Abstract
Recently, two dimensional transition metal dichalcogenides become popular research topics because of their unique crystal and electronic structure. In this work, the geometrical structure, electronic, electrical transport, redox potentials and photocatalytic properties of nonmetal (H, B, C, Si, N, P, As, O, S, Te, F, Cl, Br and I) doped monolayer MoSe2 were investigated by first principle calculations. The binding energy indicates that nonmetal doped MoSe2 are energetically favorable compared to Se vacancies, except B- and C-doped. We have found that nonmetal dopants with an even number of valence electrons doped MoSe2 have p-type conductivity. On the contrary, nonmetal dopants with an odd number of valence electrons doped MoSe2 have p-type or n-type conductivity; and they have better photocatalytic performance.
Similar content being viewed by others
Introduction
Transition metal dichalcogenides (TMDCs) have unique structural and electronic properties. Consequently, they possess various conducting properties that include insulating, semiconducting, conducting and even superconducting. And these cause potential applications in electronics, spintronics and optoelectronics1,2,3,4,5,6,7,8. TMDCs family has more than 40 compounds (including MoS2, WS2, MoSe2, WSe2 and WTe2 etc) with the formula of MX2 9,10. These TMDCs materials have been obtained by micromechanical exfoliation or liquid exfoliation (due to the weak van der Waals bonding interactions in the adjacent sandwiched layers)11,12,13, chemical vapor deposition (CVD)14 and molecular beam epitaxy (MBE)15,16. However, vacancies inevitably occur in the growth process of TMDCs17,18,19.
The introduction of dopant atoms into the vacancies is considered as a promising method of modulating their electronic, magnetic and transport properties of monolayer TMDCs20,21,22,23,24,25,26,27,28,29. Experimentally, Zn- and Co-doped MoS2 nanosheets have been realized and demonstrate potential applications in the field of electronics, optoelectronics, spintronics and photocatalysis30,31. Moreover, a series of work have reported that ultrathin N- and P-doped MoS2 nanosheets have demonstrated enhanced hydrogen evolution reaction (HER) catalysis, enhanced oxygen reduction reaction (ORR) and efficient degradation of methyl orange and RhB, respectively32,33,34,35,36. This is due to they can promote the charge transfer in the photocatalytic reaction. These results suggest that doping nonmetal (NM) atoms is an effective way to promote the catalytic performance.
However, the above articles lack the calculations on the electrical transport of NM doped systems. Moreover, systematically understanding the modification on the physical and chemical properties of the NM doped monolayer MoSe2 is necessary. In this work, we provide a comprehensive investigation on the geometrical structure, binding energy, electronic, optical and photocatalytic properties of substitutionally doped monolayer MoSe2 with a series of NM atoms, such as H, B, C, Si, N, P, As, O, S, Te, F, Cl, Br and I, by employing first-principle calculations. And we found the parity of the valence electrons of the dopants causes a dramatic difference on electrical transport, molecular state, redox potentials and photocatalytic activity.
Computational Methods
The geometrical structure, electronic and optical properties of undoped and NM doped monolayer MoSe2 are calculated within the framework of density functional theory by using the CASTEP package. Norm-conserving pseudopotentials and Perdew-Burke-Ernzerhof (PBE) function of the generalized gradient approximation (GGA) are used for the electron-ion interactions and exchange-correlation potential, respectively37,38. The adopted monolayer structure is a 4 × 4 × 1 supercell containing 16 Mo (blue ball), 31 Se (yellow ball) and 1 NM atoms (purple ball) (Fig. 1(a)). The high cutoff energy for the plane-wave basis is set at 750 eV and the Brillouin zone is sampled by a 9 × 9 × 1 k-point sampling grid. A vacuum layer of 15 Å is adopted in the direction perpendicular to the monolayer surface to avoid the interactions between periodic slabs. The structural optimization is continued until the convergence tolerance of energy, maximum force and maximum displacement are less than 5.0 × 10−6 eV/atom, 0.01 eV/Å and 1.0 × 10−3 Å, respectively.
We have calculated the binding energies (E b) of all the doped systems to assess the stability of doped MoSe2:
where E doped is the total energy of the monolayer MoSe2 with one Se atom replaced by the NM atom, E vacany is the total energy of the monolayer MoSe2 with one Se vacancy, and μ NM is the chemical potential of the dopant and is calculated with respect to the elemental bulk or gas in nature. Positive E b suggests that doped atom is energetically favorable to sit at the substitutional site of Se.
Results and Discussions
Binding energies and optimized Structures
Table 1 lists the calculated results of the doped systems. All of them (except for B- and C-doped) have positive E b, indicating that their formations are exothermic reaction and therefore stable. The E b of the VIA (O, S and Te) group doped is the largest (>2 eV). This is due to the dopants have the same valence electron structure as Se. Thus they are most stable when fill up the Se vacancy. O-doped system, overall, has the largest E b (3.78 eV), since O atom has the highest electronegativity. The E b of the VA (N, P and As) and VIIA (F, Cl, Br and I) group doped are smaller (~1 eV). This is probably due to the absent or extra one electron cost some formation energy. Doping of NM atoms with the IA (H), IIIA (B) and IVA (C and Si) groups is less energetically favorable, with E b of ~0 eV. This can be attributed to their valence electron structures have the largest difference compared with Se.
Although their E b varies a lot, their lattice structures stay constant, within 0.6%. On the other hand, the local structure and the electronic properties have been modified dramatically. The Mo-NM bond lengths (d Mo-NM) and h (the sheet thicknesses from NM atom to the reference Mo atom plane, Fig. 1(b)) of doped MoSe2 vary as much as 21.5% and 51.6%, respectively. This is mostly due to the variation of the atomic radius and electronegativity of NM atoms. More details will be discussed later.
Electronic and electrical transport properties
To explain how doped NM atoms modify the electronic properties of monolayer MoSe2, we have calculated the band structures and partial density of states (PDOS) of individual Mo, Se and NM atoms of all the systems, as shown in Figs 2 and 3, respectively. For undoped monolayer MoSe2, conduction band (CB) and valence band (VB) are composed of Mo-4d and Se-4p states, and each Mo atom can provide four electrons interacting with the surrounding six Se atoms. For IVA (C and Si) and VIA (O, S and Te) group doped systems (NM dopants with even number of valence electrons), the Fermi level (E F) of doped MoSe2 is almost unchanged (−0.04 ~ 0.06 eV, see Table 1) compared to the undoped one and is slightly above the valence band maximum (VBM), seen in Fig. 2. These phenomena are consistent with the results in C-, Si-, O-, Se- and Te-doped monolayer MoS2 or WSe2 21,28,29. Thus, NM dopants with an even number of valence electrons doped monolayer MoSe2 is still generally a p-type semiconductor.
The band structures of undoped and NM doped monolayer MoSe2. The light blue and light purple background colors are represent the band structures of the odd and even number valence electrons doped systems, respectively. NM dopants with an even number of valence electrons are generally p-type conductivity. On the contrary, NM dopants with an odd number of valence electrons can provide effective p-type or n-type conductivity.
For IIIA (B) and VA (N, P and As) group doped cases, the E F lowered about 0.18~0.50 eV compared to the undoped one. The similar results have also been reported in B- N-, P- and As-doped monolayer MoS2 or WSe2 and N-, P- and As-doped monolayer MoSe2 21,28,39,40. Thus, they are still a p-type semiconductor. For IA (H) group doped case, a half-filled impurity level (mostly composed of Mo-4d states) appears within the band gap and lifts the E F into the middle of the band gap. Thus p-type conductivity is suppressed. For VIIA (F, Cl, Br and I) group doped systems, an additional valence electron results in the donor impurity level (consisting Mo-4d and NM-p states) and the E F is lifted close to the conduction band minimum (CBM). Thus, they become an n-type semiconductor, consistent with the reported results21,28,39,40. Thus, we can conclude that NM dopants with an odd number of valence electrons doped MoSe2 can have p-type or n-type conductivity. On the contrary, NM dopants with an even number of valence electrons can only provide p-type conductivity. This suggests a possible way to realize bipolar electrical transport properties by tuning the dopants, which is essential for practical applications in optoelectronics and electronics.
We further observe the PDOS of all doped systems found that Mo-4d (green lines) and Se-4p states (red lines) are basically invariable, while the NM-p (or -s) states (blue lines) demonstrate huge difference (see Fig. 3). Therefore, we believe that the dopants can only affect the local electronic structure. This is also verified by the calculated electronic density difference. Overall, the dopants only affect the electronic distribution of the three nearest neighboring Mo atoms, other atoms are unaffected. Thus, in Fig. 4, we only demonstrate the electronic density difference of the NM and the closest Mo and Se atoms. The blue (or red) regions indicate the electron loss (or accumulation), and the red regions between the two atoms indicate that they form a covalent bond. Thus, the Se and the Mo atom are ionized; meanwhile, a covalent bond is formed between them in undoped monolayer MoSe2. It is found that Mo-NM ionic bonds are formed in the N-, O- and F-doped system, due to their large electronegativity. On the other hand, covalent bonds with different strength are formed for other dopants. Thus we believe the change of the globe electronic properties of the doped MoSe2 is due to this local change of electron distribution.
Optical and photocatalytic properties
We have also calculated the optical properties of all the systems and the results show that NM dopants slightly affect the optical absorption edge and optical absorption areas in the range of 300 to 900 nm (see Supplementary Fig. S1). On the other hand, this work also provides the highest occupied molecular state (HOMO) and lowest unoccupied molecular state (LOMO) of all the systems (show in Fig. S2). We have found that the physical location of HOMO and LUMO can be tuned by different NM dopants28,41.
For undoped MoSe2, the HOMO and LUMO are made up of Mo-4d states, thus they are located at all the Mo atoms simultaneously (Fig. S2(k)). And the photogenerated electrons (e−) and holes (h+) will be easily recombined on Mo atoms, which reduce the photocatalytic efficiency. For IVA (C and Si) group doped MoSe2, both the HOMO and LUMO mainly locate at the NM atom and its three nearest Mo atoms. For VIA (O, S and Te) group doped MoSe2, similar to undoped MoSe2, both the HOMO and LUMO mainly locate at the Mo atoms across the film. Thus, in these two cases, the photogenerated electrons e−/h+ have a good chance to recombine.
For IA (H) group and VIIA (F, Cl, Br and I) group doped MoSe2, the distribution of HOMO states is the same as the undoped MoSe2, while the LUMO states are mainly located at the NM atom and its three nearest Mo atoms. For IIIA (B) doped MoSe2, the distribution is opposite to them. For VA (N, P and As) group doped MoSe2, the HOMO states locate at around NM atom and its surrounding Mo atoms, and the LUMO states locate at Mo atoms further away from NM atoms. In these three cases, the HOMO and LUMO states are separated in real space.
O2 − ion and OH radical are important oxidants for the degradation of organic pollutants. As an example, the schematic mechanism of photocatalytic degrading process is shown in Fig. 5 for VIIA (F, Cl, Br and I) group doped MoSe2 system. Under light irradiation, the e− and h+ will be generated at the yellow and purple region, respectively. Next, O2 − ions and ·OH radicals can be reduced or oxidized by the e− or h+, simultaneously. And they can oxidize the organic pollutant into H2O and CO2. Thus, due to the separation of the HOMO and LUMO states in the real space, NM dopants with an odd number of valence electrons doped monolayer MoSe2 can suppress the recombination probability of the photogenerated e− and h+, and increase the photocatalytic performance.
The mechanism of photocatalytic degrading organic pollutant and the charge dynamics in VIIA doped monolayer MoSe2. The purple and yellow regions represent HOMO and LUMO, respectively. Under light irradiation, the photogenerated e− moves to Mo atoms and VIIA group atoms in the yellow region, while, photogenerated h+ moves only on the Mo atoms in the purple region. This represents the separation of photogenerated e−/h+ in real space.
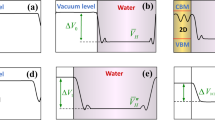
Further, the VBM and CBM position are calculated relative to vacuum level42,43, see Fig. 6 and Table 1, the oxidation potential of the photogenerated h+ is enhanced by 0.44~0.64 eV, while the reduction potential of photogenerated e− is reduces by 0.52~0.69 eV in the NM dopants with odd number of valence electrons (IA, IIIA, VA and VIIA) doped monolayer MoSe2. On the contrary, the NM dopants with even number of valence electrons even (IVA and VIA doped MoSe2 barely changed the redox potential. Thus, NM dopants with odd number of valence electrons can modify the redox potentials, and tends to generate more ·OH radical than O2 − ion in the photocatalytic reaction. This is consistent with the experimental report that the ·OH radical played a dominant role in the visible light photocatalytic degradation of RhB for the B/C-doped TiO2 44.
Conclusions
In this work, the geometrical structure, electrical transport, redox potentials, molecular state, optical and photocatalytic properties of NM doped monolayer MoSe2 are studied by first principle calculations. The E b suggests that NM doped MoSe2 are energetically favorable compared to Se vacancies, except B- and C-doped MoSe2. We have found that NM dopants with odd number of valence electrons doped monolayer MoSe2 can be p-type or n-type, while dopants with even valence electrons doped ones tend to be p-type. This provides a useful method to realize bipolar electrical transport properties by tuning the dopants for the applications in optoelectronics and electronics. We have also found NM dopants with odd number of valence electrons doped MoSe2 have better photocatalytic performance, due to they can suppress the recombination of photogenerated e− and h+, and enhance the oxidation potential of the holes.
References
Tian, T. et al. Multiscale analysis for field-effect penetration through two-dimensional materials. Nano. Lett. 16, 5044–5052 (2016).
Zhou, G. D. et al. Investigation of the behaviour of electronic resistive switching memory based on MoSe2-doped ultralong Se microwires. Appl. Phys. Lett. 109, 143904 (2016).
Kim, Y., Huang, J. L. & Lieber, C. M. Characterization of nanometer scale wear and oxidation of transition metal dichalcogenide lubricants by atomic force microscopy. Appl. Phys. Lett. 59, 3404 (1991).
Mak, K. F. et al. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 105, 136805 (2010).
Mak, K. F. et al. Control of valley polarization in monolayer MoS2 by optical helicity. Nat. Nano. 7, 494–498 (2012).
Sanchez, O. L. et al. Ultrasensitive photodetectors based on monolayer MoS2. Nat. Nano. 8, 497–501 (2013).
Zhu, Z., Peelaers, H. & Walle, C. G. V. D. Hydrogen intercalation in MoS2. Phys. Rev. B 94, 085426 (2016).
Kadioglu, Y., Gokoglu, G. & Aktürk, O. U. Effects of gold based dimers on structural and electronic properties of MoS2. Appl. Surf. Sci. 396, 455–460 (2017).
Jiang, Q. Q. et al. Facile solvent-thermal synthesis of ultrathin MoSe2 nanosheets for hydrogen evolution and organic dyes adsorption. Appl. Surf. Sci. 402, 277–285 (2017).
Ma, D. W. et al. The adsorption of CO and NO on the MoS2 monolayer doped with Au, Pt, Pd, or Ni: A first-principles study. Appl. Surf. Sci. 383, 98–105 (2016).
Radisavljevic, B., Brivio, J., Giacometti, V. & Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 6, 147–150 (2011).
Coleman, J. N. et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 331, 568–571 (2011).
Ding, Y. et al. First principles study of structural, vibrational and electronic properties of graphene-like MX2 (M = Mo, Nb, W, Ta; X = S, Se, Te) monolayers. Physica. B 406, 2254–2260 (2011).
OBrien, M. et al. Low wavenumber Raman spectroscopy of highly crystalline MoSe2 grown by chemical vapor deposition. Phys. Status. Solidi. B 252, 2385–2389 (2015).
Mo, S. K. et al. Spin-resolved photoemission study of epitaxially grown MoSe2 and WSe2thin films. J. Phys.: Condens. Matter. 28, 454001 (2016).
Zhang, Q. et al. Bandgap renormalization and work function tuning in MoSe2/hBN/Ru (0001) heterostructures. Nat. Commun. 7, 13843 (2016).
Zhou, W. et al. Intrinsic structural defects in monolayer molybdenum disulfide. Nano. Lett. 13, 2615–2622 (2013).
Zheng, H. L., Yang, B. S. & Wang, D. D. Tuning magnetism of monolayer MoS2 by doping vacancy and applying strain. Appl. Phys. Lett. 104, 132403 (2014).
Zou, X., Liu, Y. & Yakobson, B. I. Predicting dislocations and grain boundaries in two-dimensional metal-disulfides from the first principles. Nano. Lett. 13, 253–258 (2013).
Kong, L. J., Liu, G. H. & Qiang, L. Electronic and optical properties of O-doped monolayer MoS2. Comp. Mater. Sci. 111, 416–423 (2016).
Lu, S. et al. Tunable redox potential of nonmetal doped monolayer MoS2: First principle calculations. Appl. Surf. Sci. 384, 360–367 (2016).
Cheng, Y. C. et al. Prediction of two-dimensional diluted magnetic semiconductors: doped monolayer MoS2 systems. Phys. Rev. B 87, 100401 (2013).
Suh, J. et al. Doping against the native propensity of MoS2: degenerate hole doping by cation substitution. Nano. Lett. 14, 6976–82 (2014).
Mishra, R. et al. Long-range ferromagnetic ordering in manganese-doped two-dimensional dichalcogenides. Phys. Rev. B 88, 144409 (2013).
Yang, Y. et al. First-principles investigations of transition-metal doped bilayer WS2. Phys. Chem. Chem. Phys. 18, 10152–10157 (2016).
Qi, L., Wang, Y., Shen, L. & Wu, Y. H. Chemisorption-induced n-doping of MoS2 by oxygen. Appl. Phys. Lett. 108, 063103 (2016).
Ma, D. W. et al. Modulating electronic, magnetic and chemical properties of MoS2 monolayer sheets by substitutional doping with transition metals. Appl. Surf. Sci. 364, 181–189 (2016).
Lu, S. et al. The effects of nonmetal dopants on the electronic, optical, and catalytic performances of monolayer WSe2 by a first-principles study. RSC. Adv. 6, 114109–114122 (2016).
Hu, A. M. et al. Electronic structures and magnetic properties in nonmetallic element substituted MoS2 monolayer. Comp. Mater. Sci. 107, 72–78 (2015).
Xu, E. Z. et al. p-Type transition-metal doping of large-area MoS2 thin films grown by chemical vapor deposition. Nanoscale 9, 3576 (2017).
Wang, Y. R., Li, S. A. & Yi, J. B. Electronic and magnetic properties of Co doped MoS2 monolayer. Sci. Rep. 6, 24153 (2016).
Liu, P. T. et al. Flower-like N-doped MoS2 for photocatalytic degradation of RhB by visible light irradiation. Nanotechnology 27, 225403 (2016).
Hao, L. et al. Nitrogen-doped MoS2/carbon as highly oxygen-permeable and stable catalysts for oxygen reduction reaction in microbial fuel cells. J. Power. Sources. 339, 68–79 (2017).
Zhang, H. Y. et al. Small dopants make big differences: enhanced electrocatalytic performance of MoS2 monolayer for oxygen reduction reaction (ORR) by N- and P-Doping. Electrochim. Acta. 225, 543–550 (2017).
Li, R. C. et al. Nitrogen doped MoS2 nanosheets synthesized via a low temperature process as electrocatalysts with enhanced activity for hydrogen evolution reaction. J. Power. Sources. 356, 133–139 (2017).
Huang, H. et al. High-quality phosphorus-doped MoS2 ultrathin nanosheets with amenable ORR catalytic activity. Chem. Commun. 51, 7903 (2015).
Perdew, J. P., Burke & Ernzerhof, K. M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Hamann, D. R., Schluter, M. & Chiang, C. Norm-conserving pseudopotentials. Phys. Rev. Lett. 43, 1494–1497 (1979).
Dolui, K. et al. Possible doping strategies for MoS2 monolayers: An ab initiostudy. Phys. Rev. B 88, 075420 (2013).
Zhao, X. et al. Effective n-type F-doped MoSe2 monolayers. Rsc. Adv. 7, 26673 (2017).
Wang, S. et al. Structure and photocatalytic property of Mo-doped TiO2 nanoparticles. Powder. Technol. 244, 9–15 (2013).
Maytal, C. T. et al. First principles scheme to evaluate band edge positions in potential transition metal oxide photocatalysts and photoelectrodes. Phys. Chem. Chem. Phys. 13, 16644–16654 (2011).
Yu, J. G. et al. New insight into the enhanced visible-light photocatalytic activities of B-, C- and B/C-doped anatase TiO2 by first-principles. Phys. Chem. Chem. Phys. 15, 12040 (2013).
Yu, J. G. et al. Ionic liquid assisted synthesis of uniform fluorinated B/C-Codoped TiO2 nanocrystals and their enhanced visible-light photocatalytic activity. Chem. -Eur. J 19, 2433–2441 (2013).
Acknowledgements
This work is supported by the National Key Research and Development Program of China (No. 2016YFA0300803), the National Basic Research Program of China (No. 2014CB921101), the National Natural Science Foundation of China (Nos 61474061, 61674079), Jiangsu Shuangchuang Program and the Natural Science Foundation of Jiangsu Province of China (No. BK20140054). Computational resources were provided by the Jilin University.
Author information
Authors and Affiliations
Contributions
The idea was proposed by Y.F.Z. and L.H. The simulations and data analyses were performed by Y.F.Z., Y.F.Z. wrote the manuscript, C.L. and W.W. prepared the table and the figures. All authors discussed the results and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Y., Wang, W., Li, C. et al. First-principles study of nonmetal doped monolayer MoSe2 for tunable electronic and photocatalytic properties. Sci Rep 7, 17088 (2017). https://doi.org/10.1038/s41598-017-17423-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-17423-w
This article is cited by
-
First-principles study of the effect of doping on the optoelectronic properties of defective monolayers of MoSe2
Journal of Molecular Modeling (2024)
-
The structural, stability, electronic, optical and thermodynamic properties of MoX2 (X= S, Se, and Te) under hydrostatic pressures: a plasmon approach and first-principle study
Journal of Molecular Modeling (2024)
-
Role of Main Group Nonmetal Dopants on the Electronic Properties of the TcS2 Monolayer Revealed by Density Functional Theory
Journal of Electronic Materials (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.