Abstract
Galling insects are a highly sophisticated herbivore group on Caryocar brasiliense, a tree that represents the main income source for many communities. The effect of architectural diversity of C. brasiliense trees on galling insect community diversity and abundance was studied. The abundance of adult insects and galled leaves were seven and 1.6 times higher in trees with a greater height/width of canopy (RHW) ratio, respectively. Gall parasitoid richness was 1.8 times greater on trees with higher RHW. Zelus armillatus (Lepeletier & Serville) (Hemiptera: Reduviidae) and ant numbers were 5.8 and 2.7 higher on trees with the largest and smallest RHW, respectively. More complex plant architectures favored species diversity for galling insects and their natural enemies. The competition among four galling insect species for space and feeding and the evidence of “prudence strategy” were, for the first time, observed for galling insects in the Brazilian Cerrado biome.
Similar content being viewed by others
Introduction
Galling insects are a highly sophisticated herbivore group1 and many of them damage economically important plants2. They developed the ability to modify host plant tissue to produce specialized structures where their larvae could develop protected from harsh environmental conditions while feeding on a rich food source3,4.
Changes to the host plant affect interactions with galling insects. The diversity and abundance of these insects differ with higher species numbers on more architecturally complex hosts5,6,7. On the other hand, the factors affecting the galling community and population trends of galling insects need to be studied at a local scale8 especially given that host traits mediated habitat interactions.
A system with Caryocar brasiliense Camb. (Malpighiales: Caryocaraceae), common and economic cerrado (savanna) tree, and four galling herbivores in central Brazil is appropriate to evaluate the hypotheses presented9,10. Galls induced by four hymenopteran species are distinct in shape and morphology, and abundant11,12,13,14, facilitating data collection and testing of hypotheses. The canopy of a tree is a small-scale biogeographic island and an example to test this hypothesis15. A more aggressive galling insect species could affect a member of this group or even extinguish it by altering the environment, as observed for exotic versus native plants16. Biogeographic island predicts that extinction rates are higher on smaller islands because they cannot withstand high populations of organisms with the rarest species being vulnerable to extinction16. Biogeographic island involves the history of the biological processes such as colonization, speciation and extinction to explain species distribution patterns17. Smaller trees would provide smaller rather than larger islands.
Caryocar brasiliense trees are protected by Brazilian federal law and represent the main income source of many communities10. These trees remain in Cerrado lands transformed into pasture or agricultural land in a common scenario of isolated individuals in the agro-urban-landscape. The effects of host plant attributes on the diversity and abundance of galling insects and their natural enemies on C. brasiliense trees were evaluated in a pasture area. The hypotheses that more complex host individuals – larger trees - (i.e. biogeographic island) support a higher diversity of galling insects and their community was tested7,18. Therefore, we expect to find more species and individuals of galling insects, parasitoids and predators on larger trees.
Results
Architectural diversity and galling insects
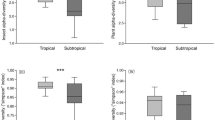
In comparison with trees with a smaller RHW, Caryocar brasiliense trees with a greater height/width canopy ratio (RHW) had 7.0 times the number of galling insects, 7.8 times more Eurytoma sp. (globoid galls) adults, 1.6 times the percentage of galled leaves, 7.2 times greater leaflet area with galls, 2.2 times more leaf area (mm2) occupied by Eurytoma globoid galls, and 3.9 times the number of Eurytoma globoid galls. On the other hand, the number of Hymenopteran discoid galls was 7.8 times higher on C. brasiliense trees with lower RHW than on those with greater RHW.
The C. brasiliense RHW did not affect diversity and richness of the galling insect, abundance of adult Bruchophagus sp. vein galls, Eulophidae spherical galls, or Hymenopteran discoid galls, the leaf area (mm2) occupied by Bruchophagus sp. vein galls, Eulophid spherical galls and hymenopteran discoid galls, width (mm) of conglomerate of globoid galls, gall numbers of Bruchophagus sp. vein galls and Euplophid spherical galls (Tables 1–3). The effect of C. brasiliense RHW on adult survival rates for Eurytoma globoid galls was not significant but they had a survival rate 2.6 times higher on higher RHW trees. The increase in the number of adults of Eurytoma sp. globoid galls and their galls reduced the number of Hymenopteran discoid galls and Bruchophagus sp. vein galls (Fig. 1).
Estimated network structures based on Spearman correlation (P < 0.05) generated for globoid galls (N.G. galls), vein galls (N.V. galls), spherical galls (N.S. galls) and discoid galls (N.D. galls), Eurytoma sp. (Ad. Eury) adults and their survivals (%) (S. Eury), Sycophila sp. (Ad. Syc), Zelus armillatus adults (Z. Armil) and ants per Caryocar brasiliense tree leaflet and height/width of canopy ratio (RHW) characteristics. Montes Claros, Brazil, autumn 2013 to autumn 2016.
Architectural diversity and natural enemies
Species richness of gall parasitoids was 1.8 times higher on Caryocar brasiliense trees with a higher RHW. The C. brasiliense RHW did not affect abundance or diversity of gall parasitoid species, but their values were 1.8 and 1.4 times greater, respectively, on higher RHW trees. The numbers of Quadrastichus sp. (Hymenoptera: Eulophidae) and Ablerus magistretti Blanchard (Hymenoptera: Aphelinidae) adults were similar between trees with low and high RHW, but the latter species was observed only on higher RHW trees.
Caryocar brasiliense RHW did not affect the number of Sycophila sp. (Hymenoptera: Eurytomidae) adults, but this insect had numbers 1.8 times higher on larger RHW trees. The effect of C. brasiliense RHW on the percentage of Sycophila sp. and Quadrastichus sp. adult survival was not observed, but both showed 2.4 times greater survival on lower and intermediate RHW trees, respectively (Tables 1–2).
The abundance, diversity and richness of predator species and the numbers of Epipolops sp. (Hemiptera: Geocoridae), Holopothrips sp. (Thysanoptera: Phlaeothripidae), spiders and Trybonia intermedius Bagnall and Trybonia mendesi Moulton (Thysanoptera: Phlaeothripidae) were similar between trees with different RWH. However, C. brasiliense trees with larger and smaller RHW had 5.8 and 2.7 times greater Zelus armillatus (Lepeletier & Serville) (Hemiptera: Reduviidae) and ant numbers, respectively (Tables 1–2).
Adult numbers and survival (%) of the parasitoid Sycophila sp. and the predator Z. armillatus increased with the frequency of Eurytoma sp. globoid galls and their galls (Fig. 1). On the other hand, the greater number of individuals of the predator Z. armillatus reduced the survival (%) of the parasitoid Sycophila sp. [Survival of Sycophila sp. (%) = 7.61 + 101.32x²√Z. armillatus − 104.00 x Z. armillatus, F = 3.34, P = 0.0477, R2 = 0.17]. Ants positively affected the numbers of Eulophid spherical galls and those of Hymenopteran discoid gall (Fig. 1).
Discussion
Architectural diversity and galling insects
The attacks more frequently of galling insects for taller C. brasiliense trees or those with wider crowns confirms the hypothesis that larger trees support more species and individuals of galling insects and their communities (i.e. parasitoids and predators)7,18 and the prediction of the plant vigour hypothesis (PVH). The PVH indicates oviposition preference of females and higher offspring performance of herbivorous insects on fast-growing plants (plant modules). Sawfly galls attacked more frequently longer shoots without galls and those with two, three, four, or five galls were successively longer than with fewer galls19. Bigger plants typically support more galling insect species5,20.
Galling insect preference for C. brasiliense trees in relation to height and canopy width are of two groups: i) Eurytoma sp. (most abundant) and ii) other three galling insect species. Eurytoma (globoid galls) preferred taller trees (RHW 3) where it showed higher gall numbers and area and consequently a higher percentages of galled leaflets and greater area with galls. The second group, Eulophidae spherical galls, Hymenopteran discoid galls, and Bruchophagus sp. vein galls did not prefer taller trees, but those with wider crowns (i.e. Hymenopteran discoid galls) generally showed higher numbers of its galls. Bruchophagus vein galls were not found on taller trees (RHW 3). The question why Eurytoma sp. attacks more frequently taller C. brasiliense trees and the other three galling insects a wider canopy may be explained by the fact that leaves on the top of taller C. brasiliense trees probably suffer a drying effect from the wind that shorter trees do not especially in regions with high temperatures and sunlight and low relative humidity10. Higher fruit production in the basal part than in the apical part of C. brasiliense canopy increased flower and fruit drop by winds10. This indicates that leaves more exposed to sunlight and winds can negatively affect their resistance to galling insects21. The higher wind and sunlight incidence harden leaves and may also explain the higher population of Eurytoma sp. on taller C. brasiliense trees. These leaves are, probably, a better food source for galling insects and Eurytoma sp. seems to predominate on them. Harsh ecophysiological conditions in the upper canopy of tropical rainforests increase gall-forming populations. Sclerophyll leaves increased with tree height while free-feeding herbivores decreased inversely22. Water and nutrient stress in the canopy tree meristem in tall wet tropical rainforests may cause leaf sclerophylly, forming a suitable ecosystem for gall-forming insects22. The other three galling insect species also prefer leaves more exposed to wind and sun12,13,14, but they preferred trees with a wider canopy than taller trees, avoiding competition with Eurytoma sp.
The pattern changes of the three galling insect species between C. brasiliense trees with low or high Eurytoma sp. density is related to sunlight and wind exposure, gall distribution on the leaf (i.e. border/interior of the plant canopy, border/near the mid vein, distal/proximal-near to petiole), and branch level (i.e. north/south)21. Most galling insects preferred the C. brasiliense leaves more exposed to the wind and sunshine, on the branch and at the leaf border and median parts11,12,13,14. However, other galling insects attacked other parts of C. brasiliense leaves in the presence of high Eurytoma sp. populations21. This suggests competition between the three galling insect species and Eurytoma sp. with the latter showing faster colonization of plants with greater biotic potential21. Caryocar brasiliense loses its leaves in Aug/Sep with new leaves appearing at the end of September, a period without rainfall and with strong wind and high sunlight10. Eurytoma sp. induces galls on young expanding C. brasiliense leaves when the wasp females were easily found ovipositing on unfolded leaves. Approximately two days later the oviposition site became reddish with a gall visible a few days later11. Plant phenology and colonization periods on C. brasiliense leaves explains the decrease of Eurytoma gall abundance as the numbers of other galling insects increased throughout the year as well as the differential temporal distribution of galling insects23. Chemical or visual markers may indicate which galling insect dominates a particular ecological niche (i.e., part of a leaf, branch or even a tree)21. The genetic differences between C. brasiliense plants and the chemical or morphological composition with different leaf parts, branch positions, sun/wind exposure or relationships with other arthropods (i.e., natural enemies)21 may also be responsible for this process or these differences.
The reduction of the galling insect communities on C. brasiliense, which acts as a biogeographic islands for these insects, can be explained by the collect of fruits without control affecting plant propagation. Caryocar brasiliense had only about 7.96 and 10.65% of individuals up to 1.0 m tall and 59.58 and 44.73% above 3.0 m (reproductive phase) in the cerrado and pasture areas, respectively10. This indicates that collectors remove nearly all fruits from the tree thus reduce C. brasiliense propagation in the cerrado of Brazil. Eurytoma sp. attack more frequently larger trees and the other three species of insect galling the smaller trees, which increases the risk of their extinction due to the inadequate renewal of C. brasiliense trees. This agrees with reports for exotic plants that can extinguish those native to smaller biogeographic islands16 and to Lepidoptera communities (i.e. generalists x specialists) in urban parks being affected by their size and human disturbance15.
Architectural diversity and natural enemies
A higher diversity of natural enemies, principally parasitoids, as was found with galling insects in the more complex host individuals, indicates that their populations depend on their prey and host and that they follow of the herbivorous insects24,25.
The natural enemies found can be divided into parasitoids and predators. The first group (i.e. Sycophila sp. and A. magistretti) showed the same trend as its hosts (galling insects) with higher richness, diversity and abundance on wider crown trees. Sycophila sp., a major Eurytoma sp.23,26 parasitoid showed a higher survival rate on lower RHW trees and Eurytoma had better fitness on higher RHW trees. The larger RHW trees had the highest number of Eurytoma sp. and Z. armillatus predators11,26. These facts showed an evidence of “prudence strategy”, whereby predators fed on parasitized prey, preserving the healthy prey as a food reserve for future generations, without endangering prey populations27. The predator Z. armillatus may prefer attacking Eurytoma sp. galls parasitized by Sycophila sp. The “prudence strategy” has been observed for Protonectarina sylveirae (Saussure) (Hymenoptera: Vespidae) with the leafminer Liriomyza sp (Diptera: Agromyzidae) and parasitized aphids (Hemiptera: Aphididae)28, but this is the first time that it is seen, evidenced, in galling insects. However, a prudence strategy would have to be demonstrated experimentally in future study. Positive relationships between indole butyric acid concentrations and successfully induced globoid galls as well as between the number of adults of the galling Eurytoma sp. and its parasitoid Sycophila sp. were found26. Selecting an enemy-free space could be the main reason why sawfly oviposition patterns with higher values on lower quality plants decreases predation and parasitism8,29,30,31.
The similar abundance, richness and diversity of the second group on C. brasiliense with different RHW is due to the fact that they are generalists with little dependence on a single prey, contrary to most parasitoids32 and these trees support greater diversity of free-feeding herbivore insects33,34,35,36. The higher percentages of defoliation and populations of defoliators and ants on C. brasiliense trees may be explained by the impact of predatory ants, bugs and spiders reducing defoliators and leaf miner insects on wider crown plants35. Variations in abundance and diversity of sucking insects and their natural enemies on wide crown trees and of the number of species and individuals of natural enemies is similar to those for Dikrella caryocar (Coelho, Leite & Da-Silva) (Hemiptera: Cicadellidae), of ants, predator thrips and lady beetles as well as with higher values on these C. brasiliense trees36.
Ants, Epipolops sp., Holopothrips sp., Sycophila sp., spiders, and Z. armillatus can be important to control Eurytoma sp. on C. brasiliense trees11,23,26,37,38. The higher numbers of the predator Z. armillatus on C. brasiliense trees at the University Campus might be due to more leaves galled by Eurytoma sp. on these trees than in pastureland and cerrado35. Zelus armillatus preyed on Eurytoma galls, which can cover up to 70% of the leaf area11,26. These galls can support higher diversity of natural enemies which can kill and cause a top down impact on natural enemies8,30,31.
Complexity of plant architecture (i.e. biogeographic island) favored the diversity of galling insect species and their parasitoids and the predator Z. armillatus. The patterns of natural enemies indicate that their populations depend on prey/hosts and that they follow those of the herbivorous insects. The competition among the four galling insect species for shelter and feeding space and the evidence of “prudence strategy” were observed for the first time for galling insects.
Methods
Study
This study was performed in the municipality of Montes Claros, Minas Gerais state, Brazil over three consecutive years (Jun 2013 through Jun 2016). The region has dry winters and rainy summers, climate Aw: tropical savanna according to Köppen39. The study was performed in pasture [S 16°46′16.1″ W 43°57′31.4″at 940 m a.s.l. altitude with dystrophic yellow red latossol soil with loamy texture34. The arthropods collected are neither an endangered nor protected species.
The pasture area has 84.2% of the soil covered by grass, 5.8%, 4.8% and 2.8% by herbs, small and tall trees, respectively with an average of 42 C. brasiliense trees per ha34.
Host Plants Studied and Galling Species
Caryocar brasiliense trees can reach over 10 m tall with a 6 m canopy width10. Its fruits have an internal mesocarp rich in oil, vitamins, and proteins with many compounds of medicinal importance. Moreover, it is used by humans for food, production of cosmetics, lubricants, and in the pharmaceutical industry40,41,42.
Adult (reproductive stage) C. brasiliense trees in the pasture area were two to nine meters tall with a two to 11 meters width canopy34. The hymenopteran galls on C. brasiliense leaves are Bruchophagus sp. vein galls (Hymenoptera: Eurytomidae), Eulophidae spherical galls (Hymenoptera), Eurytoma sp. glodoid galls (Hymenoptera: Eurytomidae) and Hymenopteran discoid galls, the morphology, natural history and tree distribution of which have been described11,12,13,14,21. Descriptions of the natural enemies and other herbivores in this C. brasiliense system were also provided21.
Study Design
The study design was completely randomized with 36 replications (36 trees). Data was collected from C. brasiliense adult trees (producing fruits) every 50 m along a 600-m transect. Insect species (i.e., rare species) data was collected over three consecutive years. No fertilizers or pesticides were used.
The distribution of galling insects and their galls, predators, and leaf percentages infested with galls (three leaflets/leaf) were recorded in 12 fully expanded leaves of 36 C. brasiliense trees (one leaf per canopy vertical and horizontal stratifications). Sampling was performed monthly in the morning (7–11 AM) by visual observation43. Insects were collected with tweezers, brushes, or aspirators and preserved in vials with 70% alcohol for identification by taxonomists. Twelve leaves per tree were collected and transported to the laboratory. Gall size was measured with a digital caliper (accurate to the nearest 0.1 mm). Leaves were scanned and their area and those of each leaf with galling species calculated using a computer program. Subsequently, leaves of each sample were placed in white plastic pots (temperature of 25 °C), and the emergence of galling insects, hyperparasitoids, inquilines and parasitoids evaluated on alternate days for 30 days. The insects emerged were collected and preserved as described for identification by taxonomists. The voucher number for spiders is IBSP 36921–36924 (Instituto Butantan, São Paulo state, Brazil) and that for insects is 1595/02 and 1597/02 (CDZOO, Universidade Federal do Paraná, Paraná state, Brazil).
Statistical Analyses
Averages were made by reducing the data to leaflet/tree. The richness and species diversity of galling insects and their parasitoids and predators were calculated per tree. The diversity was calculated using Hill´s formula44 and the species richness with Simpson indices45,46. The height and width (RHW) ratio on C. brasiliense trees and percentage survival of galling insect adults [(number of adults/number of galls)*100] and of the parasitoid adults [(number of adults/number of host adults)*100] were calculated. The ratio of height/width categories for C. brasiliense trees are: RHW 1: 1.00 to 1.64, RHW 2: 1.64 to 2.10 and RHW 3: 2.11 to 2.53 m with 12 trees per group.
The effect of RHW on ecological indexes and numbers of individuals per herbivore species and natural enemies (transformed to √x + 0.5 or arcsine for percentage data whenever necessary) was tested with ANOVA (P < 0.05) and Scott-Knott’s test (P < 0.05) and regression analysis were performed with the System of Statistical and Genetics Analysis of the Universidade Federal de Viçosa (UFV)47.
The Spearman correlation matrix, among the most significant characteristics, was calculated. The matrices were submitted to correlation networks48. The edge thickness was controlled by applying a cut off value of 0.33 (value from which the Spearman correlation became significant, meaning that only edges with \(|{{\rm{r}}}_{{\rm{ij}}}|\,\ge 0.33\) were highlighted). All the analyses were performed using the R software version 3.4.149. The correlation network procedure was performed using the package qgraph 48.
References
Shorthouse, J. D., Wool, D. & Raman, A. Gall-inducing insects- Nature’s most sophisticated herbivores. Basic. Appl. Ecol. 6, 407–411 (2005).
Fernandes, G. W. Gall forming insects: their economic importance and control. Rev. Bras. Entomol. 31, 379–398 (1987).
Price, P. W., Fernandes, G. W. & Waring, G. L. Adaptive nature of insect galls. Environ. Entomol. 16, 15–24 (1987).
Fernandes, G. W. & Price, P. W. Biogeographical gradients in galling species richness: Tests of hypotheses. Oecologia 76, 161–167 (1988).
Ferrier, S. M. & Price, P. W. Oviposition preference and larval performance of a rare bud-galling sawfly (Hymenoptera: Tenthredinidae) on willow in northern Arizona. Environ. Entomol. 33, 700–708 (2004).
Price, P. W. & Hunter, M. D. Long-term population dynamics of a sawfly show strong bottom-up effects. J. Anim. Ecol. 74, 917–925 (2005).
Espírito-Santo, M. M., Neves, F. S., Andrade-Neto, F. R. & Fernandes, G. W. Plant architecture and meristem dynamics as the mechanisms determining the diversity of gall-inducing insects. Oecologia 153, 353–364 (2007).
Price, P. W. Adaptive radiation of gall-inducing insects. Basic Appl. Ecol. 6, 413–421 (2005).
Bridgewater, S., Ratter, J. A. & Ribeiro, J. F. Biogeographic patterns, β-diversity and dominance in the Cerrado biome of Brazil. Biodivers. Conserv. 13, 2295–2318 (2004).
Leite, G. L. D., Veloso, R. V. S., Zanuncio, J. C., Fernandes, L. A. & Almeida, C. I. M. Phenology of Caryocar brasiliense in the Brazilian Cerrado Region. Forest Ecol. Manag. 236, 286–294 (2006).
Leite, G. L. D., Veloso, R. V. S., Silva, F. W. S., Guanabens, R. E. M. & Fernandes, G. W. Within tree distribution of a gall-inducing Eurytoma (Hymenoptera, Eurytomidae) on Caryocar brasiliense (Caryocaraceae). Rev. Bras. Entomol. 53, 643–648 (2009).
Leite, G. L. D., Cerqueira, V. M., d’Ávila, V. A., Magalhães, C. H. P. & Fernandes, G. W. Distribution of a leaf vein gall in Caryocar brasiliense (Caryocaraceae) tree. Rev. Caatinga 24, 186–190 (2011).
Leite, G. L. D., d’Ávila, V. A., Cerqueira, V. M., Nascimento, A. F. & Fernandes, G. W. Spatial distribution of a spherical gall (Hymenoptera, Eulophidae) on Caryocar brasiliense (Caryocaraceae). Rev. Bras. Entomol. 55, 396–400 (2011).
Leite, G. L. D., Nascimento, A. F., Jesus, F. M., Alves, S. M. & Fernandes, G. W. Within tree distribution of a discoid gall on Caryocar brasiliense. Rev. Colomb. Entomol. 37, 289–293 (2011).
Kitahara, M. & Fujii, K. An island biogeographical approach to the analysis of butterfly community patterns in newly designed parks. Res. Popul. Ecol. 91, 23–35 (1997).
Burns, K. C. Native–exotic richness relationships: a biogeographic approach using turnover in island plant populations. Ecology 97, 2932–2938 (2016).
Patiño, J. et al. A roadmap for island biology: 50 fundamental questions after 50 years of The Theory of Island Biogeography. J. Biogeogr. 44, 963–983 (2017).
Espírito-Santo, M. M., Neves, F. S., Andrade Neto, F. R., Silva, J. O. & Fernandes, G. W. Plant phenology and absence of sex-biased gall attack on three species of Baccharis. Plos One 7, e46896 (2012).
Fritz, R. S., Crabb, B. A. & Hochwender, C. G. Preference and performance of a gall-inducing sawfly: at test of the plant vigor hypothesis. Oikos 89, 555–563 (2000).
Hjältén, J., Roininen, H., Danell, K. & Price, P. W. Distribution and oviposition preference of galling sawflies in arctic Canada. Polar Biol. 26, 768–773 (2003).
Leite, G. L. D. Galling insects on Caryocar brasiliense Camb. (Caryocaraceae) in Neotropical Insect Galls (eds Fernandes, G. W. & Santos, J. C.) 179–192 (Springer, 2014).
Ribeiro, S. P. & Basset, Y. Gall-forming and free-feeding herbivory along vertical gradients in a lowland tropical rainforest: the importance of leaf sclerophylly. Ecography 30, 663–672 (2007).
Leite, G. L. D. et al. Seasonal abundance of galling insects (Hymenoptera) on Caryocar brasiliense (Malpighiales: Caryocaraceae) trees in the Cerrado. Fla. Entomol. 96, 797–809 (2013).
Oberg, S., Mayr, S. & Anddauber, J. Landscape effects on recolonisation patterns of spiders in arable fields. Agric. Ecosyst. Environ. 123, 211–218 (2008).
Venturino, E., Isaia, M., Bona, F., Chatterjee, S. & Badino, G. Biological controls of intensive agroecosystems: Wanderer spiders in the Langa astigiana. Ecol. Complex. 5, 157–164 (2008).
Leite, G. L. D., Veloso, R. V. S., Castro, A. C. R., Lopes, P. S. N. & Fernandes, G. W. Effect of AIB on quality and phytossanity of Caryocar brasiliense Camb. (Caryocaraceae) air layering. Rev. Arvore 31, 315–320 (2007).
Slobodkin, L. B. How to be a predator. Am. Zool. 8, 43–51 (1968).
Leite, G. L. D., Oliveira, I. R., Guedes, R. N. C. & Picanço, M. Predatory behaviour of Protonectarina sylveirae (Saussure) (Hymenoptera: Vespidae) in mustard. Agro-Cienc. 17, 93–96 (2001).
Fritz, R. S., Crabb, B. A. & Hochwender, C. G. Preference and performance of a galling-inducing sawfly: plant vigor, sex, gall traits and phenology. Oikos 102, 601–613 (2003).
Price, P. W. et al. Release of phylogenetic constraints through low resource heterogeneity: the case of gall-inducing sawflies. Ecol. Entomol. 29, 467–481 (2004).
McGeoch, M. A. & Price, P. W. Scale-dependent mechanisms in the population dynamics of an insect herbivore. Oecologia 144, 278–288 (2005).
Takahashi, F. Ecosystem immunity as a strategy for controlling insect pests in a biotic community. J. Crop Prod. 3, 49–61 (2008).
Leite, G. L. D. et al. Identification of the wood-borer and the factors affecting its attack on Caryocar brasiliense trees in the BrazilianSavanna. Acta Sci. Agron. 33, 589–596 (2011).
Leite, G. L. D. et al. The mortality of Caryocar brasiliense in northern Minas Gerais State, Brazil. Acta Sci. Agron. 34, 131–137 (2012).
Leite, G. L. D. et al. Habitat complexity and Caryocar brasiliense herbivores (Insecta; Arachnida; Araneae). Fla. Entomol. 95, 819–830 (2012).
Leite, G. L. D. et al. Diversity of Hemiptera (Arthropoda: Insecta) and their natural enemies on Caryocar brasiliense (Malpighiales: Caryocaraceae) trees in the Brazilian Cerrado. Fla. Entomol. 99, 239–247 (2016).
Leite, G. L. D. et al. Seasonal damage caused by herbivorous insects on Caryocar brasiliense (Caryocaraceae) trees in the Brazilian savanna. Rev. Colomb. Entomol. 38, 35–40 (2012).
Leite, G. L. D. et al. Seasonal abundance of hemipterans on Caryocar brasiliense (Malpighiales: Caryocaraceae) trees in the Cerrado. Fla. Entomol. 95, 862–872 (2012).
Vianello, R. F. & Alves, A. R. Meteorologia básica e aplicações (Universidade Federal de Viçosa, 2000).
Ferreira, L. C. & Junqueira, R. G. Microbiological evaluation of pequi (Caryocar brasiliense Camb.) preserves made from a typical Brazilian fruit. World J. Microbiol. Biotechnol. 23, 1179–1181 (2007).
Garcia, C. C., Franco, B. I. P. M., Zuppa, T. O., Antoniosi Filho, N. R. & Leles, M. I. G. Thermal stability studies of some cerrado plant oils. J. Therm. Anal. Calorim. 87, 645–648 (2007).
Khouri, J. et al. Anticlastogenic potential and antioxidant effects of an aqueous extract of pulp from the pequi tree (Caryocar brasiliense Camb). Genet. Mol. Biol. 30, 442–448 (2007).
Horowitz, A. R. Control strategy for the sweetpotato whitefly, Bemisia tabaci, late in the cotton-growing season. Phytoparasitica 21, 281–291 (1993).
Hill, M. O. Diversity and evenness: a unifying notation and its consequences. Ecology 54, 427–432 (1973).
Townsend, C. R., Bergon, M. & Harper, J. L. Fundamentos em ecologia (Artmed, 2006).
Lazo, J. A., Valdes, N. V., Sampaio, R. A. & Leite, G. L. D. Diversidad zoológica asociada a un silvopastoreo leucaena-guinea con diferentes edades de establecimiento. Pesqui. Agropecu. Bras. 42, 1667–1674 (2007).
Euclides, R. F. Sistema de análises estatisticas e genéticas (Universidade Federal de Viçosa, 1983).
Epskamp, S., Cramer, A. O. J., Waldorp, L. J., Schmittmann, V. D. & Borsboom, D. qgraph: Network Visualizations of Relationships in Psychometric Data. J. Stat. Softw. 48, 1–18 (2012).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2014).
Acknowledgements
To “Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)”, “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)”, “Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG)”,“Programa de Proteção Florestal (PROTEF)” of the “Instituto de Pesquisas Florestais (IPEF)” and “and Secretaria de Ciência e Tecnologia do Estado de Minas Gerais” for financial support. Dr. Phillip John Villani (The University of Melbourne, Australia) revised and corrected the English language used in this manuscript.
Author information
Authors and Affiliations
Contributions
G.L.D.L., J.L.S. and R.V.S.V. performed the experiments; A.M.A., C.I.M.A., G.L.D.L. and M.A.S. analyzed the data; A.M.A., C.F.W., G.L.D.L., J.C.Z. and R.V.S.V. designed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Leite, G.L.D., Veloso, R.V.d.S., Zanuncio, J.C. et al. Architectural diversity and galling insects on Caryocar brasiliense trees. Sci Rep 7, 16677 (2017). https://doi.org/10.1038/s41598-017-16954-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16954-6
This article is cited by
-
The association of beneficial and pest arthropods on Sapindus saponaria saplings fertilized with sewage sludge in a degraded area
Phytoparasitica (2023)
-
Does fertilization with dehydrated sewage sludge affect Terminalia argentea (Combretaceae) and associated arthropods community in a degraded area?
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.