Abstract
Mouse models have contributed to the bulk of knowledge on Systemic Lupus Erythematosus (SLE). Nevertheless, substantial differences exist between human and mouse immune system. We aimed to establish and characterise a SLE model mediated by human immune system. Injection of pristane into immunodeficient mice reconstituted with human immune system (humanised mice) recapitulated key SLE features, including: production of human anti-nuclear autoantibodies, lupus nephritis, and pulmonary serositis. There was a reduction in the number of human lymphocytes in peripheral blood, resembling lymphopenia in SLE patients. Concurrently, B cells and T cells were systemically hyperactivated, with a relative expansion of CD27+ and CD27−IgD− memory B cells, increased number of plasmablasts/plasma cells, and accumulation of effector memory T cells. There was also an increased production of human pro-inflammatory cytokines, including: IFN-γ, IL-8, IL-18, MCP-1, and IL-6, suggesting their role in SLE pathogenesis. Increased expression of type I IFN signature genes was also found in human hepatocytes. Altogether, we showed an SLE model that was mediated by human immune system, and which recapitulated key clinical and immunological SLE features. The advancements of humanised mice SLE model would provide an in vivo platform to facilitate translational studies and pre-clinical evaluations of human-specific mechanisms and immunotherapies.
Similar content being viewed by others
Introduction
Systemic Lupus Erythematosus (SLE) is a chronic, relapsing autoimmune disorder where the immune system targets multiple self-nuclear antigens, leading to chronic organ damage and mortality1. The systemic nature of SLE is manifested in a highly heterogenous manner, such that the Systemic Lupus Collaborating Clinics (SLICC) established 17 criteria for SLE classification, including both clinical and immunological criterion2. Clinical manifestations of SLE involve multiple organs, ranging from skin rash, neurologic dysfunction, joint synovitis, serositis and renal inflammation, known as lupus nephritis. There is no cure for SLE, and current treatments for SLE mostly relied on empirical use of NSAIDs and immunosuppressants to manage symptoms associated with SLE. Only one FDA-approved treatment targeting B cell anomalies in patients with active SLE has emerged in the past 55 years3. As such, the need for SLE treatment to reduce mortality and morbidity remains critical.
The exact etiology of SLE remains unknown, and the disease is thought to derive from multiple factors, including genetic predispositions, environmental and hormonal factors. Study of human SLE face many challenges owing to the complex nature of SLE, and the lack of definitive diagnostic and prognostic biomarkers of disease activity4,5. Moreover, human studies are generally restricted by ethical limitations to in vitro or ex vivo assays. Animal models, particularly murine, have contributed to the bulk of knowledge regarding the etiopathogenesis of SLE6. Spontaneous models using inbred strains, such as the NZB/W F1 mice7, MRL/lpr mice8, and BXSB/Yaa mice models9, possess genetic backgrounds that confers SLE susceptibility, and develop spontaneous nephritis and autoantibodies production. These spontaneous models have been particularly useful in studying the complex genetic contribution in SLE. In addition to the spontaneous models, SLE can be induced in different mice strains through a number of ways, including induced Graft versus host disease10, as well as injection of a synthetic mineral oil known as pristane (Tetramethylpentadecane, TMPD)11. Single pristane injection into various mouse strains could induce most histopathological features of SLE, and it is one of the few animal SLE models to exhibit the type I interferon (IFN) signature genes (ISG) expression, as is observed in SLE patients12. Despite the non-spontaneous nature, induced SLE model is particularly beneficial in determining the contribution of single gene/factor in SLE pathogenesis, which would require significant time and resources to backcross onto the spontaneous SLE strains.
While various mouse models have provided fundamental insights on SLE pathogenesis, they have not fully recapitulated the whole spectrum and complexity of human SLE. Importantly, substantial differences exist between mouse and human immune system13,14. Findings in mouse models may not be directly translatable to human, and have to be taken with caution, particularly in the development and evaluation of therapeutic protocols. The use of humanised mice (from hereon referred to as hu-mice), where human immune system is stably reconstituted into immunodeficient mice, has allowed in vivo studies of human immunology, particularly for human-specific infectious diseases and cancer15. However, the use of hu-mice for the study of human autoimmune diseases remained largely unsuccessful16. Several attempts to study the pathogenesis of human SLE in immunodeficient mice have been described, through engraftment of peripheral blood mononuclear cells (PBMCs) from SLE patients into immunodeficient mice17,18,19. Nevertheless, these previous models were limited by low efficiency of human cells engraftment, requirement of large numbers of PBMCs from SLE patients who are often lymphopenic, or the lack of human anti-nuclear autoantibodies production, which is widely considered as one of the hallmark symptoms of SLE. Here we described a model of human immune system-mediated SLE induced by pristane injection in hu-mice. Most of the clinical and immunological features of human SLE, including production of human anti-nuclear autoantibodies, pulmonary serositis, lupus nephritis, proteinuria, lymphopenia, lymphocytes hyperactivation and aberrant cytokine production could be recapitulated. A model of SLE induced in human immune system would allow numerous in vivo studies of human-specific SLE pathogenesis in manners that is not possible in human. Importantly, it would facilitate translational studies for the development and evaluation of targeted immunotherapy for human SLE.
Results
Early mortality in pristane-injected hu-mice
Engraftment of human CD34+ haematopoietic stem cells (HSCs) into sublethally irradiated NOD/LtSz-PrkdcscidIl2rgtm1whl/J (NSG) pups consistently achieved good human immune system reconstitution, with an average of 42.12% (±1.296 S.E.M) reconstitution level in the blood, and higher in the tissues such as spleen (82.83% ± 2.77 S.E.M), mesenteric lymph nodes (97.39% ± 0.42 S.E.M), and liver (89.03% ± 1.98 S.E.M) (Supplementary Fig. S1A).
NSG mice completely lack T, B and NK cells. However, residual mouse myeloid cells remain, albeit with impaired function20. To examine if these residual mouse cells could contribute to SLE pathogenesis in hu-mice, pristane was injected to both NSG and hu-mice. Pristane-injected NSG mice appeared healthy, and there was no mortality throughout the observation period of 20 weeks post injection. On the other hand, pristane injection into hu-mice induced significantly earlier mortality with a median survival at 13 weeks post injection (Fig. 1A). The absolute cell number counts and reconstitution levels of human CD45+ leukocytes, CD3+ T cells, CD19+ B cells, CD4+ T cells and CD8+ T cells in control and pristane treated hu-mice were shown in Supplementary Fig. S1B. This showed that the human immune system is necessary and sufficient for the induction of SLE pathogenesis, leading to increased mortality in hu-mice; and, on the other hand, the residual mouse myeloid cells did not contribute significantly to the pathogenesis.
Increased mortality and production of human anti-nuclear autoantibodies in pristane-injected hu-mice. (a) Survival curve of hu-mice (left) and NSG (right), with or without pristane injection, as indicated, was monitored for 20 weeks after treatment. Figure shown is from three independent experiments (control n = 19; pristane n = 24; NSG control n = 9; NSG pristane n = 9). (b) Level of human anti-nuclear autoantibodies in blood plasma were measured by ELISA at 8 weeks post-pristane injection in hu-mice. Control group represents data from littermate hu-mice without pristane injection. Data shown is from four independent experiments (control n = 16; pristane n = 14). **P < 0.01; ***P < 0.001.
Induction of autoantibodies production
SLE is characterised by the presence of a spectrum of autoantibodies against nuclear antigens. Pristane-induced SLE model produced the widest range of autoantibodies compared to other spontaneous SLE mouse models12. Correspondingly, pristane injection into hu-mice increased the total level of human IgG and IgM (Supplementary Fig. S2A), and induced production of a wide range of human anti-nuclear autoantibodies, including anti-dsDNA, anti-histone, anti-RNP70, anti-SM and anti-SSA IgGs (Fig. 1B). Increased production of human anti-dsDNA IgG could be detected significantly as early as four weeks post injection, and continued to increase to week 8 (Supplementary Fig. S2B). There was no significant difference in the level of anti-dsDNA IgG in female and male pristane-injected hu-mice (Supplementary Fig. S2C).
Lupus Nephritis and Pleuritis
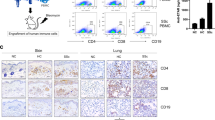
Lupus nephritis, or kidney inflammatory condition associated with SLE, affects the majority of SLE patients, and kidney failure is one of the leading causes of mortality in individuals with active SLE21. Lupus nephritis is characterised by the presence of immune deposits in the glomeruli, which induced leukocyte infiltration and severe damage, leading to protein leakage into the urine, or proteinuria22. Histological evaluation of kidneys from pristane-injected hu-mice revealed kidney inflammation and glomerular changes that are consistent with proliferative glomerulonephritis. Specifically, there was focal to diffuse global glomerular enlargement by mesangial/endocapillary proliferation and increased glomerular cellularity, which was not evident in the kidneys of pristane-injected NSG mice (Fig. 2A). Human leukocyte infiltration was further verified by immunohistochemistry staining of human CD45+ cells in the glomeruli of pristane-injected hu-mice (Supplementary Fig. S2D). Consistent with immune complex deposition observed in SLE patients, human IgG and IgM deposition was found in the glomeruli of pristane-injected hu-mice (Fig. 2B). Expectedly, this was accompanied by increased proteinuria in pristane-injected humice, which was not observed in pristane-injected NSG (Fig. 2C). The presence of kidney damage and proteinuria in pristane-injected hu-mice, but not pristane-injected NSG mice, again implicates the key role of human immune system, and the minimal contribution of residual mouse cells, in the induction of SLE pathogenesis upon pristane injection.
Lupus nephritis and pulmonary inflammation. (a) (Left) Kidney sections from hu-mice and NSG with or without pristane injection were H&E stained and pathologically evaluated. Scale bar represents 50 µm and images are representative from two independent experiments (control NSG n = 3; pristane NSG n = 3; control hu-mice n = 8; pristane hu-mice n = 12). (Right) Mean glomeruli area was measured from 50 random glomeruli from kidneys of each experimental animal (control NSG n = 3; pristane NSG n = 3; control hu-mice n = 5; pristane hu-mice n = 6). (b) Immunohistochemistry of human IgG and IgM on kidney sections from control and pristane-injected hu-mice Scale bar represents 50 µm and images are representative from two independent experiments (control n = 8; pristane n = 12). (c) Protein content in the urine of control and pristane-injected NSG, control and pristane-injected hu-mice at 10 weeks post-pristane injection was measured using Uristix reagent strips. Figure is from two independent experiments (control NSG n = 8; pristane NSG n = 3; control hu-mice n = 8; pristane hu-mice n = 7). (d) Lung sections were H&E stained and pathologically evaluated. Scale bar represents 200 µm and images are representative from two independent experiments (control n = 6; pristane n = 5). **P < 0.01.
In addition to nephritis, pulmonary manifestation affects up to 50% of individuals with SLE. The most common pulmonary manifestation associated with SLE is lung serositis (inflammation in the lining of the lung, known as pleuritis) and interstitial lung inflammation23. Pathological evaluation of lung from pristane-injected hu-mice revealed increased multifocal serosal and subpleural inflammation with fibrosis, as well as perivascular interstitial and intra-alveolar mononuclear cell infiltrate (Fig. 2D). Altogether, the presence of lupus nephritis, pulmonary serositis, and autoantibodies production showed that pristane injection could induce SLE pathogenesis that is mediated by the human immune system in hu-mice.
Lymphopenia in pristane-injected hu-mice
Lymphopenia is a common feature and one of the diagnostic criteria in SLE24. It, however, is not often recapitulated in most SLE animal models; instead, many well-studied SLE mouse models displayed massive lymphoproliferation6. Pristane injection into hu-mice induced a dramatic drop in the reconstitution percentage and absolute number of human immune cells in the blood over a period of 12 weeks (Fig. 3A; Supplementary Fig. S3A). Consistent with lymphopenia observed in SLE patients25, there was a significant reduction in the absolute number of human T cells, B cells and NK cells. B cell numbers were significantly reduced as early as four weeks post pristane injection, while the number of T cells and NK cells were reduced at a later stage at week 8 and 10, respectively (Fig. 3B). Notably, pristane injection to hu-mice induced an accumulation of both human and mouse immune cells in the peritoneal lavage (Supplementary Fig. S3B), and a mild spleen enlargement with a significant increase in the number of mouse, but not human immune cells (Supplementary Fig. S3C,D).
Lymphopenia in pristane-injected hu-mice. (a) Reconstitution level (left) and absolute number (right) of human immune cells in the peripheral blood of control and pristane-injected hu-mice were measured by flow cytometry at the indicated time points. (b) Absolute number of human T cells, B cells and NK cells in the peripheral blood was measured based on surface expression of human CD3, CD19 and CD56, respectively. Absolute cell numbers/μl blood was calculated using counting beads (Materials and Methods). Data shown was from four independent experiments (control n = 20; pristane n = 26). **P < 0.01; ***P < 0.001; ****P < 0.0001.
Lymphocyte hyperactivation in hu-mice
As antibody producing cells, B cells have been the focus of many studies and targeted immunotherapies for SLE, not only for their role in producing autoantibodies, but also in antigen presentation and cytokine expression26. Various B cell anomalies have been reported in SLE patients, including lymphopenia, hyperactivation, relative expansion of memory B cells, as well as increased frequency of antibody-producing plasma cells26. To determine B cell abnormality in pristane-injected hu-mice, B cell subsets and activation markers in peripheral blood as well lymphoid organs were analysed by flow cytometry. Indeed, pristane injection induced systemic B cell hyperactivation, as indicated by the increased expression of B cell activation marker CD86, in peripheral blood, spleen, mesenteric lymph nodes and peritoneal lavage (Fig. 4A). There was increased frequency (Fig. 4B and Supplementary Fig. S4A) and number (Supplementary Fig. S4B) of CD19+CD20−CD27hiCD38hi plasmablasts/plasma cells in the peripheral blood and spleen of pristane-injected hu-mice. The CD27−IgD− B cell population has been reported to consist of a distinct memory B cells population, and is highly expanded in SLE patients27. Parallel to the observation in SLE patients, there was a relative expansion in the frequency of CD27+ memory B cells and CD27−IgD− B cell population (Fig. 4C); and a reduction in the CD27−IgD+ naïve/transitional B cells compartment (Supplementary Fig. S4C).
B cell hyperactivation and anomalies. (a) Human B cell in the peripheral blood (PB), peritoneal lavage (lav), spleen and lymph node (LN) of control and pristane-injected hu-mice were analysed by FACS at 8 weeks after pristane injection. B cells activation was measured by expression of B cell activation marker, CD86. Histogram (left) showed representative staining of CD86 on CD19+CD20+ B cells in PB and peritoneal lavage of control and pristane-injected hu-mice. Relative expression level was quantified as Staining Index (right) Figure shown is from four independent experiments (control n = 18; pristane n = 14). (b) Human plasma cells/plasmablasts were identified by their surface markers CD19loCD20−CD27+CD38hi and were quantified as percentage of human CD45. Figure is from three independent experiments (control n = 11; pristane n = 10). (c) CD19+CD20+ B cell subsets were identified by their surface markers CD27 and IgD, as indicated, and were quantified as percentage of CD19+CD20+ B cells. Figure shown is from three independent experiments (control n = 14; pristane n = 12). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
In addition to its role in supporting antibodies production, T cells majorly contribute to the inflammatory response in SLE through inflammatory cytokine production and tissue infiltrations that often lead to organ damage28. Various signaling abnormalities in T cells lead to chronic T cell activation, reduced peripheral tolerance, and accumulation of autoreactive memory T cells29. Similar to patients with active SLE, pristane injection in hu-mice induced activation of both CD4+ and CD8+ T cells30,31. There was a marked reduction of both CD4+ and CD8+ T cells with naïve phenotype (CCR7+CD45RA+), and increased proportion of T cells with an effector memory phenotype (CCD7−CD45RA−) in the peripheral blood, spleen, mesenteric lymph nodes and peritoneal lavage (Fig. 5A), indicating a systemic inflammation condition. Accordingly, T cell activation was also evident in the increased proportion of both CD4+ and CD8+ T cells expressing HLA-DR (Fig. 5B), a human-specific lymphocyte activation marker32. Hence, we conclude that both T cell and B cell activation play a significant role in the pathogenesis of induced SLE in hu-mice. Moreover, SLE induction in hu-mice exhibited lymphocyte anomalies with high resemblance to the anomalies observed in SLE patients.
T cell hyperactivation and accumulation of memory cells. (a) Human T cells in the peripheral blood (PB), peritoneal lavage (Lav), spleen (SP) and lymph node (LN) of control and pristane-injected hu-mice were analysed by FACS at 8 weeks after pristane injection. Proportion of human CD4 and CD8 naïve, effector, and memory T cells were quantified according to the surface expression of CCR7 and CD45RA, as indicated. Pie chart shown is the mean value from four independent experiments (control n = 18; pristane n = 14). Statistical analysis is attached in Supplementary Table S1. (b) (Left) Flow plot showed representative staining of HLA-DR in CD4 and CD8 T cells in the PB. (Right) Percentage of HLA-DR expressing cells were quantified from the total CD4 or CD8 T cells population, as indicated. Figure shown is from three independent experiments (control n = 14; pristane n = 12). ***P < 0.001; ****P < 0.0001.
Production of human pro-inflammatory cytokines
Both T cells and B cells contribute to SLE pathogenesis through aberrant cytokines production, which modulates the innate and adaptive immune responses33. To measure the involvement of human cytokines in pristane-induced SLE in hu-mice, human pro-inflammatory cytokine levels were measured in blood plasma as well as peritoneal lavage fluid. There was a significant increase in the level of inflammatory cytokines such as IFN-γ, IL-18, IL-8, MCP-1 and IL-6 (Fig. 6A), which indicates their role in the SLE pathogenesis.
Aberrant production of human pro-inflammatory cytokines. (a) Levels of human proinflammatory cytokines, as indicated, in the blood serum and peritoneal lavage fluid (lavage) of control and pristane-injected hu-mice were measured by a multiplex bead-based immunoassay at 8 weeks after pristane injection. Figure shown is from five independent experiments (control n = 19; pristane n = 17).Ct: control; Pr: pristane. (b) Gene expression level of type I IFN target genes in human hepatocytes in control and pristane-injected humice at four weeks after pristane injection. Data is representative of three independent experiments (control n = 10; pristane n = 10). *P < 0.05; ***P < 0.001; ****P < 0.0001
Furthermore, it has been shown in our previous publications that with the transplantation of human fetal liver CD34+ cells, humanized mice develop a ~5% chimerism of human hepatocytes in the liver34,35. Consistent with the expression of type I IFN signature genes (ISG) expression in mouse pristane model, we observed increased expression of human ISGs (Mx1, Mx2, and ISG15) in the human hepatocytes of pristane-injected hu-mice (Fig. 6B). Taken together, systemic lymphocytes hyperactivation coupled with aberrant production of pro-inflammatory cytokines and increased expression of ISGs, suggests that pristane injection into hu-mice induces a chronic systemic inflammation similar as was seen in SLE.
Discussion
Hu-mice containing stable engraftment of human immune system in immunodeficient mouse have proven to be instrumental in the study of human immunology, particularly for human-specific infectious diseases and cancer models15. Nevertheless, hu-mice have not been widely used in the study of human autoimmune diseases, and previous attempts to model human SLE in humanised mice met with various limitations17,18,19. Despite its artificial nature of induction, pristane-induced SLE model strongly recapitulates many of the human SLE symptoms in mice. Here we show that pristane injection into hu-mice could induce SLE pathogenesis that is mediated by human immune system, and recapitulate major clinical and immunological features found in SLE patients.
Different hu-mice models could be generated from different sources of human HSCs engrafted to various immunodeficient mice strains. To date, NSG mice used in this study are among the best immunodeficient recipient strains for human cell engraftment: they have a long life span; completely devoid of mature lymphocytes and NK cells; and support stable development of human HSCs into mature and functional immune cells20. Engraftment of human CD34+ HSCs into irradiated NSG pups, as described here, consistently yielded good human immune cells reconstitution, with an average of 40% human immune cells reconstitution in the blood. The lymphoid tissues were, as expected, largely devoid of mouse immune cells, and were mostly comprised of human immune cells (Supplementary Fig. S1A). Still, residual mouse myeloid cells remain present in NSG, and pristane injection notably induced increased number of mouse immune cells in the spleen and peritoneal lavage (Supplementary Fig. S2). As such, we cannot completely rule out the contribution of the residual mouse myeloid cells in the induction and pathogenesis of SLE. Nevertheless, the absence of mouse mature lymphocytes, together with the lack of kidney damage and mortality when pristane was injected to NSG mice alone, strongly suggest that the residual mouse immune cells did not significantly contribute the SLE pathogenesis and that human immune cells were the key component in the pathogenesis of SLE in hu-mice (Figs 1A, 2A). Still, many approaches to completely eradicate these residual mouse cells are currently being generated, which would tremendously improve the application of hu-mice particularly in the studies of autoimmunity16.
Production of anti-nuclear autoantibodies is one of the characteristic features of SLE. Previous attempt to model human SLE by engraftment of PBMCs from SLE patients into immunodeficient mice did not induce increased production of human IgG and anti-dsDNA antibodies17. Pristane injection into hu-mice significantly increased the total level of human IgG and IgM, and importantly, induced the production of a wide range of human anti-nuclear autoantibodies, including anti-dsDNA, anti-histone, anti-RNP70, anti-SM and anti-SSA antibodies (Fig. 1B). In the current study, we could not assess the difference in disease severity between hu-mice derived from male and female HSC donors, which would require a much larger cohort of HSC donors. However, there was no significant difference in the level of anti-dsDNA production in male and female pristane-injected hu-mice (Supplementary Fig. S2C, which suggests that gender predominance in SLE is largely attributed to the genotype of the immune system. Furthermore, anti-dsDNA antibodies, one of the definitive SLE diagnostic criteria, could be significantly detected as early as 4 weeks post-pristane injection in hu-mice (Supplementary Fig. S2B). This could be highly beneficial for pre-clinical evaluation of novel therapies against SLE.
Lymphopenia is one of the clinical criteria in SLE diagnosis2, and it is detected in more than 90% of patients with active SLE36. Consistently, pristane injection into hu-mice induced a dramatic loss of human T cells, B cells and NK cells in the peripheral blood within 12 weeks post-pristane injection (Fig. 3). The exact cause of lymphopenia in SLE patients is still unknown, but it has been attributed to the excess level of type I IFN in these individuals37.
Immunological analysis of peripheral blood and lymphoid tissues in pristane injected hu-mice revealed hyperactivation of both B cells and T cells, resembling the systemic chronic inflammation state in SLE patients. Relative expansion of CD27+ memory B cells and CD27−IgD− population is consistent with observation in patients where B cell lymphopenia in SLE prejudicially affects CD19+CD27−IgD+ naïve B cells (Fig. 4C and Supplementary Fig. S4C)38. Additionally, consistent with findings in patients with active SLE38,39, increased number and frequency of antibody producing plasmablasts/plasma cells could be found in the peripheral blood and lymphoid tissues of pristane-injected hu-mice (Fig. 4B and Supplementary Fig. S4B). T cell abnormalities in SLE include abnormal signaling transduction, leading to lower threshold of T cells activation28 and amplification of inflammation response resulting in organ damage29. In concordance to the aberrant T cells activation in SLE, pristane injection into hu-mice induced the activation of both CD4+ T cells and CD8+ T cells, as shown by accumulation of CCR7−CD45RA− effector memory cells, and increased expression of HLA-DR, a human-specific lymphocyte activation marker (Fig. 5). Human CD45+ leukocytes were also found infiltrating into the glomeruli, thus contributing to the pathological damage seen in the kidney and other organs (Supplementary Fig. S2D)40.
Type I IFN has been shown to play a crucial role in SLE pathogenesis41. We were not able to detect increased level of human type I IFN (specifically, IFN-α), nor increased expression of ISGs in the blood of pristane-injected humice (data not shown). This might be attributed to the imperfect development and function of the myeloid subsets in humanised mice42. However, we did observe increased expression of human IFN signature genes in human hepatocytes in pristane-injected humice (Fig. 6B). A number of other human pro-inflammatory cytokines were also increased upon pristane injection (Fig. 6A). Increased level of IL-6 is in line with B cell hyperactivation, maturation into plasma cells and induction of autoantibodies production43. Production of IFN-γ and IL-17 signify the role of Th1 and Th17 cells in SLE44. IL-18 and MCP-1 have both been found to be elevated in the serum/plasma and urine of SLE patients, and was positively correlated to disease activity and renal involvement; both cytokines have been proposed as biomarkers for disease activity and renal involvement45,46. IL-8, which has no murine homologue, has been found to be significantly higher in the serum and bronchoalveolar lavage of SLE patients, and its level is positively correlated to disease activity47,48. Hu-mice SLE model could be particularly useful for studies of human-specific genes/factors, such as IL-8, in the induction and pathogenesis of SLE and as potential immunotherapy targets. Furthermore, recent advancements in genome editing have opened up huge applications potential with genetic manipulation. Genetic manipulation of human HSCs49 to be engrafted into humanised mice could be a revolutionary tool in studying the contribution of a single gene/factor in the pathogenesis of various human diseases, including SLE.
While hu-mice have proven to be instrumental in translational research involving human specific immune responses, several limitations remain in the current generation of humanised mice. As mentioned above, these limitations include the presence of residual mouse myeloid cells; suboptimal myeloid subsets reconstitution and function; and impaired B cells maturation and antibody production. Injection of pristane to hu-mice induced production of anti-nuclear autoantibodies (Fig. 1B), however, the level of these autoantibodies was much lower compared to those in SLE patients. Efforts to improve hu-mice have focused on improving the myeloid subset and adaptive immunity in the humanised immune system, which would particularly enhance the level of antibodies production. Different methods to supplement hu-mice with human-tendentious cytokines such as transgenic/knock-in mice and hydrodynamic injection based gene delivery have been developed to further improve human immune cell functions42,50. Future improvements on the adaptive and humoral immune response in hu-mice would certainly further improve the application of hu-mice in the study of human autoimmunity and other human diseases.
Our work provides a proof-of-concept study that hu-mice, particularly those derived from HSC transplantation, is able to recapitulate many key features of SLE immunopathology, and thus useful for SLE research. Furthermore, many human specific immunological responses were observed in this model, including HLA-DR expression on T cells, production of human specific genes (IL-8), and lymphopenia. Thus we anticipate that this new SLE model can provide a closest-to-human in vivo platform for the study of human specific mechanisms as well as immunotherapy target identification and evaluation.
Methods
Isolation of human CD34+ HSCs
Human fetal HSC specimens were obtained from aborted fetuses at 16–23 weeks of gestation in accordance with the institutional ethical guidelines of Kadang Kerbau Women’s and Children’s Hospital (KKH). All patients gave written informed consent for the donation of their fetal tissues for research. Isolation of human CD34+ HSCs were as described previously35. Briefly, human CD34+ HSCs were purified by magnetic-based cell sorting using EasySep CD34-positive selection kit (StemCell Technologies) under sterile condition, with purity of 90% and above. All methods involving human tissue samples were performed in accordance to the relevant guidelines and regulations, with approval from SingHealth and National Health Care Group Research Ethics Committees Singapore (CIRB Ref: 2012/064/B).
Generation of hu-mice and SLE model
NSG mice were purchased from The Jackson Laboratory, and bred in pathogen-free animal facility at Biological Resource Centre (BRC) in Agency for Science, Technology and Research (A*STAR). All animal experimental procedures were approved and done in accordance to Institutional Animal Care and Use Committee (IACUC) guidelines of A*STAR Singapore. NSG pups within 3 days after birth were sublethally irradiated with 1 Gy γ-ray and transplanted with 1 × 105 human CD34+ HSCs by intra-hepatic injections. Human immune cells reconstitution level was determined at 12 weeks post-transplantation by flow cytometry of the peripheral blood, and was calculated as percentage of human CD45+ cells among total cells expressing CD45 (both mouse and human).
Less than 6% of the total hu-mice population displayed a human reconstitution level lower than 10%, and were excluded from the study (Supplementary Fig. S1A). A total of 18 NSG and 82 hu-mice (engrafted from 5 different HSC donors) were used in this study, consisting of 8 male and 10 female NSG, and 37 male and 45 female hu-mice. Experimental mice were chosen randomly, regardless of sex and reconstitution level (Supplementary Fig. S1B). Pristane (Sigma Aldrich) was injected intra-peritoneally to 12–13 weeks old hu-mice and NSG.
Human immunoglobulin (Ig) and autoantibodies measurement
Levels of human IgG, IgM, and anti-nuclear (anti-dsDNA, anti-histone, anti-RNP70, anti-SM and anti-SSA) IgGs in the plasma of control and pristane-injected hu-mice were measured by ELISA quantification kits according to manufacturer’s instruction (From Bethyl laboratories, and Alpha Diagnostic Inc, respectively).
Histopathology and immunohistochemistry
Lungs and kidneys from control and pristane-injected hu-mice and NSG were harvested at eight weeks post-injection, fixed with 10% formalin and embedded in paraffin for processing into 5 µm tissue sections. Rehydrated lung and kidney sections were stained with Hematoxylin & Eosin (H&E) (Thermo Scientific), and evaluated by a pathologist who was blinded of the samples’ identity. Glomerular enlargement was quantified by area measurement of 50 random glomeruli from kidneys of each experimental animal. Images were captured using brightfield slide scanner (Axio Scan Z1) and processed by Zen software (Carl Zeiss). For immunohistochemistry (IHC), kidney sections were subjected to heat-mediated antigen retrieval with sodium citrate (pH6) buffer prior to staining with appropriate antibodies. IHC staining was performed using the SuperPicture 3rd Gen IHC Detection Kit (Life Technologies) according to manufacturer’s instruction. Primary antibodies used in the study include anti-human IgG, anti-human IgM (Bethyl Laboratories), and anti-human CD45 (AbCAM). Anti-mouse, anti-rabbit and anti-goat HRP conjugated secondary antibodies were purchased from Life Technologies.
Proteinuria
Urine was collected from control and pristane-injected hu-mice in the morning into a clean eppendorf tube, and when necessary stored in −80 °C for long-term storage. Urine was centrifuged at maximum speed to eliminate any sediment, and supernatant was dropped into urine analysis strip (Uristix, Siemen) according to manufacturer’s instruction.
Multiplex quantification of human cytokines
Blood plasma and peritoneal lavage fluid were collected from control and pristane-injected hu-mice at eight weeks post-injection. Plasma was collected through centrifugation of peripheral blood, while peritoneal fluid was collected by flushing the peritoneal cavity with 3 ml of PBS/2% FCS. Level of various human cytokines in the plasma and peritoneal lavage fluid was measured using multiplex bead-based cytometry array (BioLegend Legendplex) according to manufacturer’s instruction, acquired on LSR II flow cytometer (BD Biosciences) and data was analysed using Legendplex software.
Flow cytometry
For flow cytometry analysis, peripheral blood, peritoneal lavage cells and lymphoid tissues from control and pristane-injected hu-mice were processed to obtain single cell suspensions. Spleen, mesenteric lymph nodes and liver were digested with collagenase IV (Gibco) with an addition of DNaseI (Sigma Aldrich) to prevent cell clumping, and filtered to obtain single cells suspension. When necessary, cell suspensions were subjected to red blood cell lysis (Gibco). Cells were stained with fluorescent conjugated antibodies to human cell surface markers, as follows: CD3 (clone UCHT1; BioLegend), CD4 (clone SK3; BD Biosciences), CD8 (clone SK1; BioLegend), CD10 (clone HI10a; BD Biosciences), CD19 (clone SJ25C1; BD Biosciences), CD20 (clone 2H7; BioLegend), CD25 (clone BC96; BioLegend), CD27 (clone L128, BD Biosciences), CD38 (clone HB-7, Biolegend), CD45 (HI30; BioLegend, BD Biosciences), CD45RA (clone HI100; BioLegend), CD56 (clone HCD56; BioLegend), CD69 (clone FN50; BD Biosciences), CD86 (clone FUN-1; BD Biosciences), CD197 (clone A20, BD Biosciences), IgD (clone IA6-2; BioLegend), HLA-DR (clone L243; BD Biosciences) and mouse CD45.1 (clone A20; BD Biosciences and Biolegend). CountBrightTM (ThermoFisher) Absolute Counting Beads was used for quantification of absolute cell numbers, according to manufacturer’s instruction. Viable cells were distinguished by DAPI staining, and cell doublets were excluded through FSC-H and FSC-A gating. Samples were acquired on LSR II flow cytometer (BD Biosciences) and analysed with FlowJo (Tree Star). Relative expression level was measured in Staining Index, where the formula is [(MFI (Mean Fluorescence Intensity)(pos)/MFI(neg)*2 s.d(neg)]51.
Liver perfusion and enrichment of human EGFR+ hepatocyte
Liver perfusion was done as described previously35. Human EGFR+ cells were selected using PE selection kit (StemCell Technologies) according to manufacturer’s instruction. The enriched cells were then used for RNA extraction. The purity of enriched EGFR+ cells was >90% and the yield of live EGFR+ cells recovered from 16 weeks old control and pristine-treated mice ranged from ~400,000 to 800,000 cells/mouse.
Gene expression profiling by quantitative real-time PCR
RNA was extracted from human EGFR+ hepatocyte using the RNeasy Mini kit (Qiagen) and cDNA synthesis was performed using RT kit (Qiagen), with normalized amount of total RNA across samples. Quantitative RT-PCR (qPCR) was performed on Applied Biosystems 7500 real time pCR system (Applied Biosystems) using Sso-Eva-green reagent (Bio-rad) and custom-made primers (IDT) specific for human genes. The gene expression was normalised to house keeping gene, human GAPDH. The list of primers are attached (Supplementary Table S2)
Statistical Analysis
All statistical tests were performed using 2-tailed unpaired nonparametric test (Mann-Whitney). Survival curve comparison was performed with the Log-Rank (Mantel-Cox) test. Plots and statistical values were built and calculated using GraphPad Prism software. Error bars indicated SEM, and P < 0.05 was considered as significant.
References
Tsokos, G. C. Systemic lupus erythematosus. N Engl J Med 365, 2110–2121, https://doi.org/10.1056/NEJMra1100359 (2011).
Petri, M. et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64, 2677–2686, https://doi.org/10.1002/art.34473 (2012).
Navarra, S. V. et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 377, 721–731, https://doi.org/10.1016/S0140-6736(10)61354-2 (2011).
Eisenberg, R. Why can’t we find a new treatment for SLE? J Autoimmun 32, 223–230, https://doi.org/10.1016/j.jaut.2009.02.006 (2009).
Croker, J. A. & Kimberly, R. P. SLE: challenges and candidates in human disease. Trends Immunol 26, 580–586, https://doi.org/10.1016/j.it.2005.09.001 (2005).
Perry, D., Sang, A., Yin, Y., Zheng, Y. Y. & Morel, L. Murine models of systemic lupus erythematosus. J Biomed Biotechnol 2011, 271694, https://doi.org/10.1155/2011/271694 (2011).
Theofilopoulos, A. N. & Dixon, F. J. Murine models of systemic lupus erythematosus. Adv Immunol 37, 269–390 (1985).
Andrews, B. S. et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains.. J Exp Med 148, 1198–1215 (1978).
Maibaum, M. A., Haywood, M. E., Walport, M. J. & Morley, B. J. Lupus susceptibility loci map within regions of BXSB derived from the SB/Le parental strain. Immunogenetics 51, 370–372 (2000).
Via, C. S. Advances in lupus stemming from the parent-into-F1 model. Trends Immunol 31, 236–245, https://doi.org/10.1016/j.it.2010.02.001 (2010).
Satoh, M. & Reeves, W. H. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J Exp Med 180, 2341–2346 (1994).
Reeves, W. H., Lee, P. Y., Weinstein, J. S., Satoh, M. & Lu, L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol 30, 455–464, https://doi.org/10.1016/j.it.2009.06.003 (2009).
Shay, T. et al. Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc Natl Acad Sci USA 110, 2946–2951, https://doi.org/10.1073/pnas.1222738110 (2013).
Mestas, J. & Hughes, C. C. Of mice and not men: differences between mouse and human immunology. J Immunol 172, 2731–2738 (2004).
Shultz, L. D., Brehm, M. A., Bavari, S. & Greiner, D. L. Humanized mice as a preclinical tool for infectious disease and biomedical research. Ann N Y Acad Sci 1245, 50–54, https://doi.org/10.1111/j.1749-6632.2011.06310.x (2011).
Koboziev, I. et al. Use of Humanized Mice to Study the Pathogenesis of Autoimmune and Inflammatory Diseases. Inflamm Bowel Dis 21, 1652–1673, https://doi.org/10.1097/MIB.0000000000000446 (2015).
Andrade, D. et al. Engraftment of peripheral blood mononuclear cells from systemic lupus erythematosus and antiphospholipid syndrome patient donors into BALB-RAG-2-/- IL-2Rgamma-/- mice: a promising model for studying human disease. Arthritis Rheum 63, 2764–2773, https://doi.org/10.1002/art.30424 (2011).
Duchosal, M. A., McConahey, P. J., Robinson, C. A. & Dixon, F. J. Transfer of human systemic lupus erythematosus in severe combined immunodeficient (SCID) mice. J Exp Med 172, 985–988 (1990).
Mauermann, N., Sthoeger, Z., Zinger, H. & Mozes, E. Amelioration of lupus manifestations by a peptide based on the complementarity determining region 1 of an autoantibody in severe combined immunodeficient (SCID) mice engrafted with peripheral blood lymphocytes of systemic lupus erythematosus (SLE) patients. Clin Exp Immunol 137, 513–520, https://doi.org/10.1111/j.1365-2249.2004.02559.x (2004).
Shultz, L. D. et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 174, 6477–6489 (2005).
Cervera, R. et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 82, 299–308, https://doi.org/10.1097/01.md.0000091181.93122.55 (2003).
Borchers, A. T. et al. Lupus nephritis: a critical review. Autoimmun Rev 12, 174–194, https://doi.org/10.1016/j.autrev.2012.08.018 (2012).
Keane, M. P. & Lynch, J. P. III. Pleuropulmonary manifestations of systemic lupus erythematosus. Thorax 55, 159–166 (2000).
Rivero, S. J., Diaz-Jouanen, E. & Alarcon-Segovia, D. Lymphopenia in systemic lupus erythematosus. Clinical, diagnostic, and prognostic significance. Arthritis Rheum 21, 295–305 (1978).
Erkeller-Yusel, F., Hulstaart, F., Hannet, I., Isenberg, D. & Lydyard, P. Lymphocyte subsets in a large cohort of patients with systemic lupus erythematosus. Lupus 2, 227–231, https://doi.org/10.1177/096120339300200404 (1993).
Dorner, T., Giesecke, C. & Lipsky, P. E. Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther 13, 243, https://doi.org/10.1186/ar3433 (2011).
Jacobi, A. M. et al. Activated memory B cell subsets correlate with disease activity in systemic lupus erythematosus: delineation by expression of CD27, IgD, and CD95. Arthritis Rheum 58, 1762–1773, https://doi.org/10.1002/art.23498 (2008).
Mak, A. & Kow, N. Y. The pathology of T cells in systemic lupus erythematosus. J Immunol Res 2014, 419029, https://doi.org/10.1155/2014/419029 (2014).
Crispin, J. C., Kyttaris, V. C., Terhorst, C. & Tsokos, G. C. T cells as therapeutic targets in SLE. Nat Rev Rheumatol 6, 317–325, https://doi.org/10.1038/nrrheum.2010.60 (2010).
Blanco, P. et al. Increase in activated CD8+ T lymphocytes expressing perforin and granzyme B correlates with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum 52, 201–211, https://doi.org/10.1002/art.20745 (2005).
Fritsch, R. D. et al. Abnormal differentiation of memory T cells in systemic lupus erythematosus. Arthritis Rheum 54, 2184–2197, https://doi.org/10.1002/art.21943 (2006).
Viallard, J. F. et al. HLA-DR expression on lymphocyte subsets as a marker of disease activity in patients with systemic lupus erythematosus. Clin Exp Immunol 125, 485–491 (2001).
Wong, C. K., Ho, C. Y., Li, E. K. & Lam, C. W. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus 9, 589–593, https://doi.org/10.1191/096120300678828703 (2000).
Chen, Q. et al. Human fetal hepatic progenitor cells are distinct from, but closely related to, hematopoietic stem/progenitor cells. Stem Cells 31, 1160–1169, https://doi.org/10.1002/stem.1359 (2013).
Keng, C. T. et al. Characterisation of liver pathogenesis, human immune responses and drug testing in a humanised mouse model of HCV infection. Gut 65, 1744–1753, https://doi.org/10.1136/gutjnl-2014-307856 (2016).
Ng, W. L., Chu, C. M., Wu, A. K., Cheng, V. C. & Yuen, K. Y. Lymphopenia at presentation is associated with increased risk of infections in patients with systemic lupus erythematosus. QJM 99, 37–47, https://doi.org/10.1093/qjmed/hci155 (2006).
Kamphuis, E., Junt, T., Waibler, Z., Forster, R. & Kalinke, U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood 108, 3253–3261, https://doi.org/10.1182/blood-2006-06-027599 (2006).
Odendahl, M. et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol 165, 5970–5979 (2000).
Odendahl, M. et al. Perturbations of peripheral B lymphocyte homoeostasis in children with systemic lupus erythematosus. Ann Rheum Dis 62, 851–858 (2003).
Bagavant, H. & Fu, S. M. Pathogenesis of kidney disease in systemic lupus erythematosus. Curr Opin Rheumatol 21, 489–494, https://doi.org/10.1097/BOR.0b013e32832efff1 (2009).
Banchereau, J. & Pascual, V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 25, 383–392, https://doi.org/10.1016/j.immuni.2006.08.010 (2006).
Chen, Q., Khoury, M. & Chen, J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc Natl Acad Sci USA 106, 21783–21788, https://doi.org/10.1073/pnas.0912274106 (2009).
Tackey, E., Lipsky, P. E. & Illei, G. G. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus 13, 339–343, https://doi.org/10.1191/0961203304lu1023oa (2004).
Shah, K. et al. Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis Res Ther 12, R53, https://doi.org/10.1186/ar2964 (2010).
Favilli, F. et al. IL-18 activity in systemic lupus erythematosus. Ann N Y Acad Sci 1173, 301–309, https://doi.org/10.1111/j.1749-6632.2009.04742.x (2009).
Singh, R. G., Usha, Rathore, S. S., Behura, S. K. & Singh, N. K. Urinary MCP-1 as diagnostic and prognostic marker in patients with lupus nephritis flare. Lupus 21, 1214–1218, https://doi.org/10.1177/0961203312452622 (2012).
Lit, L. C., Wong, C. K., Tam, L. S., Li, E. K. & Lam, C. W. Raised plasma concentration and ex vivo production of inflammatory chemokines in patients with systemic lupus erythematosus. Ann Rheum Dis 65, 209–215, https://doi.org/10.1136/ard.2005.038315 (2006).
Ding, D., Mehta, H., McCune, W. J. & Kaplan, M. J. Aberrant phenotype and function of myeloid dendritic cells in systemic lupus erythematosus. J Immunol 177, 5878–5889 (2006).
Mandal, P. K. et al. Efficient ablation of genes in human hematopoietic stem and effector cells using CRISPR/Cas9. Cell Stem Cell 15, 643–652, https://doi.org/10.1016/j.stem.2014.10.004 (2014).
Rongvaux, A. et al. Human hemato-lymphoid system mice: current use and future potential for medicine. Annu Rev Immunol 31, 635–674, https://doi.org/10.1146/annurev-immunol-032712-095921 (2013).
Maecker, H. T., Frey, T., Nomura, L. E. & Trotter, J. Selecting fluorochrome conjugates for maximum sensitivity. Cytometry A 62, 169–173, https://doi.org/10.1002/cyto.a.20092 (2004).
Acknowledgements
This study was supported by the National Research Foundation Fellowship Singapore NRF-NRFF2017-03 to Qingfeng Chen and Joint Council Office Development Programme 1334k00082, Agency for Science, Technology and Research (A*STAR), Singapore to Qingfeng Chen. Jerry KY Chan received salary support from Singapore’s Ministry of Health’s National Medical Research Council (NMRC/CSA(SI)/008/2016). The authors thank Dr. Sabry Hamza and Dr. Aaron Zefrin Fernandis from Merck Research Laboratories, MSD, Singapore, for offering consultations and support.
Author information
Authors and Affiliations
Contributions
M.G. designed and performed experiments, analysed and interpreted data, and prepared the manuscript; Z.H., M.L., S.Y.T., W.W.S.T., X.Y.C., C.B.O. and S.D. performed experiments; Y.F., E.L., K.T.E.C., T.C.T. and J.K.Y.C. contributed the research tools and reagents and prepared the manuscript; Q.C. conceived the study, supervised the project and prepared the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gunawan, M., Her, Z., Liu, M. et al. A Novel Human Systemic Lupus Erythematosus Model in Humanised Mice. Sci Rep 7, 16642 (2017). https://doi.org/10.1038/s41598-017-16999-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16999-7
This article is cited by
-
Regulatory T cells expressing CD19-targeted chimeric antigen receptor restore homeostasis in Systemic Lupus Erythematosus
Nature Communications (2024)
-
B cell development and antibody responses in human immune system mice: current status and future perspective
Science China Life Sciences (2024)
-
CD4+CD69+ T cells and CD4+CD25+FoxP3+ Treg cells imbalance in peripheral blood, spleen and peritoneal lavage from pristane-induced systemic lupus erythematosus (SLE) mice
Advances in Rheumatology (2019)
-
The Post-GWAS Era: How to Validate the Contribution of Gene Variants in Lupus
Current Rheumatology Reports (2019)
-
Model organism data evolving in support of translational medicine
Lab Animal (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.