Abstract
The inferior frontal and superior temporal areas in the left hemisphere are crucial for human language processing. In the present study, we investigated the magnetic mismatch field (MMF) evoked by voice stimuli in 3- to 5-year-old typically developing (TD) children and children with autism spectrum disorder (ASD) using child-customized magnetoencephalography (MEG). The children with ASD exhibited significantly decreased activation in the left superior temporal gyrus compared with the TD children for the MMF amplitude. If we classified the children with ASD according to the presence of a speech onset delay (ASD - SOD and ASD - NoSOD, respectively) and compared them with the TD children, both ASD groups exhibited decreased activation in the left superior temporal gyrus compared with the TD children. In contrast, the ASD - SOD group exhibited increased activity in the left frontal cortex (i.e., pars orbitalis) compared with the other groups. For all children with ASD, there was a significant negative correlation between the MMF amplitude in the left pars orbitalis and language performance. This investigation is the first to show a significant difference in two distinct MMF regions in ASD – SOD children compared with TD children.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) comprises a set of neurodevelopmental disorders characterized by deficits in social communication and restrictive and repetitive patterns of behaviour, interests, and activities. Language abilities are highly variable in individuals with ASD, with difficulties that range from mild to severe impairments in pragmatics and/or social communication1. Currently, language level is considered to be a continuous, rather than categorical variable2. Intriguingly, accumulating electrophysiological evidence suggests that deficits in the discrimination of rapid sound changes are associated with impaired speech processing in children with ASD3 as well as developmental language disorders4,5,6,7.
Auditory mismatch negativity (MMN) or its magnetic mismatch field (MMF)8 is quantified by subtracting the average waveform generated in response to standard stimuli from the average deviant waveform; MMN typically peaks between 100 to 250 ms from the onset of the stimulus change9. The sources of the MMN have been identified as the bilateral temporal regions in the primary and secondary auditory cortices, with contributions from the frontal regions10,11,12. In healthy populations, MMN/MMF is considered an indicator of change detection and has been used to probe speech discrimination13,14,15,16. Using source-localizing methods, MMN/MMF has been reported to be generated by temporal and frontal lobe sources, with the former being associated with change detection and the latter with involuntary switching of attention to sound changes10. Therefore, MMN/MMF have been used as neurophysiological markers for the integrity of auditory sensory memory and automatic change detection6,17.
In previous studies in children, the MMN latency ranged between 100 and 400 ms, depending on the age, cognitive skill and stimulus type3,18,19,20,21,22. MMN has been considered a suitable method to investigate speech development, even in infants, because MMN is elicited at all ages and is enhanced by native speech but not unfamiliar speech contrasts beginning at the age of 6 months15,23. Atypical MMN/MMF responses have also been reported in populations with developmental language disorders4,24 as well as children with ASD3,22,25,26,27,28,29,30,31,32,33,34,35,36,37.
Numerous auditory electrophysiological studies have primarily focused on MMF in older children with ASD (older than 6 years); however, to our knowledge, no previous magnetoencephalography (MEG) studies have focused on analysis of the MMF source in younger children with ASD (age 6 and under).
In the present study, we investigated the MMF evoked by voice changes during speech (i.e., a change in the fundamental frequency contour) in young children with ASD (aged 3–5 years) and a speech onset delay (AS-SOD), children with ASD without a speech onset delay (AS-NoSOD), and age-matched typically developing (TD) control participants. The aim of this study was to investigate regional activity in the brain during a speech perception task in order to explain the phenotypic heterogeneity in language development among children with ASD. Based on the finding that the atypical processing of auditory information is associated with language impairment in ASD, we hypothesized that young pre-school-aged children with ASD and a speech onset delay (SOD) exhibit atypical MMF compared with TD children and young children with ASD who do not have a SOD. We also hypothesized that atypical MMF would be associated with language performances at the time of recording, considering subjects’ ages and cognitive levels.
Results
As shown in Table 1, forty-seven children with ASD and 46 TD children participated in the study.
Comparison of the MMF amplitudes in the 100–200 ms time window between the TD group and all ASD groups
As shown in Fig. 1a,b, unpaired t- tests identified significant differences in the left superior temporal gyrus (t = 3.547, P = 0.001) and the left transverse temporal gyrus (t = 3.176, P = 0.002) in 20 regions of interest (ROIs) between the two groups (Bonferroni’s correction, alpha = 0.05/20 = 0.0025). As a complementary analysis (alpha = 0.05), the ANCOVA for these two ROIs, which included possible confounding factors (i.e., age38 and cognitive skill39) as covariances, identified significant differences in the left transverse temporal gyrus (F = 8.407, P = 0.005) and the left superior temporal gyrus (F = 11.227, P = 0.001) between the two groups. As another complementary analysis (alpha = 0.05) to test hemispheric lateralization, a repeated two-way ANOVA for these two ROIs, in which the group (i.e., TD and ASD) is between factor and the right/left (i.e., MMF from right or left hemisphere) is within factor, identified a significant interaction between these two factors in the left superior temporal gyrus (F = 4.415, P = 0.038) and left transverse temporal gyrus (F = 5.032, P = 0.027).
Comparison of the MMF source amplitude in the 100–200 ms time window. Significant differences were identified the left superior temporal gyrus (a) and left transverse temporal gyrus (b) between the TD children and children with ASD. There was a significant difference in the left superior temporal gyrus (c) between the TD children and ASD children with and without SOD. SOD, speech onset delay.
Comparison of the MMF amplitudes in the 100–200 ms time window in the TD children and children with ASD with and without SOD
As shown in Fig. 1c, one-way ANOVA identified a significant difference in the left superior temporal gyrus between groups (F = 6.448, P = 0.002) (Bonferroni’s correction, alpha = 0.05/20 = 0.0025). A post hoc analysis (alpha = 0.05) identified significant differences between the TD children and the children with AS-NoSOD (P = 0.037), as well as between the TD children and the children with AS-SOD (P = 0.005). The ANCOVA (alpha = 0.05), which included age and cognitive skill as covariates, indicated a significant difference in the left superior temporal gyrus (F = 5.856, P = 0.004). The MMF amplitude and t-value for each sampling time point (1 ms) in the left superior temporal gyrus are presented in Fig. 2a–d.
MMF source amplitude at each sampling point in the left superior temporal gyrus and left pars orbitalis. T values were calculated between the groups. Significant differences were indicated by a red coloured area. In the left superior temporal gyrus, (a) ASD vs TD, (b) ASD-NoSOD vs TD, (c) ASD-SOD vs TD and (d) ASD-NoSOD vs ASD-SOD. In the left pars orbitalis, (e) ASD vs TD, (f) ASD-NoSOD vs TD, (g) ASD-SOD vs TD and (h) ASD-NoSOD vs ASD-SOD. SOD, speech onset delay.
Comparison of the MMF amplitudes in the 200–350 ms time window between TD children and all ASD groups
Unpaired t-tests did not identify a significant difference in 20 ROIs in the 200–350 ms time window between the two groups (Bonferroni’s correction, alpha = 0.05/20 = 0.0025).
Comparison of the MMF amplitudes in the 200–350 ms time window in TD children and children with ASD with and without SOD
As shown in Fig. 3, the one-way ANOVA revealed a main effect of the group on the left pars orbitalis (F = 6.932, P = 0.002) (Bonferroni’s correction, alpha = 0.05/20 = 0.0025). A post hoc analysis (alpha = 0.05) identified significant differences between the TD and ASD-SOD groups (P = 0.014), as well as between the AS-NoSOD and AS-SOD groups (P = 0.002). The ANCOVA (alpha = 0.05), which included age and cognitive skill, indicated a significant difference in the left pars orbitalis among the three groups (F = 6.957, P = 0.002). The MMF amplitude and t-value for each sampling time point (1 ms) in the left pars orbitalis are presented in Fig. 2e–h.
Comparison of the MMF amplitudes in the left pars orbitalis in the 200–350 ms time window among the three groups. One-way ANOVA identified significant differences among the three groups (F = 6.932 P = 0.002, α = 0.0025). The post hoc test identified significant differences between the TD children and children with ASD-SOD (P = 0.014, α = 0.05), as well as between the children with ASD-NoSOD and children with ASD-SOD (P = 0.002, α = 0.05).
Relationship between the MMF amplitudes and language performance
In the two regions with significant differences in the MMF amplitude among the three groups (i.e., the left superior temporal gyrus for the time window of 100–200 ms and left pars orbitalis for the time window of 200–350 ms), a Pearson’s correlation coefficient was used to investigate the relationship between the MMF amplitude and language performances (i.e., receptive and expressive vocabulary) for TD and ASD group, respectively. Bonferroni’s correction was applied for the two ROIs (i.e., alpha = 0.05/2 = 0.025).
In the TD children, Pearson’s correlation coefficient did not indicate a significant correlation between the MMF source amplitude in the left superior temporal gyrus and language performances (r = −0.287, P = 0.065 and r = −0.149, P = 0.323 for receptive and expressive vocabulary, respectively) or between the MMF source amplitude in the left pars orbitalis and language performance (r = −0.046, P = 0.770 and r = 0.267, P = 0.073 for receptive and expressive vocabulary, respectively).
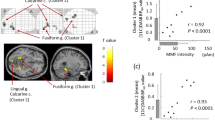
In children with ASD, Pearson’s correlation coefficient did not indicate a significant correlation between the MMF source amplitude in the left superior temporal gyrus and language performances (r = 0.110, P = 0.446 and r = 0.165, P = 0.266 for receptive and expressive vocabulary, respectively), however, there were significant correlations between the MMF amplitude in the left pars orbitalis and receptive vocabulary (r = −0.359, P = 0.014) and expressive vocabulary (r = −0.406, P = 0.005) (Fig. 4).
Relationship between the MMF amplitude in the left pars orbitalis and language performance. Scatter plots for expressive vocabulary. An open circle indicates the TD children and a closed circle indicates the children with ASD. The broken line indicates the regression line for the TD children and the bold line indicates the regression line for the children with ASD. Pearson’s correlation coefficient indicated a significantly negative correlation between the log-transformed MMF amplitude and the expressive vocabulary performance in the children with ASD (r = −0.406, P = 0.005, α = 0.025), but not in the TD children.
In addition, we employed a multiple linear regression analysis to predict the MMF amplitude (i.e., dependent variable) using group (i.e., TD and ASD), language performance (receptive or expressive vocabulary) and interaction term (i.e., group × language performance) as predictors to consider the potential interaction between group and language performance. We employed an alpha level of 0.05 for these complementary analysis using multiple linear regression analysis. As a result from expressive vocabulary ability, the multiple regression model suitably fitted the data (R = 0.364 F = 4.540, P = 0.005). Moreover, the interaction term (i.e., group × expressive vocabulary ability) showed significant in the left pars orbitalis (β = −0.337, P = 0.002). This result demonstrated that children with ASD and TD children have different relationships between MMF amplitude and expressive vocabulary ability. On the other hand, as a result from receptive vocabulary ability, the multiple regression model failed to fit the data (R = 0.291 F = 2.584, P > 0.05). Since there was a significant interaction between group and expressive vocabulary performance, we employed a stepwise multiple linear regression analysis to predict MMF amplitude (i.e., the dependent variable), using age and cognitive skill (first step) and expressive vocabulary (second step) as predictors (i.e., three independent variables) to consider the potential effects of age or cognitive skill on TD and ASD, respectively. We employed an alpha level of 0.05 for these complementary analyses using multiple linear regression. Expressive vocabulary was found to be significantly associated with MMF amplitude in the left pars orbitalis in the second step in children with ASD (Step 2, F = 3.031, β = −0.481, P = 0.012; Table 2). However, in TD children, there was no significant relationship between the MMF amplitude in the left pars orbitalis and any independent variable (i.e. expressive vocabulary, age, cognitive skills).
Discussion
The current study investigated the cortical pre-attentive discrimination of changes in speech tone in 3- to 5-year-old TD children and children with ASD. The amplitude of the early MMF (i.e., 100–200 ms) component was decreased in the left temporal auditory areas (i.e., the superior temporal and transverse temporal gyri) in the children with ASD compared with the TD children. This finding agrees with previous studies using electroencephalography (EEG) and MEG. In a previous EEG study of adult subjects, individuals with ASD did not exhibit a MMN response to emotional syllables and exhibited a reduced MMN to non-vocal sounds40. In addition, although our target age range (3–5 years) is missing from previous MMN/F studies, a large number of previous EEG/MEG studies have focused on MMN/F in older children with ASD3,22,25,27,28,29,30,31,32,33,34,35,36,37,41,42, of which many of the findings were highly inconsistent. Some studies reported larger MMN in children with ASD22,29,33,36, whereas other studies reported smaller27,34,37 or even normal MMN35,42 in children with ASD than in age-matched TD children. In part, these inconsistencies have been explained by differences in stimuli (e.g., vowel, tone, frequency change or intensity change)22,29,30,31, attentional condition (e.g., attended or unattended)32, time windows of focus (e.g., early MMN or late MMN including P3a)22,30,33,35,36 and age ranges36. The present study is the first to focus on younger children (3- to 5-year-olds) and replicated the decreased MMF amplitude reported in some previous studies in older children with ASD27,34,37 regardless of SOD. Moreover, this investigation is the first to show a reduction in the MMF amplitude in the left but not right hemisphere in children with ASD. This finding may be attributed to atypical brain lateralization in children with ASD, which was supported by recent MEG auditory-evoked field (AEF) studies in younger (3–7 year-old)43 and older (8–17 year-old)44 children with ASD.
In TD children, the Pearson’s correlation coefficient failed to demonstrate a significant correlation between the MMF source amplitude in the left superior temporal gyrus and receptive vocabulary ability (r = −0.287, P = 0.065), however, a higher MMF source amplitude tended to be associated with lower receptive vocabulary ability. Interpretation of this result is difficult because a deficit of MMN in children with specific language impairment was reported45. Further studies are necessary to conclude whether this finding could be replicated with a larger sample size in children with this age range.
If we divided the children with ASD into two groups according to the presence of SOD, the children with ASD-SOD exhibited enhanced cortical activation in the left inferior frontal gyrus (i.e., pars orbitalis) in the late MMF (i.e., 200–350 ms) compared with the two other groups. Consistent with our results, previous studies reported enhanced MMN or P3a (which is a late component of MMN and is thought to reflect an involuntary attention switch)22,30,31,36 in children with ASD. Vlaskamp et al. reported that children with ASD (age range: 8–12 year-old) exhibited a significant increase in P3a (latency: around 250 ms) in response to deviant stimuli compared with TD controls30, whereas another study reported a significant decrease in P3a in 7–11 year-old children (latency: around 340 ms)22. Intriguingly, Ferri et al. reported a higher P3a (latency: 220–240 ms) amplitude in ASD subjects during childhood (8 year-old), while the opposite was observed during young adulthood. These results, in conjunction with our findings, suggested that the enhanced late-component of MMF (time window: 200–350) in ASD subjects is a robust finding in younger children (3–5 year-old) with a delay in speech onset. A limitation of the present study is that it remains unclear whether the enhanced MMF in the frontal area is a signature of a developmental language impairment per se or mental retardation in the context of ASD; however, our findings from the multiple regression analysis (cognitive skill was employed as a confounding factor) suggest that the enhanced MMF observed in response to changes in speech tone is a neurophysiological feature of children with ASD and a developmental language impairment. Because auditory information processing in ASD is biased towards low-level information46, children with ASD and a language impairment may perceive the speech tone variations as low-level information and may fail to interpret the meaning it connotes. Therefore, these children have difficulties understanding variations in speech tone. Similarly, with respect to general speech comprehension, excellent pitch-perception skills may bias auditory processing towards perceptual, low-level information at the cost of processing speech at a higher level. One recent model claims that the excellent perception of low-level information at the cost of social skills in individuals with ASD47 may explain the enhanced frontal MMF activities (i.e., enhanced involuntary switching of attention to sound change) associated with lower language performance in children with ASD. Regarding the left inferior frontal cortex, interestingly, a previous fMRI study of school-age to adolescent subjects with ASD indicated that activation in the left inferior frontal gyrus increased during song stimulation compared with controls48. The left inferior frontal cortex may play an important role in the detection of rapid pitch changes in subjects with ASD at the cost of language performance.
There are several limitations of this study. First, we did not include subjects with a language impairment who did not present ASD symptoms; therefore, we could not conclude that the significant differences in the left temporal and frontal areas are specific neurophysiological markers for ASD-SOD. Second, we used only human voice stimuli; therefore, we cannot generalize our findings to other types of auditory stimulation. Third, there is a significant difference in current language abilities between ASD-SOD and ASD-NoSOD; therefore, the results from the regression analysis for current language abilities might be confounded by the history of speech onset delay. Fourth, we could not determine the precise location of the source of the magnetic field, but could have if we used individual anatomical images, such as those obtained from MRI. Future studies using child-friendly and open-type MRI devices to obtain individual fine brain structures are necessary to reduce the level of uncertainty in source level estimation. Despite these limitations, this investigation is the first to report differences in the brain source activity evoked by changes in speech tone between 3- to 5-year-old TD children and children with ASD with and without SOD.
Materials and Methods
Participants
The clinical group included 47 children who were recruited from Kanazawa University and prefectural hospitals in the Kanazawa or Toyama area. ASD was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders (4th edition) (DSM-IV)49, the Diagnostic Interview for Social and Communication Disorders (DISCO)50, and the Autism Diagnostic Observational Schedule, Generic (ADOS-G)51; these assessments were conducted by a psychiatrist and a clinical speech therapist. Based on the criteria for SOD52, the children with ASD were divided into two groups based on the presence (AS-SOD) or absence of SOD (AS-NoSOD). Speech acquisition was considered typical if the parents reported that the child spoke his or her first single words before 24 months of age and his or her first two-word phrases before 33 months of age. Based on these criteria, 23 participants were defined as AS-SOD and 24 participants were defined as AS-NoSOD. The 46 TD children were matched to the ASD participants’ gender, age, and head circumference. No TD children had a history of SOD. Cognitive skills were assessed using the Japanese translation of the Kaufman Assessment Battery for Children (K-ABC)53. All participants were administered receptive and expressive language tests to investigate the relationship between the auditory brain response (i.e., the MMF) and language performance. Receptive vocabulary and comprehension were measured for each child using the Picture Vocabulary Test-Revised (PVT-R)54. The PVT-R is used to assess language comprehension and is similar to the Peabody Picture Vocabulary Test-Revised (PPVT-R). When an examiner speaks a word to a child in the PVT-R, the child chooses one of four pictures that represents the appropriate spoken word on each page. Expressive vocabulary was measured using the expressive vocabulary task in the K-ABC. In the expressive vocabulary task, the examiner shows the child several pictures, and the child provides the name of the objects. All participants had normal hearing according to their available medical records.
The parents agreed to allow their child to participate in the study and had full knowledge of the experimental nature of the research. Written informed consent was obtained prior to participation in the study. The Ethics Committee of Kanazawa University Hospital approved the methods and procedures, which were performed in accordance with the Declaration of Helsinki. The demographic data for all participants are presented in Table 1.
Auditory-evoked field stimuli and procedures
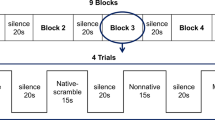
We used typical oddball sequences consisting of standard stimuli (456 times, 83%) and deviant stimuli (90 times, 17%). The stimuli consisted of the Japanese syllable “ne” pronounced two different ways (Fig. 5). A repetitive series of utterances of “ne” pronounced with a flat tone (/ne/) was used as the standard. This stimulus carries no intonational information. As a deviant stimulus, we used “ne” pronounced with a high falling tone (/Ne/), which carries intonational information (e.g., attention-seeking, emotional, declarative, or interrogative intonation). We used this syllable because /ne/ is a sentence-ending particle in Japanese and conveys prosodic information55. The syllable /ne/ is often used in mother-child conversations and expresses a speaker’s request for acknowledgement or empathy from the listener56. A female native Japanese speaker produced the /ne/ sounds, which were recorded using a condenser microphone (NT1-A; Rode, Silverwater, NSW, Australia) and a personal computer. The interstimulus interval (ISI) was 818 ms. Both stimuli had an intensity level of approximately 65 dB (A-weighted) at the head position against a background noise level of 43 dB. The intensity was measured using an integrating sound level metre (LY20; Yokogawa, Tokyo, Japan).
Magnetoencephalography recordings
MEG data were recorded using a 151-channel SQUID (Superconducting Quantum Interference Device) whole-head coaxial gradiometer MEG system for children (PQ 1151 R; Yokogawa/KIT, Kanazawa, Japan) in a magnetically shielded room (Daido Steel, Nagoya, Japan) installed at the MEG Center of Ricoh Company, Ltd. (Kanazawa, Japan). One researcher remained in the room to encourage the children and prevent them from moving during the analysis. The location of the head relative to the helmet of the MEG device was measured using 3 coils attached on the head surface as fiducial points with respect to the landmarks (bilateral mastoid processes and nasion).
Data analysis
The band pass-filtered MEG data (0.16–200 Hz) were collected at a sampling rate of 1000 Hz. The continuous MEG data were subsequently epoched into 100-ms pre-stimulus intervals and 900-ms post-stimulus intervals and baseline-corrected using the pre-stimulus interval. Epochs contaminated by muscle, heartbeat or eye blink artefacts that contained field amplitudes greater than ±4 pT were excluded from the analysis. Brainstorm57, which is documented and freely available for download online under the GNU general public license (http://neuroimage.usc.edu/brainstorm), was used for the subsequent analyses. Typical eye blinks and heartbeats were manually identified in the raw data for each participant to correct for blink and heartbeat artefacts. The pattern search function in Brainstorm was used to scan the raw data to identify other blinks and compute the average eye-blink topography across the MEG sensors. An eye-blink was modelled by the topography of its first PCA component. In addition to the heartbeat activity, the average heartbeat topography was also computed and modelled using the first PCA component. The trials of each type of stimuli were subsequently averaged after baseline correction (−50 to 0 ms). The mean averaging time for each stimulus (i.e., rare and frequent) was 76 ± 11 (mean ± standard deviation). The MMF responses were calculated by subtracting the average response to the standard stimuli (flat tone /ne/) from the average response to the deviant stimuli (falling tone /Ne/).
Brain template
We could not obtain individual brain structural data because it is difficult to perform MRI recordings on young children without sedation. Instead, we estimated the brain structures based on the individual head surface shapes of the participants using a modified version of the estimation algorithm developed in our previous study58 and superimposed the coordinate system of the MEG on the resulting anatomical information. Our algorithm was developed to identify an optimal structural image from 98 brain examples using head surface points59.
Analysis of the MMF source
We estimated the signal source of the MMF using the individually estimated anatomies of the children. The anatomical locations of the activating regions were based on the Desikan-Killiany gyrus atlas provided by FreeSurfer (open-source software: http://surfer.nmr.mgh.harvard.edu/)60. Source reconstruction was performed with Brainstorm57. To estimate the brain sources, we used an anatomically constrained MEG approach that places an anatomical constraint on the estimated sources by assuming that the recorded brain activity of each individual lies in the cortical mantle61. The lead field was then computed using the overlapping spheres algorithm62, with a cortical surface tessellated with 15,000 vertices. The inverse solution was calculated for each individual using Tikhonov-regularized minimum-norm estimates (MNE)63. A noise covariance matrix was calculated from the MEG recordings obtained in the −50 to 0 ms time window.
Selection of regions of interest
The MNE source maps were obtained for each participant and group and averaged onto the cortical regions corresponding to the Desikan-Killiany gyrus atlas. The definition of the anatomical ROIs was based on the prediction that MMF generators would be located primarily in the temporal, frontal and parietal regions9,64,65,66,67,68. Twenty ROIs of the total of 68 ROIs of Desikan-Killiany gyrus atlas were selected for further analysis. Two temporal windows (100–200 ms and 200–350 ms) were selected in each ROI.
Statistical analysis
Statistical analyses were conducted using SPSS (Statistical Package for the Social Sciences) for Windows, version 20.0 (IBM, Tokyo, Japan). For the 20 ROIs defined in the two time windows, differences in the log-transformed MMF amplitude were tested using unpaired t-tests (i.e., TD versus ASD) and one-way analysis of variance (ANOVA) (i.e., TD versus AS-SOD versus AS-NoSOD). Based on the number of ROIs, which were predefined according to previous studies9,64,65,66,67,68, Bonferroni’s correction was applied for the alpha level (i.e., alpha = 0.05/20 = 0.0025). If there was a significant difference in the MMF component amplitude among groups, for ANOVA (i.e., TD versus AS-SOD versus AS-NoSOD) result, we used post-hoc t test (alpha = 0.05). In addition, we included an analysis of covariance (i.e., ANCOVA) that included clinical variables (i.e., age and cognitive skills assessed by the K-ABC) as covariates to consider the potential confounding factors, such as an aging effect, on the AEF component in young children69. We employed an alpha level of 0.05 for this complementary analysis. We also used multiple linear regression analysis to predict MMF amplitude (i.e., the dependent variable) using group (i.e., TD and ASD), language performance (receptive or expressive vocabulary) and interaction term (i.e., group × language performance) as predictors to consider the potential interaction between group and language performance. We employed an alpha level of 0.05 for these multiple linear regression analyses. If a significant interaction between group and language performance was found, we employed stepwise multiple linear regression analysis to predict MMF amplitude (i.e., the dependent variable), using age and cognitive skill (first step) and language performance (i.e., receptive or expressive vocabulary) (second step) as predictors (i.e., three independent variables) for TD and ASD, respectively. We employed an alpha level of 0.05 for this complementary analysis.
Data Availability
The datasets used and analyzed during current study are available from the corresponding author on reasonable request.
References
Tager-Flusberg, H., Joseph, R. & Folstein, S. Current directions in research on autism. Ment Retard Dev Disabil Res Rev. 7, 21–29 (2001).
American_Psychiatric_Association. Diagnostic and Statistical Manual of Mental Disorders (5th ed.) (Washington, DC., 2013).
Roberts, T. P. et al. Auditory Magnetic Mismatch Field Latency: A Biomarker for Language Impairment in Autism. Biol Psychiatry. (2011).
Bishop, D. V. M. Using mismatch negativity to study central auditory processing in developmental language and literacy impairments: Where are we, and where should we be going? Psychol Bull. 133, 651–672 (2007).
Cheour, M., Leppanen, P. H. T. & Kraus, N. Mismatch negativity (MMN) as a tool for investigating auditory discrimination and sensory memory in infants and children. Clinical Neurophysiology. 111, 4–16 (2000).
Kujala, T., Tervaniemi, M. & Schroger, E. The mismatch negativity in cognitive and clinical neuroscience: theoretical and methodological considerations. Biol Psychol. 74, 1–19 (2007).
Kujala, T. & Naatanen, R. The mismatch negativity in evaluating central auditory dysfunction in dyslexia. Neurosci Biobehav Rev. 25, 535–543 (2001).
Sams, M. Cortical Responses to Changes in Auditory-Stimuli - Magnetoencephalographic Studies. Acta Oto-Laryngol. 124–131 (1991).
Naatanen, R., Paavilainen, P., Rinne, T. & Alho, K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin Neurophysiol. 118, 2544–2590 (2007).
Rinne, T., Alho, K., Ilmoniemi, R. J., Virtanen, J. & Naatanen, R. Separate time behaviors of the temporal and frontal mismatch negativity sources. Neuroimage. 12, 14–19 (2000).
Alho, K. Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes. Ear Hear. 16, 38–51 (1995).
Doring, C. et al. Mismatch negativity: Alterations in adults from the general population who report subclinical psychotic symptoms. Eur Psychiatry. 34, 9–16 (2016).
Eulitz, C. & Lahiri, A. Neurobiological evidence for abstract phonological representations in the mental lexicon during speech recognition. J Cogn Neurosci. 16, 577–583 (2004).
Mathiak, K., Hertrich, I., Lutzenberger, W. & Ackermann, H. Encoding of temporal speech features (formant transients) during binaural and dichotic stimulus application: A whole-head magnetencephalography study. Cognitive Brain Res. 10, 125–131 (2000).
Naatanen, R. et al. Language-specific phoneme representations revealed by electric and magnetic brain responses. Nature. 385, 432–434 (1997).
Tavabi, K., Elling, L., Dobel, C., Pantev, C. & Zwitserlood, P. Effects of place of articulation changes on auditory neural activity: a magnetoencephalography study. PLoS One. 4, e4452 (2009).
Naatanen, R., Paavilainen, P., Rinne, T. & Alho, K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin Neurophysiol. 118, 2544–2590 (2007).
Korpilahti, P., Krause, C. M., Holopainen, I. & Lang, A. H. Early and late mismatch negativity elicited by words and speech-like stimuli in children. Brain Lang. 76, 332–339 (2001).
Kuuluvainen, S., Alku, P., Makkonen, T., Lipsanen, J. & Kujala, T. Cortical speech and non-speech discrimination in relation to cognitive measures in preschool children. Eur J Neurosci. 43, 738–750 (2016).
Pihko, E. et al. Magnetic fields evoked by speech sounds in preschool children. Clin Neurophysiol. 116, 112–119 (2005).
Shafer, V. L., Yu, Y. H. & Datta, H. Maturation of speech discrimination in 4- to 7-yr-old children as indexed by event-related potential mismatch responses. Ear Hear. 31, 735–745 (2010).
Lepisto, T. et al. The discrimination of and orienting to speech and non-speech sounds in children with autism. Brain Res. 1066, 147–157 (2005).
Kuhl, P. K. et al. Phonetic learning as a pathway to language: new data and native language magnet theory expanded (NLM-e). Philos T R Soc B. 363, 979–1000 (2008).
Paul, I., Bott, C., Heim, S., Wienbruch, C. & Elbert, T. Phonological but not auditory discrimination is impaired in dyslexia. Eur J Neurosci. 24, 2945–2953 (2006).
Oram Cardy, J. E., Flagg, E. J., Roberts, W. & Roberts, T. P. Delayed mismatch field for speech and non-speech sounds in children with autism. Neuroreport. 16, 521–525 (2005).
Kuhl, P. K., Coffey-Corina, S., Padden, D. & Dawson, G. Links between social and linguistic processing of speech in preschool children with autism: behavioral and electrophysiological measures. Dev Sci. 8, F1–F12 (2005).
Tecchio, F. et al. Auditory sensory processing in autism: a magnetoencephalographic study. Biol Psychiatry. 54, 647–654 (2003).
Lepisto, T. et al. Auditory perception and attention as reflected by the brain event-related potentials in children with Asperger syndrome. Clin Neurophysiol. 117, 2161–2171 (2006).
Lepisto, T. et al. The perception of invariant speech features in children with autism. Biol Psychol. 77, 25–31 (2008).
Vlaskamp, C. et al. Auditory processing in autism spectrum disorder: Mismatch negativity deficits. Autism Res (2017).
Kujala, T. et al. Speech-feature discrimination in children with Asperger syndrome as determined with the multi-feature mismatch negativity paradigm. Clin Neurophysiol. 121, 1410–1419 (2010).
Dunn, M. A., Gomes, H. & Gravel, J. Mismatch negativity in children with autism and typical development. J Autism Dev Disord. 38, 52–71 (2008).
Korpilahti, P. et al. Processing of affective speech prosody is impaired in Asperger syndrome. J Autism Dev Disord. 37, 1539–1549 (2007).
Seri, S., Cerquiglini, A., Pisani, F. & Curatolo, P. Autism in tuberous sclerosis: evoked potential evidence for a deficit in auditory sensory processing. Clin Neurophysiol. 110, 1825–1830 (1999).
Ceponiene, R. et al. Speech-sound-selective auditory impairment in children with autism: they can perceive but do not attend. Proc Natl Acad Sci USA 100, 5567–5572 (2003).
Ferri, R. et al. The mismatch negativity and the P3a components of the auditory event-related potentials in autistic low-functioning subjects. Clin Neurophysiol. 114, 1671–1680 (2003).
Abdeltawwab, M. M. & Baz, H. Automatic Pre-Attentive Auditory Responses: MMN to Tone Burst Frequency Changes in Autistic School-Age Children. J Int Adv Otol. 11, 36–41 (2015).
Gage, N. M., Siegel, B. & Roberts, T. P. Cortical auditory system maturational abnormalities in children with autism disorder: an MEG investigation. Brain Res Dev Brain Res. 144, 201–209 (2003).
Ikeda, K., Hashimoto, S., Hayashi, A. & Kanno, A. ERP evaluation of auditory sensory memory systems in adults with intellectual disability. Int J Neurosci. 119, 778–791 (2009).
Fan, Y. T. & Cheng, Y. Atypical mismatch negativity in response to emotional voices in people with autism spectrum conditions. PLoS One. 9, e102471 (2014).
Berman, J. I. et al. Multimodal Diffusion-MRI and MEG Assessment of Auditory and Language System Development in Autism Spectrum Disorder. Frontiers in neuroanatomy. 10, 30 (2016).
Kemner, C., Verbaten, M. N., Cuperus, J. M., Camfferman, G. & van Engeland, H. Auditory event-related brain potentials in autistic children and three different control groups. Biol Psychiatry. 38, 150–165 (1995).
Yoshimura, Y. et al. Atypical brain lateralisation in the auditory cortex and language performance in 3- to 7-year-old children with high-functioning autism spectrum disorder: a child-customised magnetoencephalography (MEG) study. Mol Autism. 4, 38 (2013).
Flagg, E. J., Cardy, J. E., Roberts, W. & Roberts, T. P. Language lateralization development in children with autism: insights from the late field magnetoencephalogram. Neurosci Lett. 386, 82–87 (2005).
Davids, N. et al. The nature of auditory discrimination problems in children with specific language impairment: an MMN study. Neuropsychologia. 49, 19–28 (2011).
Mottron, L., Dawson, M., Soulieres, I., Hubert, B. & Burack, J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord. 36, 27–43 (2006).
Mottron, L. et al. Veridical mapping in the development of exceptional autistic abilities. Neurosci Biobehav Rev. 37, 209–228 (2013).
Lai, G., Pantazatos, S. P., Schneider, H. & Hirsch, J. Neural systems for speech and song in autism. Brain. 135, 961–975 (2012).
American_Psychiatric_Association (ed.) Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (Washington D.C., 1994).
Wing, L., Leekam, S. R., Libby, S. J., Gould, J. & Larcombe, M. The Diagnostic Interview for Social and Communication Disorders: background, inter-rater reliability and clinical use. J Child Psychol Psychiatry. 43, 307–325 (2002).
Lord, C. et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 30, 205–223 (2000).
Samson, F., Zeffiro, T. A., Doyon, J., Benali, H. & Mottron, L. Speech acquisition predicts regions of enhanced cortical response to auditory stimulation in autism spectrum individuals. J Psychiatr Res. 68, 285–292 (2015).
Kaufman, A. & Kaufman, N. Kaufman Assessment Battery for Children: Administration and Scoring Manual. Circle Pines. MN: American Guidance Service (1983).
Ueno, K., Nagoshi, S. & Konuki, S. Picture Vocabulary Test-Revised., Success/Bell Co Ltd, Hiroshima, Japan (2008).
Anderson, V., Hiramoto, M. & Wong, A. Prosodic Analysis of the Interactional Particle Ne in Japanese Gendered Speech. Japanese/Korean Linguistics. 15, 43–54 (2007).
Kajikawa, S., Amano, S. & Kondo, T. Speech overlap in Japanese mother-child conversations. J Child Lang. 31, 215–230 (2004).
Tadel, F., Baillet, S., Mosher, J. C., Pantazis, D. & Leahy, R. M. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput Intell Neurosci. 2011, 879716 (2011).
Hayashi, N. et al. Algorithm for estimation of brain structural location from head surface shape in young children. Neuroreport. 23, 299–303 (2012).
Hasegawa, C. et al. Mu rhythm suppression reflects mother-child face-to-face interactions: a pilot study with simultaneous MEG recording. Sci Rep. 6, 34977 (2016).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 31, 968–980 (2006).
Dale, A. M. et al. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 26, 55–67 (2000).
Huang, M. X., Mosher, J. C. & Leahy, R. M. A sensor-weighted overlapping-sphere head model and exhaustive head model comparison for MEG. Phys Med Biol. 44, 423–440 (1999).
Baillet, S., Friston, K. & Oostenveld, R. Academic software applications for electromagnetic brain mapping using MEG and EEG. Comput Intell Neurosci. 2011, 972050 (2011).
Lin, Y. Y. et al. Plastic phase-locking and magnetic mismatch response to auditory deviants in temporal lobe epilepsy. Cereb Cortex. 17, 2516–2525 (2007).
Tse, C. Y. & Penney, T. B. On the functional role of temporal and frontal cortex activation in passive detection of auditory deviance. Neuroimage. 41, 1462–1470 (2008).
Opitz, B., Rinne, T., Mecklinger, A., von Cramon, D. Y. & Schroger, E. Differential contribution of frontal and temporal cortices to auditory change detection: fMRI and ERP results. Neuroimage. 15, 167–174 (2002).
Lappe, C., Steinstrater, O. & Pantev, C. A beamformer analysis of MEG data reveals frontal generators of the musically elicited mismatch negativity. PLoS One. 8, e61296 (2013).
Cheng, C. H., Baillet, S., Hsiao, F. J. & Lin, Y. Y. Effects of aging on the neuromagnetic mismatch detection to speech sounds. Biol Psychol. 104, 48–55 (2015).
Edgar, J. C. et al. The maturation of auditory responses in infants and young children: a cross-sectional study from 6 to 59 months. Front Neuroanat. 9, 131 (2015).
Acknowledgements
We are very grateful to the participants who participated in this study. This study was supported by the Centre of Innovation Programme from the Japan Science and Technology Agency, JST, a Grant-in-Aid for Specially Promoted Research (Research Number 24000012) from MEXT, and a Grant-in-Aid for Scientific Research (C) (Research Number 16K10247). We declare that this work is free from financial limitations or other relationships that might cause a conflict of interest.
Author information
Authors and Affiliations
Contributions
Y.Y. conceived and designed the study, collected and analysed the MEG data, evaluated the clinical symptoms, and authored the manuscript. M.K. and H.K. recruited participants and performed clinical evaluation. H.H., C.H., T.I. and D.N.S. collected MEG data. N.H., T.T. and G.B.R. contributed to analysis and interpretation of data. M.O. and Y.M. contributed to conceive and design the study. All authors read, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoshimura, Y., Kikuchi, M., Hayashi, N. et al. Altered human voice processing in the frontal cortex and a developmental language delay in 3- to 5-year-old children with autism spectrum disorder. Sci Rep 7, 17116 (2017). https://doi.org/10.1038/s41598-017-17058-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-17058-x
This article is cited by
-
Autism Spectrum Disorder and auditory sensory alterations: a systematic review on the integrity of cognitive and neuronal functions related to auditory processing
Journal of Neural Transmission (2023)
-
Markers for the central serotonin system correlate to verbal ability and paralinguistic social voice processing in autism spectrum disorder
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.