Abstract
Many previous magnetic resonance imaging (MRI) studies have documented sex differences in brain morphology, but the patterns of sexual brain differences in transgender women – male sex assigned at birth – with a diagnosis of gender dysphoria (TW) have been rarely investigated to date. We acquired T1-weighted MRI data for the following four (n = 80) groups: treatment-naïve TW (TNTW), TW treated with cross-sex hormones for at least one year (TTW), cisgender men, and cisgender women (cisgender individuals as controls). Differences in whole-brain and regional white matter volume and grey matter volume (GMV) were assessed using voxel-based morphometry. We found lower global brain volumes and regional GMVs in a large portion of the posterior-superior frontal cortex in the cisgender women group than in the TTW and cisgender men groups. Additionally, both transgender groups exhibited lower bilateral insular GMVs than the cisgender women group. Our results highlight differences in the insula in both transgender groups; such differences may be characteristic of TW. Furthermore, these alterations in the insula could be related to the neural network of body perception and reflect the distress that accompanies gender dysphoria.
Similar content being viewed by others
Introduction
Transgender people (frequently referred to as trans people) experience incongruence between their personal sense of gender identity and their sex assigned at birth1. According to the DSM-5 classification, trans people who suffer significant distress associated with their gender perception are diagnosed with gender dysphoria, which is characterized by distress accompanying the incongruence between one’s sex assigned at birth and the gender with which one identifies. Although not all individuals express discomfort as a result of this discordance, many may experience personal anguish if hormone and/or surgical intervention is not available2. Considering that the aetiology of gender dysphoria is unknown, it has been postulated that foetal sexual brain differentiation during the second half of pregnancy does not correspond to the development of the rest of the body in transgender people. This assumption implies that neuroanatomical features reflective of the causative processes that determine gender dysphoria may be detectable in the brain3.
Many neuroimaging studies to date have investigated sex differences in brain morphology. Initial investigations in this field have demonstrated regional brain volume differences between men and women through magnetic resonance imaging (MRI) based on brain regions of interest (ROIs) defined manually or with semiautomated techniques4,5. The introduction of voxel-based morphometry (VBM) has made it feasible to conduct voxel-wise comparisons in an automated fashion across the whole brain. Many subsequent VBM studies have reported findings indicating sexual differences in the human brain regarding the volumes of specific structures6,7. In addition, the results of brain volumetric studies have been complemented by MRI studies that have investigated sex differences in regard to measurements of cortical thickness across the whole brain8,9.
In a recently published meta-analysis of MRI studies on this subject using VBM or tensor-based morphometry (which also affords measures of regional brain volumes and grey matter density), Ruigrock et al. concluded that sexual differences in brain volumes, most notably in the insula (related to human consciousness), limbic structures (emotion, behaviour and motivation, among others), basal ganglia (numerous functions, including control of voluntary motor movements, learning and cognition), thalamus (sensory and motor signals to the cerebral cortex), language-related cortical regions and cerebellum (motor control and cognitive functions), may be detected with MRI10. Furthermore, previous VBM studies have also reported findings of white matter volume (WMV) sex differences in the cerebellum, cerebral lobes and corpus callosum7,11.
All MRI studies in this field to date have suggested that the sex/gender of the brain does not present such well-defined characteristics presented by the genitalia12,13,14. A recent study showed that this distinction would only be possible if sex/gender differences in brain characteristics were highly dimorphic and if brains were inherently congruent with male or female sex/gender. Although this study noted differences in sex/gender and behaviour, the human brain has a variety of individual characteristics that may each be more masculine or feminine. Consequently, human brains cannot be classified into two distinct categories of “male brain” versus “female brain”15. Likewise, there is a growing a social trend considering gender as a continuum, rather than distinct male and female genders16.
The possible brain anatomical features associated with transgender women − male sex assigned at birth − with gender dysphoria diagnosis (TW) have been rarely investigated to date. The first MRI study of TW used a ROI-based method, focusing on the corpus callosum, and did not show differences between TW and cisgender men (CM) or cisgender women (CW) controls17. A decade later, one study reported that the corpus callosum pattern in TW was congruent with gender identity18. To date, four VBM studies investigating differences in regional grey matter volume (GMV) have compared transgender people with cisgender controls. The first of these studies on adult TW showed that the right putamen had a greater GMV in treatment-naïve TW (TNTW) than in CM19. The second study demonstrated greater GMV in the temporo-parietal junction, the inferior frontal cortex and the insular cortex of the right hemisphere in TNTW than in cisgender controls, as well as between-group volumetric differences of the putamen and the thalamus20. The third VBM study showed lower regional GMV in the left angular gyrus and the inferior parietal lobule in the transgender group21. The fourth study evaluated adolescents and noted lower volume of the cerebellum (bilaterally) and the hypothalamus in transgender girls than in cisgender boys22. Two other studies examined cortical thickness measurements in TNTW and demonstrated consistent results, that is, higher cortical thickness in TNTW than in CM controls23,24. Although reported findings have been heterogeneous to date, the above VBM studies detected GMV variations in the angular gyrus, the insula and the putamen of TW19,20,21. These brain regions were hypothesized to be relevant in transgender people because they are related to the neural network of body perception20,21.
Only one previous VBM study of TNTW has assessed regional WMV and verified that TNTW exhibit lower WMV in the precentral area than CW20. On the other hand, the white matter (WM) microstructure of TW has been evaluated using diffusion tensor imaging (DTI) in previous MRI studies. One study showed that the fractional anisotropy of several fasciculi in TNTW was halfway between those of CM and CW controls25. Another study did not find differences in fractional anisotropy between TNTW and cisgender controls, although the mean diffusivity in CW and TNTW was higher than that in CM26. Overall, these MRI studies have provided some evidence supporting the view that there may be regional GMV and WMV differences between TW and cisgender controls. However, the patterns detected thus far are not clearly associated with female brain characteristics, thus raising the possibility that TW-related brain volume differences are not necessarily related to signs of brain feminization27.
All the abovementioned VBM studies of adult TW were performed before cross-sex hormone treatment (CHT). However, it is important to note that most TW have a history of prolonged use of sex steroids. Three VBM studies have investigated the influence of circulating sex steroids on cisgender individuals and have reported the following findings: (i) sex hormones influence the organization of the grey matter (GM) in cortical regions during adolescence and adulthood6, (ii) increased levels of circulating testosterone during puberty in boys are associated with sexual differences in the amygdala and the hippocampus28, and (iii), increased levels of 17β-oestradiol in girls are related to sexual differences in GM29. Another ROI-based MRI study investigated the influence of CHT for four months in TW and demonstrated the presence of brain volume reductions towards female proportions30. In another MRI study, cortical thickness in TW was assessed prior to and after six months of CHT, and evidence that sex steroids are associated with reduced cortical and subcortical structures was reported31.
To further analyse possible brain anatomical features associated with TW and the influence of CHT, the present cross-sectional MRI study aimed to investigate differences in whole-brain and regional GMV and WMV by VBM in two TW groups: TNTW and TW after at least one year of CHT (TTW). These TW groups were compared with CM and CW as control groups. Moreover, by applying the small-volume correction (SVC) method, we intended to replicate the findings of previous MRI investigations for the following brain regions: the angular gyrus, the insula and the putamen19,20,21. The corpus callosum, which has also been previously reported to present changes in TW18, was additionally assessed by SVC in the current study.
Results
Sample characteristics
Table 1 shows the sociodemographic and clinical data for all groups. TW and controls did not differ in age, but the schooling level was significantly lower in TNTW than in controls. The total testosterone level was significantly lower in TTW than in TNTW (p < 0.001), although the oestradiol level was not significantly different between the two groups (p = 0.892). No changes in secondary sexual characteristics, which were evaluated by physical examination, were reported for the TNTW group.
Imaging data
Total brain volumetric differences
The total brain volumes of the four groups are shown in Table 2. There were no significant between-group differences in the total brain volumes between the TW groups. All brain compartments showed reduced global volumes in the CW group compared with the three other groups (Table 2).
Regional GMV differences
In the whole-brain exploratory analysis, statistical parametric mapping (SPM) for ANCOVA comparing regional GMVs between groups showed one large voxel cluster corresponding to a between-group difference with a peak statistical significance in the left superior medial frontal gyrus [Brodmann area (BA) 6–1240 voxels, peak voxel at x = 0, y = −7, z = 67; Z = 5.09, pFWE = 0.01 corrected for multiple comparisons] that extended bilaterally to the supplementary motor cortex and the paracentral lobule. Post hoc two-group comparisons (see Table 3 for details) revealed that the GMV of this large voxel cluster encompassing these three brain regions was greater in the TTW group than in the CW group. Although not significant after correcting for multiple comparisons, the GMV of the same voxel cluster also tended to be higher in the TNTW group than in the CW group (peak voxel at x = 0, y = −12, z = 75; Z = 2.42, p = 0.002 uncorrected for multiple comparisons). Finally, CM also exhibited significantly higher GMVs in the bilateral paracentral lobule, the bilateral supplementary motor cortex and the left precentral gyrus than CW (Table 3).
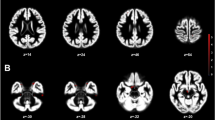
We further evaluated the SPM for ANCOVA comparing regional GMVs in a priori-selected brain regions between groups using the SVC approach, and we observed additional significant findings in the left insula [BA 13–243 voxels, peak voxel at x = −41, y = −9, z = 6; Z = 3.78, pFWE = 0.023 corrected for multiple comparisons]. Post hoc two-group comparisons showed lower GMVs in the bilateral insula in both TW groups than in the CW group (Fig. 1) and a lower GMV in the left insula in the TTW group than in the CM group (Table 3). No additional GMV differences were observed in any other a priori-selected brain regions.
Findings showing clusters of lower grey matter volumes in the insular cortex of both hemispheres, highlighted in yellow: (a) in treatment-naïve TW compared with cisgender women controls and (b) in hormone-treated TW compared with cisgender women controls. Region-of-interest masks of the left and right insular cortex were superimposed on the images. Foci of significance are overlaid on axial brain slices spatially normalized into an approximation to the Talairach and Tournoux stereotactic atlas, and the numbers associated with each frame represent standard coordinates in the z-axis. All voxel clusters shown in the figures retained statistical significance after family-wise error correction for multiple comparisons (pFWE < 0.05, with small-volume correction over the insula) and had a minimum extent threshold of 30 voxels. Statistical details are given in Table 3. The coloured bar represents T-values. (TW = transgender women diagnosed with gender dysphoria; R = right).
Regional WMV differences
No significant regional WMV differences were observed among the groups in the exploratory whole-brain analysis (pFWE < 0.05). Further evaluation of the SPM for ANCOVA comparing regional WMVs in a priori-selected brain regions between groups using the SVC approach revealed no statistically significant clusters (pFWE < 0.05).
Discussion
The present VBM study investigated GMV and WMV patterns in two TW groups that were well-characterized regarding their sexual orientation and non-conformity to their sex assigned at birth from an early age. The TNTW and TTW subjects were recruited before beginning CHT and at least one year after beginning CHT, respectively, which allowed us to differentiate brain volume findings related to TW or CHT.
The lower total brain volume in CW observed here is consistent with a previously reported profile of sexual differences in the brain10. The finding that the three other groups presented total brain volumes larger than the CW group indicates that these brain volume differences are congruent with the sex assigned at birth in both TW groups27. Additionally, no differences in total brain volume were found between the TTW group and the TNTW and CM groups in the present cross-sectional study, although overall effects of CHT on reductions in total intracranial volume (TIV) and total GMV have been observed in other longitudinal MRI studies of TW undergoing CHT30,31.
Regarding regional GMV, we found lower GMV bilaterally in the insula in both TW groups, in contrasts to the results reported by Savic and Arver (2011), who found higher GMV in the right insula of the TW group relative to controls20. Likewise, Zubiarre-Elorza et al. (2013) verified greater cortical thickness in the right insula in TNTW than in CM controls23. Another MRI study conducted by Zubiarre-Elorza et al. (2014) reported decreased right insular volumes in TW undergoing CHT31. Our finding in the insula is unlikely directly due to CHT because the lower GMV of the insula was observed for both TW groups compared with the CW group. Furthermore, brain volume and cortical thickness are widely considered as complementary brain measurements32,33.
Manzouri et al.34 recently conducted a morphological and functional MRI study including treatment-naïve transgender men - female sex assigned at birth - and cisgender controls. The GMV differences found in the transgender men group followed patterns related to the sex assigned at birth. However, the authors also demonstrated weaker functional connections from the pregenual anterior cingulate to the insular cortex and to the temporo-parietal junction in the transgender men than in controls. These findings also suggest that transgender men differ from cisgender people with respect to the own-body image neural network, which may be a neurobiological substrate related to transgender men34. Similarly, Savic and Arver (2011) and Simon et al. (2013) suggested that regional GMV differences detected in transgender people emphasize the brain regions related to the body perception network20,21.
It is worth mentioning that an important diagnostic criterion for gender dysphoria is the distress that accompanies the incongruity between the body and gender identity, as the secondary sexual characteristics do not belong to the gender with which one identifies35. Decreased insular volumes have been reported in depressed subjects with melancholic symptoms36, and these changes in insular and prefrontal cortical volumes may be specifically associated with the manifestation of psychotic symptoms in major depressive disorder37. The insula is related to all subjective sensations and is the possible foundation of interoception38, including body awareness39. Furthermore, the insula connects the distinct functional systems involved in processing emotions, sensory-motor skills and cognition40. Two studies have evaluated functional connectivity in transgender people and found (i) a pattern of neural connectivity that inferred suffering due to incongruity between sex assigned at birth and gender identity41, and (ii) that the connectivity between the right insula and the somatosensory cortex was negatively related to ratings on a well-being scale in regard to gender dysphoria42. Another study examined brain structural connectivity and observed unique differences in regional network efficiency in the insular area in trans people43. These observations, together with our findings of reduced GMVs in the insula in both hemispheres in two independent TW groups, suggest that such regional GMV differences could be characteristics associated with TW. Furthermore, these alterations in the insula could be related to the neural network of body perception and reflect the distress that accompanies gender dysphoria. The hypothesis that insular volume variations in TW individuals reflect the distress of these individuals should be considered in future studies including large TW samples by evaluating significant correlations between GMV and symptom severity ratings. In addition, it is well known that the symptoms of gender dysphoria improve with CHT35. As a consequence of symptom relief, the TTW group could present a greater insular volume than the TNTW group. However, this possibility was not verified in the current study, which suggests that GMV differences in the insular cortex in both TW groups may be related to a more general “transgender trait”.
One important difference between our VBM study and the study performed by Savic and Arver (2011) is that the latter investigated non-androphilic TW (sexually attracted to females, both males and females, or neither gender)20 according to the Blanchard classification, which is based on the sex assigned at birth of TW44, while our study investigated androphilic TW (sexually attracted to males). Therefore, one might speculate that the differences observed in the insular cortex could be linked to the sexual orientation of TW. It should be noted, however, that sexual orientation and gender identity are distinct phenomena. For this reason, overlapping VBM findings associated with these two conditions are highly speculative. Another VBM study assessing hetero- and homosexual CM did not identify any regional GMV differences between groups. Homosexual CW presented lower GMV in the perirhinal cortex than heterosexual CW45. Considering these speculative findings, further MRI studies comparing androphilic TW with non-androphilic TW are required to reveal further information on this topic.
We also detected lower regional GMV in a large portion of the posterior-superior frontal cortex in the CW controls than in both TW groups and the CM controls. Because our statistical approach was primarily designed to identify regional GMV variations that could be related to gender dysphoria, direct post hoc two-group comparisons between the cisgender controls in our study should not be considered as comprehensive indicators of a predominant profile between the two control groups regarding regional brain volume differences. In addition, the lower GMV detected in the posterior-superior frontal cortex in the CW group is consistent with previously described regional patterns of sexual differences of the human brain10,46. Significantly higher GMV in the posterior-superior frontal cortex was also detected only in the TTW and CM groups relative to the CW group. Conversely, the difference in regional GMV in this frontal region in the TNTW group relative to the CW group was not significant after correcting for multiple comparisons. These results of the present study may indicate that this brain region in TNTW tends to present female characteristics, although this conclusion should be interpreted with caution.
Based on the abovementioned interpretation, the finding of higher GMV in the posterior-superior frontal cortex in the TTW group might seem contradictory, given the overall feminizing effects of CHT. Nevertheless, previous studies have shown increased GMV in several cortical regions in postmenopausal women under oestrogen replacement therapy47, including higher GM density in paracentral and precentral areas48 and in the superior frontal gyrus46. Such findings are believed to reflect the influence of hormonal treatment on brain neuroplasticity.
Overall, our results suggest that the impact of CHT on brain volume may vary across regional and global levels, as we found no difference in total brain volume between the two TW groups, despite the fact that the TTW had undergone prolonged use of sex steroids. A similar study conducted by Hoekzema et al. (2014) revealed that the neuroanatomical characteristics of treatment-naïve transgender adolescents and transgender adolescents undergoing CHT corresponded to those of individuals of the same sex assigned at birth.
Such results are in contrast to the findings of previous studies of TW using repeated MRI measurements before and during CHT. Hulshoff Pol et al. (2006) reported reduced global brain volumes in TW after four months of CHT towards the volumes expected for CW30. Similar to our TW sample, Zubiaurre-Elorza et al. (2014) investigated TW after at least six months of hormonal treatment and reported reduced cortical thicknesses and volumes of subcortical structures in the right hemisphere (specifically in the pallidum and the thalamus) and an enlarged ventricular system31. A recent study showed increased ventricle and reduced right hippocampal volumes in TW after four months of CHT49. These differences between our results and those of previous investigations may be because the present study employed a cross-sectional design, while the previous studies were longitudinal investigations of the same individuals and, thus, may have been more sensitive to detecting CHT-related global volume changes over time in TW.
Our findings, as well as the results of all previous brain imaging studies that have evaluated the influence of CHT, suggest that the effects of CHT on brain volume may differ at global and regional levels and may vary across brain structures. Moreover, the different results obtained for the two TW groups in our study suggest that the association of brain volumes with gender dysphoria and CHT may be complex, and furthermore, the direction of associations of brain volume with these two dimensions may vary.
Conflicting VBM-based findings among MRI studies of TW regarding subcortical GM structures have been reported to date. In the first VBM study to evaluate TW, Luders et al. (2009) identified a greater GMV in the right putamen19. Through SVC-based analyses, Savic and Arver (2011) also reported that TW subjects exhibit a greater GMV in the right putamen than control subjects; conversely, ROI-based measurements indicated reduced volumes of the putamen in TW relative to controls20. In the present study, the SVC-based analysis did not reveal significant group differences in voxels located specifically in the basal ganglia (although relatively larger clusters of lower GMV in the insula extending towards the right putamen were observed in both TW groups, as shown in Fig. 1). Finally, the paucity of between-group differences in the WM analyses in our study suggests that brain volume differences related to the effects of both TW and CHT predominate in the GM compartment of the brain.
Some methodological limitations of our study must be considered. First, because this study employed a cross-sectional design, it was not possible to determine which changes were pre-existing and which resulted from CHT. Second, our sample size can be considered small (n = 80) and may not confer confidence in the results obtained, although the sample size was similar to those in previous MRI studies in this field20,23,43. Third, variability existed in the duration of CHT among individuals in the TTW group. Finally, the stage of the menstrual cycle of the CW was not considered at the time of MRI scanning, which may be a relevant factor considering that brain structures are influenced by hormonal physiological variations50.
In conclusion, significant regional brain volume differences in this VBM study were detected in TW compared with cisgender controls. In TTW, the observed brain volume changes suggested a possible influence of CHT on brain neuroplasticity46,47. Finally, we present a novel finding of GMV alterations in the insula in the two independent TW groups, which may be a characteristic of TW. These alterations in the insula could be related to the neural network of body perception and may reflect the distress that accompanies gender dysphoria.
Methods
Sample
Inclusion criteria for all TW were as follows: (a) absence of changes (except for changes resulting from the use of CHT by TTW) in secondary male sex characteristics according to the Marshall and Tanner criteria51, as evaluated by a physical examination performed by endocrinologists at HC-FMUSP; (b) a history of non-conformity to birth sex since the early stages of child development; (c) compatibility with gender dysphoria diagnostic criteria as per the DSM-5 and based on semi-structured interviews conducted by three mental health professionals (one psychiatrist and two psychologists); and (d) exclusive sexual attraction to males according to the Kinsey scale of sexual orientation52, as ascertained by actively asking TW about their sexual attraction (i.e., reports on desire, thoughts, fantasies and/or sexual activity with men, including romantic ideas). Specific inclusion criteria for the TNTW group were as follows: the absence of any hormonal treatment history and the expressed desire to be treated with CHT. The procedures performed in TNTW included evaluations of the plasma levels of oestradiol, follicle-stimulating hormone, luteinizing hormone, testosterone and free testosterone on the day of MRI. For TTW, the specific inclusion criterion was currently receiving CHT for at least one year, as recommended by the Endocrinology Service at HC-FMUSP. This treatment regimen consisted of the administration of one or two 0.625-mg conjugated oestrogen tablets and one 50-mg cyproterone acetate pill per day53. Blood hormone levels of the TTW group were evaluated every semester. Hormone dosages of all TW were calculated in the Hormones Laboratory and Molecular Genetics LIM-42 at HC-FMUSP via immunofluorometric assays using AutoDELFIA kits (PerkinElmer Inc., Waltham, MA, USA). For the cisgender controls, the inclusion criterion was heterosexual orientation, as determined by the Kinsey scale of sexual orientation52.
Exclusion criteria based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID)54 for all groups included a history of psychiatric disorders and prior use of psychopharmacological agents. Additional exclusion criteria included the presence of medical conditions or neurological disorders that could affect the central nervous system, contraindications to MRI, major medical illnesses, and substance abuse.
The study sample included 80 individuals between 18 and 49 years of age divided into the following four groups (n = 20 each): TNTW, TTW, CW and CM (cisgender individuals as healthy controls). The selection of TNTW was based on a waiting list of those individuals who identified themselves as transgender women and who were awaiting treatment at HC-FMUSP, contacts with non-governmental organizations that provide assistance to transgender people and contacts with mental health expert clinicians who practice in São Paulo and who were able to refer patients for the study. The TTW group was selected from among those patients who were already receiving treatment at HC-FMUSP. Healthy voluntary controls were verbally invited among community and staff members at HC-FMUSP.
All participants were fully informed about the study and signed a consent form. This study followed the principles of the Declaration of Helsinki and was approved by the Ethics Committee at the Medical School Hospital, University of São Paulo (HC-FMUSP), Brazil.
Image acquisition and processing
All MRI scans were acquired using a 1.5 T Siemens Espree system (Siemens, Erlangen, Germany) at the Institute of Psychiatry, HC-FMUSP. Morphological brain data were acquired using a T1-weighted magnetization-prepared rapid gradient echo sequence (MPRAGE) with the following parameters: TR = 2,400 ms; TE = 3.65 ms; NEX = 1; field of view (FOV) = 240 mm; flip angle = 8o; matrix = 192 × 192 pixels; slice thickness = 1.2 mm (no gap between slices); and voxel size = 1.25 × 1.25 × 1.2 mm, resulting in 160 slices covering the whole brain.
All images were reviewed by a neuroradiologist with the purpose of identifying artefacts during image acquisition and the presence of silent gross brain alterations. VBM processing was performed using the Statistical Parametric Mapping (SPM) program, version 8 (Wellcome Trust Centre of Neuroimaging, London, United Kingdom), implemented in MATLAB R2012a (MathWorks, Sherborn, Massachusetts). First, all anatomical images were reoriented; the mm coordinate of the anterior commissure matched the x y z origin (0, 0, 0), and the orientation approximated Montreal Neurological Institute (MNI) space. The images were then segmented into GM, WM and cerebrospinal fluid (CSF) partitions using the unified segmentation procedure described by Ashburner and Friston55. The Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) algorithm was then used to spatially normalize the segmented images. This procedure maximizes sensitivity and the accuracy of localization by registering individual structural images to an asymmetric T1-weighted template derived from participants’ structural images rather than a standard T1-weighted template based on a different sample56.
These fully normalized images were resliced through trilinear interpolation to a final voxel size of 1.5 × 1.5 × 1.5 mm³. An additional “modulation” step consisted of multiplying each spatially normalized GM and WM image by its relative volume before and after normalization. This step ensured that the total amount of GM and WM in each voxel was preserved. Finally, the resulting GM and WM images were smoothed using an 8-mm isotropic kernel at full width at half maximum (FWHM) to ensure normal distribution of the data as required by subsequent statistical parametric tests.
Statistical analysis
Demographical and clinical data
The Statistical Package for the Social Sciences (SPSS) for Windows, version 18.0 (SPSS, Chicago, IL, USA) was used. All statistical tests were conducted with a 5% significance level. Age and education in years were compared between groups using the Kruskal-Wallis test. Differences in hormonal levels between the TW groups were verified by the Mann-Whitney U-test. Fisher’s exact test was used to evaluate handedness.
Neuroimaging data
Total GM, WM and CSF volumes were calculated by the MATLAB get_totals script (http://www.cs.ucl.ac.uk/staff/g.ridgway/vbm/get_totals.m) implemented for SPM8 using the segmented images on the native space for each subject. Between-group differences in total GM, CSF and WM volumes, total tissue volume (TV, calculated as GM + WM), and TIV (calculated as GM + WM + CSF) were tested with analysis of variance (ANOVA) using SPSS. Age and years of education were included as covariates in such comparisons.
Regarding the VBM analyses, we initially performed exploratory voxel-wise comparisons of regional GMVs and WMVs among the 4 groups across the whole brain using an analysis of covariance (ANCOVA) model in SPM (F test). Given the between-group differences in years of education, this variable was included as a covariate in such comparisons. Although the mean age was not significantly different in between groups, age was also entered as a further confounding covariate. The age range of the overall sample was relatively wide (from 18 to 49 years), and in such situations, previous VBM studies have shown that it is appropriate to include age as a confounding covariate in between-group comparisons57. The measures of total GM or WM in the brain were also entered as confounders in the respective ANCOVA. Only voxels with values above an absolute threshold of p = 0.05 for differentiating GM and WM from other tissues were included in the analyses. Significant ANCOVA findings were followed up with post hoc tests for two-group comparisons (t tests). The resulting statistics were thresholded at a p < 0.001 (uncorrected for multiple comparisons) level of significance (Z = 3.09) and displayed as SPMs in standard anatomical space. Only clusters with a minimum of 30 voxels were reported (30 voxels correspond to approximately 100 mm3; this threshold has been used in previous studies21,58 and offers an easy-to-understand size scale for the reader). Findings in these exploratory analyses were considered significant if they survived family-wise error (FWE) correction for multiple comparisons over the whole brain (pFWE ≤ 0.05).
Subsequently, each statistical map was inspected for the presence of clusters of significant differences in brain regions where volumetric differences had been predicted a priori through the SVC method, with the purpose of constraining the total number of voxels included in the analysis. Each GM region was spatially delimited by applying masks onto the SPMs, based on the anatomical volumes of interest that are available within the automatic anatomical labelling SPM toolbox (http://www.gin.cnrs.fr/AAL). All the anatomical masks were used separately in each hemisphere. SVC was applied over the following brain regions: the angular gyrus, the insula and the putamen. These regions were predicted a priori to show differences in VBM studies of TNTW19,20,21. For SVC-based analyses of WM regions18, ROIs over the corpus callosum (body, genu, and splenium) were defined based on the JHU DTI-based WM atlases59,60,61. Findings for these hypothesis-driven, SVC-based analyses were reported as significant if they survived FWE correction for multiple comparisons (pFWE ≤ 0.05) over the search volume of each ROI, with a cluster size threshold of 30 contiguous voxels.
References
World Professional Association for Transgender Health. Standards of care for the health of transsexual, transgender, and gender nonconforming people (7th ed.) (Minneapolis, WPATH, 2012).
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.) (American Psychiatric Publishing, 2013).
Bao, A.-M. & Swaab, D. F. Sexual differentiation of the human brain: Relation to gender identity, sexual orientation and neuropsychiatric disorders. Frontiers in Neuroendocrinology 32, 214–226, https://doi.org/10.1016/j.yfrne.2011.02.007 (2011).
Allen, L. S., Richey, M. F., Chai, Y. M. & Gorski, R. A. Sex differences in the corpus callosum of the living human being. J Neurosci 11, 933–942 (1991).
Goldstein, J. M. et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex 11, 490–497 (2001).
Witte, A. V., Savli, M., Holik, A., Kasper, S. & Lanzenberger, R. Regional sex differences in grey matter volume are associated with sex hormones in the young adult human brain. Neuroimage 49, 1205–1212, https://doi.org/10.1016/j.neuroimage.2009.09.046 (2010).
Good, C. D. et al. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage 14, 685–700, https://doi.org/10.1006/nimg.2001.0857 (2001).
Sowell, E. R. et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex 17, 1550–1560, https://doi.org/10.1093/cercor/bhl066 (2007).
Lv, B. et al. Gender consistency and difference in healthy adults revealed by cortical thickness. Neuroimage 53, 373–382, https://doi.org/10.1016/j.neuroimage.2010.05.020 (2010).
Ruigrok, A. N. et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev 39, 34–50, https://doi.org/10.1016/j.neubiorev.2013.12.004 (2014).
Lai, M.-C. et al. Biological sex affects the neurobiology of autism. Brain 136, 2799–2815, https://doi.org/10.1093/brain/awt216 (2013).
Witelson, S. F. Neural sexual mosaicism: sexual differentiation of the human temporo-parietal region for functional asymmetry. Psychoneuroendocrinology 16, 131–153 (1991).
Cahill, L. Why sex matters for neuroscience. Nat Rev Neurosci 7, 477–484 (2006).
McCarthy, M. M. & Arnold, A. P. Reframing sexual differentiation of the brain. Nat Neurosci 14, 677–683 (2011).
Joel, D. et al. Sex beyond the genitalia: The human brain mosaic. Proceedings of the National Academy of Sciences of the United States of America 112, 15468–15473, https://doi.org/10.1073/pnas.1509654112 (2015).
Meyer-Bahlburg, H. F. L. et al. Gender Assignment, Reassignment and Outcome in Disorders of Sex Development: Update of the 2005 Consensus Conference. Hormone Research in Paediatrics 85, 112–118 (2016).
Emory, L. E., Williams, D. H., Cole, C. M., Amparo, E. G. & Meyer, W. J. Anatomic variation of the corpus callosum in persons with gender dysphoria. Arch Sex Behav 20, 409–417 (1991).
Yokota, Y., Kawamura, Y. & Kameya, Y. Callosal Shapes at the Midsagittal Plane: MRI Differences of Normal Males, Normal Females, and GID. Conf Proc IEEE Eng Med Biol Soc 3, 3055–3058, https://doi.org/10.1109/iembs.2005.1617119 (2005).
Luders, E. et al. Regional gray matter variation in male-to-female transsexualism. Neuroimage 46, 904–907, https://doi.org/10.1016/j.neuroimage.2009.03.048 (2009).
Savic, I. & Arver, S. Sex dimorphism of the brain in male-to-female transsexuals. Cereb Cortex 21, 2525–2533, https://doi.org/10.1093/cercor/bhr032 (2011).
Simon, L. et al. Regional grey matter structure differences between transsexuals and healthy controls–a voxel based morphometry study. PLoS One 8, e83947, https://doi.org/10.1371/journal.pone.0083947 (2013).
Hoekzema, E. et al. Regional volumes and spatial volumetric distribution of gray matter in the gender dysphoric brain. Psychoneuroendocrinology 55, 59–71, https://doi.org/10.1016/j.psyneuen.2015.01.016 (2015).
Zubiaurre-Elorza, L. et al. Cortical thickness in untreated transsexuals. Cerebral Cortex (New York, N.Y.: 1991) 23, 2855–2862, https://doi.org/10.1093/cercor/bhs267 (2013).
Luders, E. et al. Increased Cortical Thickness in Male-to-Female Transsexualism. J Behav Brain Sci 2, 357–362, https://doi.org/10.4236/jbbs.2012.23040 (2012).
Rametti, G. et al. White matter microstructure in female to male transsexuals before cross-sex hormonal treatment. A diffusion tensor imaging study. J Psychiatr Res 45, 199–204, https://doi.org/10.1016/j.jpsychires.2010.05.006 (2011).
Kranz, G. S. et al. White matter microstructure in transsexuals and controls investigated by diffusion tensor imaging. J Neurosci 34, 15466–15475, https://doi.org/10.1523/jneurosci.2488-14.2014 (2014).
Guillamon, A., Junque, C. & Gómez-Gil, E. A Review of the Status of Brain Structure Research in Transsexualism. Archives of Sexual Behavior 45, 1615–1648, https://doi.org/10.1007/s10508-016-0768-5 (2016).
Neufang, S. et al. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex 19, 464–473, https://doi.org/10.1093/cercor/bhn100 (2009).
Peper, J. S. et al. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology 34, 332–342, https://doi.org/10.1016/j.psyneuen.2008.09.012 (2009).
Hulshoff Pol, H. et al. Changing your sex changes your brain: influences of testosterone and estrogen on adult human brain structure. European Journal of Endocrinology 155, S107–S114, https://doi.org/10.1530/eje.1.02248 (2006).
Zubiaurre-Elorza, L., Junque, C., Gómez-Gil, E. & Guillamon, A. Effects of cross-sex hormone treatment on cortical thickness in transsexual individuals. J Sex Med 11, 1248–1261, https://doi.org/10.1111/jsm.12491 (2014).
Hutton, C., Draganski, B., Ashburner, J. & Weiskopf, N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage 48, 371–380, https://doi.org/10.1016/j.neuroimage.2009.06.043 (2009).
Kong, L. et al. Comparison of grey matter volume and thickness for analysing cortical changes in chronic schizophrenia: A matter of surface area, grey/white matter intensity contrast, and curvature. Psychiatry Research: Neuroimaging 231, 176–183, https://doi.org/10.1016/j.pscychresns.2014.12.004 (2015).
Manzouri, A., Kosidou, K. & Savic, I. Anatomical and Functional Findings in Female-to-Male Transsexuals: Testing a New Hypothesis. Cerebral Cortex (2015).
Fisher, A. D. et al. Cross-Sex Hormonal Treatment and Body Uneasiness in Individuals with Gender Dysphoria. The Journal of Sexual Medicine 11, 709–719, https://doi.org/10.1111/jsm.12413 (2014).
Soriano-Mas, C. et al. Cross-Sectional and Longitudinal Assessment of Structural Brain Alterations in Melancholic Depression. Biological Psychiatry 69, 318–325, https://doi.org/10.1016/j.biopsych.2010.07.029 (2011).
Busatto, G. F. Structural and functional neuroimaging studies in major depressive disorder with psychotic features: a critical review. Schizophr Bull 39, 776–786, https://doi.org/10.1093/schbul/sbt054 (2013).
Craig, A. D. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci 10, 59–70, https://doi.org/10.1038/nrn2555 (2009).
Heydrich, L. & Blanke, O. Distinct illusory own-body perceptions caused by damage to posterior insula and extrastriate cortex. Brain 136, 790–803 (2013).
Chang, L. J., Yarkoni, T., Khaw, M. W. & Sanfey, A. G. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex 23, 739–749, https://doi.org/10.1093/cercor/bhs065 (2013).
Ku, H. L. et al. Brain signature characterizing the body-brain-mind axis of transsexuals. PLoS One 8, e70808, https://doi.org/10.1371/journal.pone.0070808 (2013).
Lin, C. S. et al. Neural network of body representation differs between transsexuals and cissexuals. PLoS One 9, e85914, https://doi.org/10.1371/journal.pone.0085914 (2014).
Hahn, A. et al. Structural Connectivity Networks of Transgender People. Cerebral Cortex 25, 3527–3534 (2015).
Blanchard, R. The classification and labeling of nonhomosexual gender dysphorias. Arch Sex Behav 18, 315–334 (1989).
Ponseti, J. et al. Homosexual women have less grey matter in perirhinal cortex than heterosexual women. PLoS One 2, e762, https://doi.org/10.1371/journal.pone.0000762 (2007).
Lord, C., Engert, V., Lupien, S. J. & Pruessner, J. C. Effect of sex and estrogen therapy on the aging brain: a voxel-based morphometry study. Menopause 17, 846–851, https://doi.org/10.1097/gme.0b013e3181e06b83 (2010).
Boccardi, M. et al. Effects of hormone therapy on brain morphology of healthy postmenopausal women: a Voxel-based morphometry study. Menopause 13 (2006).
Robertson, D. et al. Effects of estrogen therapy on age-related differences in gray matter concentration. Climacteric 12, 301–309, https://doi.org/10.1080/13697130902730742 (2009).
Seiger, R. et al. Subcortical gray matter changes in transgender subjects after long-term cross-sex hormone administration. Psychoneuroendocrinology 74, 371–379, https://doi.org/10.1016/j.psyneuen.2016.09.028 (2016).
Catenaccio, E., Mu, W. & Lipton, M. L. Estrogen- and progesterone-mediated structural neuroplasticity in women: evidence from neuroimaging. Brain Structure and Function 1–23, https://doi.org/10.1007/s00429-016-1197-x (2016).
Marshall, W. A. & Tanner, J. M. Variations in the Pattern of Pubertal Changes in Boys. Archives of Disease in Childhood 45, 13–23 (1970).
Kinsey, A. C., Pomeroy, W. R. & Martin, C. E. Sexual behavior in the human male. 1948. Am J Public Health 93, 894–898 (2003).
Costa, E. M. & Mendonca, B. B. Clinical management of transsexual subjects. Arq Bras Endocrinol Metabol 58, 188–196 (2014).
First, M., Spitzer, R., Gibbon, M. & Williams, J. Structured clinical interview for DSM-IV-TR Axis I disorders, research version, non-patient edition. (SCID-I/NP). (Biomedics Research, New York State Psychiatric Institute, 2002).
Ashburner, J. & Friston, K. J. Unified segmentation. Neuroimage 26, 839–851, https://doi.org/10.1016/j.neuroimage.2005.02.018 (2005).
Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113, https://doi.org/10.1016/j.neuroimage.2007.07.007 (2007).
Barnes, J. et al. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage 53, 1244–1255, https://doi.org/10.1016/j.neuroimage.2010.06.025 (2010).
Ferreira, L. K., Diniz, B. S., Forlenza, O. V., Busatto, G. F. & Zanetti, M. V. Neurostructural predictors of Alzheimer’s disease: A meta-analysis of VBM studies. Neurobiology of Aging 32, 1733–1741, https://doi.org/10.1016/j.neurobiolaging.2009.11.008 (2011).
Mori, S., Wakana, S., Nagae-Poetscher, L. & Van Zijl, P. MRI Atlas of Human White Matter. (Amsterdam, Elsevier, 2005).
Wakana, S. et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36, 630–644, https://doi.org/10.1016/j.neuroimage.2007.02.049 (2007).
Hua, K. et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 39, 336–347, https://doi.org/10.1016/j.neuroimage.2007.07.053 (2008).
Acknowledgements
We thank Dr. Elaine Frade Costa, Dr. Sorahia Domenice and the team for the physical evaluations conducted on transgender women. This research was funded by FAPESP-Brazil (2012/19165-7).
Author information
Authors and Affiliations
Contributions
Conception and design of study: G.S., G.F.B., C.H.N.A. Acquisition of data (laboratory or clinical): G.S., F.L.S.D., T.M.C.A., M.H.S., M.C., C.M.A.P., P.P.S., P.S., N.A.C. Data analysis and/or interpretation: G.S., G.F.B., F.L.S.D., C.H.N.A. Drafting of manuscript and/or critical revision: G.S., G.F.B., F.L.S.D., T.M.C.A., M.H.S., M.C., C.H.N.A. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Spizzirri, G., Duran, F.L.S., Chaim-Avancini, T.M. et al. Grey and white matter volumes either in treatment-naïve or hormone-treated transgender women: a voxel-based morphometry study. Sci Rep 8, 736 (2018). https://doi.org/10.1038/s41598-017-17563-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-17563-z
This article is cited by
-
Accurate sex prediction of cisgender and transgender individuals without brain size bias
Scientific Reports (2023)
-
Biological sex classification with structural MRI data shows increased misclassification in transgender women
Neuropsychopharmacology (2020)
-
What Drives Support for Transgender Rights? Assessing the Effects of Biological Attribution on U.S. Public Opinion of Transgender Rights
Sex Roles (2020)
-
What has sex got to do with it? The role of hormones in the transgender brain
Neuropsychopharmacology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.