium levels were repeated in followed up UC patients after 3–6 months of treatment.
Diagnosis of UC
Diagnosis of Ulcerative colitis was made based on the European Crohn’s and Colitis Organization (ECCO) guidelines, employing a combination of clinical, endoscopic and histological features17.
Clinical response
Defined as decline in Mayo score by ≥3 at follow up
Clinical remission
Mayo score <3
Mucosal healing
Mayo endoscopy subscore of 1 or less, with a reduction of at least 1 point from baseline
Measurement of Fecal calprotectin levels
Stool samples were collected and frozen at −20 °C. Fecal Calprotectin levels were measured by ELISA based methods using KAPEPKT849 kit (DIAsource ImmunoAssays S.A. – Belgium). Normal cut off value was taken <47 μg/g of stool.
Measurement of Urine electrolyte levels
5 ml of 24-hour urine samples were collected and stored at 4–8 °C until further analysis. The analysis was done by electrolyte analyzer (XI- 921; Caretium medical instrument co. ltd, Shenzen China).
In vitro study: Effect of potassium on the expression profile of Th17 cells
T cell isolation and stimulation
Peripheral venous blood was obtained from healthy volunteers in compliance with the AIIMS Institutional ethics committee protocols. Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll-Paque plus (GE Healthcare, Piscataway, NJ) gradient centrifugation. The PBMCs were stained with CD4- Allophycocyanin (APC), CD25-phycoerythrin-Cy7 (PE/Cy7) and CD45 RA- Phycoerithrin (PE) (Biolegend, San Diego, USA). Naive T cells (CD4+ CD25− CD45RA+) were sorted; by high speed flow cytometry with FACS AriaIII (BD Biosciences, San Jose, CA) to >95% post sort purity. Cells were cultured at 105 cells/well in a 96 well U bottom plate for 6 days in serum free X vivo medium (Lonza, Walkersville, MD) and stimulated with plate bound anti CD3(UCHT1; 5 ug/ml) and soluble anti CD28 (28.2; 1 ug/ml). In Th polarization assay, at the start of the culture, cells were differentiated with recombinant IL-2 (50 U/ml) for Th0, TGFβ1 (5 ng/ml), IL-1β (12.5 ng/ml), IL-6, IL-21 and IL-23 (all at 25 ng/ml) for Th17 in the presence and absence of potassium chloride.
Flow-cytometry
For intracellular staining, cells cultured under Th17 condition on Day 7 were restimulated with PMA (phorbol 12-myristate 13-acetate; 50 ng/ml; Sigma-Aldrich), ionomycin (500 ng/ml; Sigma-Aldrich) and Golgi stop (BD Biosciences) for 4 h at 37 °C. Cells were stained with live-dead stain for 5 min at 4 °C to gate-out dead cells. The cells were then fixed and made permeable with Intracellular PermWash Buffer (Biolegend) for 15 min on ice followed by intracellular cytokine staining. Data were acquired on a FACSVerse (BD Biosciences) and analysed using FlowJo software (Tree Star, Inc., Ashland, OR).
Data record and statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) or median (inter-quartile range) as appropriate. Categorical variables were compared using chi squared test. The continuous variables among controls and cases were compared using unpaired t test or Man Whitney U test depending upon normal or non-normal distribution. The comparison between same group of patients at different time points was done using paired t test or Wilcoxon rank-sum test. A P value of ≤0.05 was considered as statistically significant. Data analysis was done using SPSS version 24.
Results
Fifty-six patients of ulcerative colitis were screened for inclusion and total of 48 patients fulfilled the inclusion criteria. Of these 30 patients had follow up assessment after a mean of 4.2 ± 2.7 months (Fig. 1).
Baseline clinical and demographic characteristics
The median age of patients was 40 (IQR:28–46) years and that of controls was 43 (IQR: 35–47) years. Males were slightly more than females both among the patients (17:13) and controls (11:7). The median duration of disease was 3 (IQR: 2–10) years (Table 1). Four patients (13.3%) had E1 disease, 16 patients had E2 disease (53.3%) and 10 (33.3%) patients had E3 disease. Eight patients had mild (25%), and 22 (68.7%) had moderate to severe disease activity.
Comparison of fecal calprotectin and 24 hours urinary potassium levels among UC patients and controls
The level of intestinal inflammation was assessed by median fecal calprotectin level (370.28 ± 430.47 vs 12.44 ± 27.51, p < 0.001) which was found to be significantly higher in UC patients than controls (Table 2). Further a higher FCP value was accompanied by a significantly lower 24 hour urinary potassium levels in UC patients than controls (26.6 ± 16.9 vs 46.89 ± 35.91, p = 0.006).
Comparison of baseline and follow up fecal calprotectin, 24-hours urinary potassium, CRP and Mayo score in UC patients who had clinical response
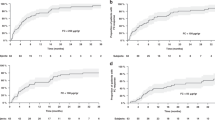
Of 30 patients, 22 had clinical response (Mayo score decline by \(\ge \)3). Among the patients who had clinical response, the median Mayo score [8 (7–9) to 2 (1.7–3.2), p < 0.001] and FCP levels decreased [302 (53–595) to 28 (5–261), p = 0.01] significantly at follow up, whereas no such trend was observed in patients who did not have clinical response (Table 3). 24-hour urinary potassium levels were significantly higher at follow up among patients with clinical response ([21.4 (14.2–39.7) to 36.5 (20.5–61.6), p = 0.04 (Fig. 2). There was no difference in the CRP levels at baseline and at follow up in both the groups. The per-patient change in Mayo score, FCP and urinary potassium in patients with clinical response vs those who did not have are depicted in Fig. 3.
Comparison of baseline and follow up fecal calprotectin, 24 hours urinary potassium, CRP and Mayo score in UC patients who had clinical remission
Of 30 patients, 12 achieved clinical remission (Mayo score <3). Patients with clinical remission at follow up had a significantly decreased median Mayo score [8 (5.5–9) to 2 (0.25–2), p < 0.01] along with a decreased FCP levels [312 (88–655) to 15.5 (1.8–256), p = 0.16], although the difference did not achieve statistical significance (Table 4). However, 24-hour urinary potassium levels were significantly higher at follow up among patients with clinical remission [18.7 (9.1–34.3) to 36.5 (23.4–70.5), p = 0.05] (Fig. 4).
Comparison of baseline and follow up fecal calprotectin, 24-hours urinary potassium, CRP and Mayo score in UC patients who had mucosal healing vs those who did not have
Of 30 patients, 19 achieved mucosal healing (Mayo endoscopic sub-score ≤1). Patients with mucosal healing at follow up had a significantly decreased median Mayo score [8 (7–9) to 2 (1–3), p < 0.01] and decreased FCP levels [332 (106–700) to 30 (7–260), p = 0.02] (Table 5). However, 24 hour urinary potassium levels were increased at follow up among patients with mucosal healing [21.6 (10.7–46.1) to 38.8 (18.3–61.1), p = 0.12] although the difference did not achieve statistical significance.
Correlation of change in Mayo score with change in fecal calprotectin levels and change in 24-hour urinary potassium levels
There was a statistically significant correlation between change in Mayo score and change in urinary potassium levels (r = 0.38, p = 0.04). However, there was no correlation between FCP change and urinary potassium change (r = −0.028, p = 0.89), as well as change in Mayo score and FCP change (−0.068, p = 0.73).
Potassium induces Foxp3+ CD4+ T cells under Th17 polarizing condition
To investigate the role of potassium on inflammatory or tolerogenic responses of CD4+ T cells, we stimulated sorted CD4+ T cells with Th17 polarizing conditions in the presence of increasing doses of potassium (10, 20 and 40 nM). As hypothesized, potassium significantly reduced the IL-17 and IFNγ expression on Th17 cells (Fig. 5(b) and (c)). Surprisingly, it also promoted the Foxp3 expression (Fig. 5(a)) in a dose dependent manner. These data suggest that potassium suppresses the inflammatory response while promoting anti-inflammatory FOXP3+ CD4+ T cells under inflammatory environment (Th17).
Sorted CD4+ T cells were stimulated for 6 days in the presence of anti CD3, anti CD28, hIL-2, Th17 polarizing condition in the presence of different doses of potassium and analysed for intracellular (a) Foxp3 (b) IFNγ and (c) IL-17. Percentages of IFNγ+, IL-17+ and Foxp3+ are represented as mean ± S.E.M of five independent experiments. *P < 0.05, **P < 0.001.
Discussion
The immunomodulatory action of the electrolytes- Na+ and K+ on inflammation has been studied in patients with cardiovascular risk18, cancer and chronic kidney disease patients and off late in IBD patients. Salt is an integral part of modern diet across the globe. However, in recent years, high salt intake has been associated with altered immune system leading to activated effector T cell responses. Both murine and human studies have depicted the role of NaCl in inducing Th17 related molecules while suppressing the Treg function via SGK1 signaling, thereby developing a more severe colitis in mice and aggravated disease activity in humans19. On the contrary, potassium enhanced the generation of FoxP3+ Treg cells in the presence of TGFβ1 and reinforced the Foxp3 expression in Th17 cells by activating Smad2/3 and inhibiting Smad 7 expression20. In mouse model of CKD, potassium supplementation down-regulates NF-κB and TGF β pathways and up-regulates Smad 7, thereby shifting the balance against pro-inflammatory state21. In another human report, potassium supplementation reduced IL-17A which was markedly enhanced by a high salt diet by inhibiting p38/MAPK-SGK1 pathway22. A recent study linked potassium with IBD and showed that dietary potassium, but not sodium, was inversely correlated with risk of Crohn’s disease in two large prospective cohorts of US women20. With a vast knowledge about the pro-inflammatory role of sodium, the role of potassium still has to be elucidated in IBD. With this background, we investigated the role of urinary potassium as a marker of intestinal inflammation in patients with ulcerative colitis. Also, as the previous two studies have highlighted the anti-inflammatory role of potassium, it would be very interesting to further take an intestinal inflammatory model such as ulcerative colitis patients and investigate the association of potassium with the disease severity in these patients.
The first major finding in our study was that the levels of 24-hour urinary potassium were significantly lower whereas FCP levels were significantly higher in patients with UC as compared to controls, thereby substantiating our hypothesis that levels of potassium had an inverse association with the disease activity in state of inflammation.
The second major finding was the significant increase of urinary potassium levels among UC patients who had a clinical response (decline in Mayo score ≥3) at follow up. This was accompanied by a significant parallel decline in Mayo score and fecal calprotectin levels. However, patients with no clinical response at follow up, had no change either in FCP levels or urinary potassium levels. These findings again strengthened the relationship of potassium to inflammation in IBD and revealed that the potassium levels increased with a decline in inflammation whereas they remained constant in patients who did not respond to therapy. Further studies are warranted to investigate whether there is a decline in potassium levels with concurrent relapse in the patient. This would further strengthen the hypothesis that urinary potassium estimation could be served as a marker of disease activity in IBD. Although the numbers are small, the results provide a signal which needs to be explored further. Similar trends were observed among patients who had clinical remission and mucosal healing.
We then corroborated our findings by studying the in vitro effect of potassium on inflammatory responses of CD4+ T cells. We observed that even in the face of Th17 polarizing conditions potassium significantly increased the expression of FoxP3 from Th17 cells in a dose dependent manner which was similar to a previous study by Khalili et al.20. However, our main focus was to check the effect of potassium on the major pro-inflammatory cytokines which are involved in aggravating the intestinal inflammation. We found that potassium reduced the expression of IL-17 and interferon \(\gamma \) which strenghthened our findings that potassium established a tolerogenic response while suppressing the inflammation inducing cytokines.
This is the first proof of concept study that documents an association between disease activity in UC and urinary potassium levels. However, there are several limitations associated with this study. The patient numbers were small, but we had the follow-up results for all the patients, and even with this small number we had significant results. We did not control the dietary pattern but measured the urinary potassium which indirectly reflects the amount of the electrolyte present in the body. This potential confounder can be offset by the fact that the patients served as their own control and measuring potassium levels in the same patient at different time points (baseline and follow up) would also negate the minimal effect of diet on urinary potassium levels. Also, diet has a major influence on the urinary sodium levels, whereas potassium levels remain largely unaffected by dietary perturbations23. There was also an evident difference in the changes in urinary potassium between responders and non-responders, and the change in urinary potassium correlated with change in Mayo score (r = 0.38, p = 0.04). 24-hour urine collection has its own fallacies in being cumbersome for patients and there could be chances of error in collection. Therefore, estimating changes in spot urine samples could be a better approach in this direction24. We also did not assess the post-therapy serum albumin and 24-hour urinary excretion of albumin in our patients, as assessment of nutritional and volume status after therapy (i.e. post-therapy albumin and total 24 hour urinary excretion) would vary between responders and non-responders.
In conclusion, we have shown that urinary potassium level is inversely associated with the disease activity in ulcerative colitis and in vitro data suggests that it suppresses inflammation by reducing the IL-17 and IFNγ and inducing the Foxp3 expression on Th17 cells. This study has laid a platform for further research in this field exploring the potential of urinary potassium as a biomarker for intestinal inflammation.
References
Magro, F. et al. Review of the disease course among adult ulcerative colitis population-based longitudinal cohorts. Inflamm. Bowel Dis. 18, 573–583 (2012).
Vermeire, S., Van Assche, G. & Rutgeerts, P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut 55, 426–431 (2006).
Fengming, Y. & Jianbing, W. Biomarkers of inflammatory bowel disease. Dis. Markers 2014, 710915 (2014).
Henriksen, M. et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut 57, 1518–1523 (2008).
Srivastava, S. et al. Serum human trefoil factor 3 is a biomarker for mucosal healing in ulcerative colitis patients with minimal disease activity. J. Crohns Colitis 9, 575–579 (2015).
Giaffer, M. H. Labelled leucocyte scintigraphy in inflammatory bowel disease: clinical applications. Gut 38, 1–5 (1996).
Lin, J.-F. et al. Meta-analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm. Bowel Dis. 20, 1407–1415 (2014).
Yilmaz, R. et al. Dietary salt intake is related to inflammation and albuminuria in primary hypertensive patients. Eur. J. Clin. Nutr. 66, 1214–1218 (2012).
Costa, A. P. R. et al. High sodium intake adversely affects oxidative-inflammatory response, cardiac remodelling and mortality after myocardial infarction. Atherosclerosis 222, 284–291 (2012).
Kleinewietfeld, M. et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496, 518–522 (2013).
Wei, Y. et al. High salt diet stimulates gut Th17 response and exacerbates TNBS-induced colitis in mice. Oncotarget 8, 70–82 (2017).
Hernandez, A. L. et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J. Clin. Invest. 125, 4212–4222 (2015).
Jantsch, J. et al. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab. 21, 493–501 (2015).
Binger, K. J. et al. High salt reduces the activation of IL-4- and IL-13-stimulated macrophages. J. Clin. Invest. 125, 4223–4238 (2015).
Silverberg, M. S. et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. J. Can. Gastroenterol. 19(Suppl A), 5A–36A (2005).
Schroeder, K. W., Tremaine, W. J. & Ilstrup, D. M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 317, 1625–1629 (1987).
Dignass, A. et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J. Crohns Colitis 6, 965–990 (2012).
Young, D. B., Lin, H. & McCabe, R. D. Potassium’s cardiovascular protective mechanisms. Am. J. Physiol. 268, R825–837 (1995).
Monteleone, I. et al. Sodium chloride-enriched Diet Enhanced Inflammatory Cytokine Production and Exacerbated Experimental Colitis in Mice. J. Crohns Colitis 11, 237–245 (2017).
Khalili, H. et al. Identification and Characterization of a Novel Association between Dietary Potassium and Risk of Crohn’s Disease and Ulcerative Colitis. Front. Immunol. 7, 554 (2016).
Wang, W. et al. Renal inflammation is modulated by potassium in chronic kidney disease: possible role of Smad7. Am. J. Physiol. Renal Physiol. 293, F1123–1130 (2007).
Wen, W. et al. Potassium supplementation inhibits IL-17A production induced by salt loading in human T lymphocytes via p38/MAPK-SGK1 pathway. Exp. Mol. Pathol. 100, 370–377 (2016).
Holbrook, J. T. et al. Sodium and potassium intake and balance in adults consuming self-selected diets. Am. J. Clin. Nutr. 40, 786–793 (1984).
Mente, A. et al. Validation and comparison of three formulae to estimate sodium and potassium excretion from a single morning fasting urine compared to 24-h measures in 11 countries. J. Hypertens. 32, 1005–1014; discussion 1015 (2014).
Acknowledgements
This work was supported by Intramural All India Institute of Medical Sciences (AIIMS) grant.
Author information
Authors and Affiliations
Contributions
S.G., R.R.: Study design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision and final approval of the manuscript. S.K., S.M.: Analysis and interpretation of data, drafting of the manuscript, critical revision and final approval of the manuscript. S.B., D.P.Y., S.J., A.K.S., M.N.W., G.M.: Acquisition of data, drafting of the manuscript, critical revision and final approval of the manuscript. A.A.: Study concept and analysis, Lab experiments supervision, critical revision and final approval of the manuscript. V.A.: study concept and design, analysis and interpretation of data; study supervision, drafting of the manuscript, critical revision and final approval of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Goyal, S., Rampal, R., Kedia, S. et al. Urinary potassium is a potential biomarker of disease activity in Ulcerative colitis and displays in vitro immunotolerant role. Sci Rep 7, 18068 (2017). https://doi.org/10.1038/s41598-017-18046-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-18046-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.