Abstract
Smoking-cessation therapy reduces the risk of smoking-related diseases, but is successful only in a fraction of smokers. There is growing evidence that genetic variations in nicotinic acetylcholine receptor (nAChR) subunits influence the risk of nicotine dependence and the ability to quit smoking. To investigate the role of polymorphisms in nAChR genes on smoking quantity and the outcome of smoking-cessation therapies, we carried out an association study on 337 smokers who underwent pharmacotherapy with varenicline, bupropion, nicotine replacement therapy (NRT) alone, or NRT plus bupropion. Smoking habit and abstention were assessed from the number of cigarettes smoked per day (CPD) and the exhaled CO (eCO), at baseline and up to 12 months. We genotyped seven polymorphisms in genes encoding the nAChR subunits CHRNA4, CHRNA5, and CHRNB2. At baseline, both CPD and eCO were associated with polymorphisms in the CHRNA5 locus (rs503464, rs55853698, rs55781567 and rs16969968; P < 0.01). rs503464, a variant in the 5′-UTR of CHRNA5, was also associated with short-, mid- and long-term responses to therapy (P = 0.011, P = 0.0043, P = 0.020, respectively), although after correction for multiple testing only the association at the mid-term assessment remained significant (FDR = 0.03). These data support the role of individual genetic makeup in the ability to quit smoking.
Similar content being viewed by others
Introduction
Cigarette smoking is the leading cause of avoidable morbidity and mortality in the world1. Tobacco use increases the risk of death from many common diseases, including cardiovascular and non-neoplastic pulmonary diseases as well as different cancers2,3. Quitting smoking before the age of 40 reduces the risk of dying from smoking-related disease by about 90%3,4. However, quitting is difficult, as approximately only 6% of smokers manage to quit on their own annually5.
The ability to quit smoking is negatively influenced by nicotine dependence, as heavy smokers are more likely to fail to cease smoking than light smokers6. Genetic studies have widely demonstrated that smoking cessation and nicotine addiction are genetically determined7, and several chromosomal loci have been associated with these phenotypes8,9,10,11,12. Of note, three such loci contain clusters of genes coding for six nicotinic acetylcholine receptor (nAChR) subunits: CHRNB3-CHRNA6 on chromosome 8p11, CHRNA5-CHRNA3-CHRNB4 on chromosome 15q25, and CHRNA4 on chromosome 20q13. Single nucleotide variations (SNPs) in these loci have repeatedly been shown to be significantly associated with nicotine dependence (reviewed in13). SNPs in another nAChR subunit, CHRNB2 on chromosome 1, are also believed to associate with nicotine dependence14.
The nAChRs are cation channels activated by acetylcholine and expressed in the nervous system, muscles and lungs. These receptors are pentameric proteins composed of various combinations of subunits named α1–10, β1–4, γ δ and ε. nAChRs bear the prime responsibility for tobacco addiction15, since they are also activated by nicotine, the cigarette’s major biologically active substance16. nAChRs are therefore targets of smoking-cessation therapies, the most effective of which are nicotine replacement therapy (NRT), varenicline, and bupropion17. Varenicline binds α4β2-containing nAChR receptors as a partial agonist18, while bupropion is a noncompetitive antagonist of nAChRs19. The efficacy of these treatments is still limited and variable20. This variability could be due to genetic differences in the nAChRs, as several pharmacogenetic studies have found significant associations between genetic polymorphisms in nAChR subunit genes and the success of pharmacological smoking-cessation treatments (reviewed in12,21). More recently, King et al.12 found associations between abstinence during varenicline treatment and SNPs in CHNRA4, CHNRA5 (and other genes in the chromosome 15q25 locus) and CHNRB2, but no association between abstinence under bupropion treatment and nAChR SNPs. Bergen et al.22 reported associations between NRT success and two SNPs in the chromosome 15q25 locus, but not with a SNP in CHNRB2.
To further evaluate the possibility that genetic variations in nAChR subunits influence smoking habit and the effectiveness of smoking-cessation therapies, we studied a cohort of adult smokers who sought pharmacological treatment in our institute. For the present study, we genotyped seven selected SNPs in three nAChR subunits. In CHNRA4, we studied rs2236196, previously associated with abstinence during varenicline treatment12 but not with smoking cessation during NRT23. In CHRNA5, we investigated five SNPs, including the non-synonymous coding variant rs16969968, suggested to affect the risk of nicotine dependence by altering the function of α5-containing nAChRs24, as well as three SNPs in the 5′-UTR (rs503464, rs55853698, and rs55781567) and one 22-bp insertion/deletion in the promoter (rs3841324), all suggested to be involved in nicotine dependence by modulating CHRNA5 subunit mRNA levels25,26,27,28,29,30. Finally, in CHRNB2, we genotyped rs2072661, previously associated with nicotine dependence14 but not with response to pharmacological or behavioural therapies for smoking cessation22. The overall aim of our study was to identify genetic variants that can predict the intensity of the smoking habit and the effectiveness of pharmacological smoking-cessation therapies.
Results
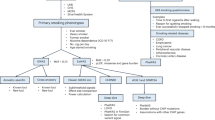
The study considered 337 Italian adults (197 men and 140 women) who participated in smoking-cessation programs (Table 1). Before beginning treatment, they consumed a median of 20 cigarettes per day (CPD) and had a median expired CO level (eCO) of 20 parts per million (ppm). Two thirds of participants had received varenicline, while the others were treated with bupropion, NRT, or both.
One month after the start of treatment, at the short-term evaluation, the majority of patients (76.3%) had stopped smoking (Fig. 1). At the mid- and long-term evaluations, corresponding to three and 12 months after the start of treatment, the percentage of patients who did not smoke had decreased to 64.4% and 47.2%, respectively, due to smoking relapse. Indeed, 92 patients who had quit at the short-term follow-up restarted smoking within one year of the start of treatment. There was no association between the type of therapy and the success of quitting and abstaining from smoking, at any time point considered (Table 2).
Polymorphisms in CHRNA5 are associated with nicotine dependence
Genotype frequencies of all seven nAChR polymorphisms were found to respect the Hardy-Weinberg equilibrium in our patient series. To test the association of these polymorphisms with nicotine dependence, we considered two nicotine addiction-related phenotypes: CPD and eCO at baseline. Four SNPs in the CHRNA5 locus (e.g. rs503464, rs55853698, rs55781567 and rs16969968) were found to be significantly associated with both CPD and eCO (Table 3). Of note, the associations of these four SNPs with CPD and eCO remained statistically significant after the correction for multiple testing (FDR < 0.05). No significant association with either eCO or CPD was found for rs3841324 in CHRNA5, rs2072661 in CHRNB2, or rs2236196 in CHRNA4.
For the four significantly associated SNPs, linear regression showed two patterns for the impact of the minor allele (Supplementary Figure S1, Table 3). The SNP rs503464 had a negative β value in the association with CPD (P = 0.6 × 10−3), indicating that as the number of minor alleles (A) increased, the number of cigarettes smoked decreased. On the contrary, the other three significant SNPs (rs55853698, rs55781567 and rs16969968) had β > 0, meaning that an increase in the number of minor alleles was associated with an increase in the number of cigarettes smoked. Regarding eCO, the β values for each significant SNP were concordant with those from the analysis of CPD, meaning that, depending on the number of minor alleles, the variation in eCO goes in the same direction as the variation in the number of cigarettes smoked per day.
rs503464 in the 5′-UTR of CHRNA5 is associated with smoking cessation
We then evaluated the association of nAChR SNPs with the response to smoking-cessation therapy (Table 4). Only one SNP, rs503464, a variant in the 5′ UTR of CHRNA5, was associated with the response (logistic regression, P < 0.05). This association was seen at all three follow-up visits, with an OR < 1, meaning that increasing the number of minor alleles (A) confers a lower probability of continuing smoking. This association appears to be free of confounding by nicotine dependence, since baseline eCO was a covariate in the logistic regression. After correction for multiple testing, however, only the association with mid-term response to smoking-cessation therapies remained statistically significant (FDR < 0.05). At the mid-term follow-up, the percentage of patients who abstained from smoking increased progressively with the number of minor alleles at rs503464, so that 10 of the 11 cases with AA genotype (91%) were not smoking at the 3-month visit (Supplementary Figure S2).
We repeated the logistic regression for rs503464 by grouping genotypes according to a dominant model for the minor allele (comparing individuals carrying at least one copy of the minor allele with homozygotes for the common allele). Also with this analysis, the OR for rs503464 was less than one at all three evaluation points, indicating that subjects having at least one minor allele (A) had a lower probability of continuing smoking than subjects carrying the common allele (Fig. 2).
Individuals carrying at least one minor allele (A) of rs503464 have a lower probability of continuing smoking than subjects carrying the common allele (OR < 1). Plot of the log-transformed OR (diamond) and 95% confidence intervals at short-, mid- and long-term evaluations after smoking cessation therapies (P = 0.020, P = 0.0030, and P = 0.012, respectively). Logistic regression was carried out using sex and therapy as covariates and considering a dominant model for the minor allele.
Discussion
In this study, we tested the involvement of nAChR subunit polymorphisms in nicotine dependence and the response to smoking-cessation therapy, and found an association of CHRNA5 SNPs with both phenotypes. In particular, the coding SNP rs16969968 and the 5′-UTR SNPs (i.e. rs503464, rs55853698, rs55781567) were significantly associated with both baseline CPD and eCO, taken as measures of nicotine dependence. rs503464 also associated with response to smoking-cessation therapies at all three time points examined. No association with nicotine dependence or smoking-cessation success was found for the two tested SNPs in CHRNA4 and CHRNB2.
Our data are in agreement with the repeatedly reported association of rs16969968 with nicotine dependence31 and also confirm the observed association of rs55853698 with smoking quantity32. Additionally, we detected a significant association for two 5′-UTR polymorphisms of CHRNA5 gene (rs503464 and rs55781567) that had never been associated with nicotine dependence. rs503464 was tested for association with nicotine dependence in Bierut et al.24, but no significant association was found. To the best of our knowledge, rs55781567 has never been reported to associate with nicotine dependence, but only with CHRNA5 expression levels. Therefore, our results support the influence of individual genetic constitution on smoking intensity. Indeed, both the coding polymorphism rs16969968, altering the protein structure of the α5 subunit, and the regulatory variations upstream of the initiation codon of CHRNA5 mRNA (rs503464, rs55853698, and rs55781567) were shown here to have a role in the genetic predisposition to nicotine dependence. The latter three SNPs (evaluated as a haplotype together with the promoter ins/del variation rs3841324) were associated with CHRNA5 expression levels in normal lung tissue27 and were also reported to have a functional role in modulating CHRNA5 promoter transcriptional activity in vitro in neuroblastoma cells25; these findings suggest that these polymorphisms play a functional role in nicotine dependence by influencing CHRNA5 mRNA level in the lungs and possibly in the brain.
We found a novel association of rs503464 also with the success of smoking-cessation therapy that was consistent along time and independent of baseline eCO, used as an estimate of nicotine dependence. This result suggests that smoking-cessation phenotype is associated with the alteration of CHRNA5 mRNA levels, being significantly associated with the regulatory SNP rs503464, rather than with variations in the amino acid sequence of the α5 subunit. Further investigations are needed to understand the mechanism through which the modification of CHRNA5 mRNA level influences the ability to quit smoking during smoking-cessation therapy. The reported association between the upregulation of brain nAChRs and the success of quitting smoking33 supports the hypothesis that modulation of CHRNA5 levels affects the ability to quit smoking. After multiple testing correction, only for the association of rs503464 with smoking-cessation therapy mid-term response reached statistical significance. Replication of our results in a wider and independent population series would allow to strengthen our findings, also overcoming the lack of information about the ancestry of the genotyped subjects who, however, all had Italian residency.
All four CHRNA5 regulatory polymorphisms (rs3841324, rs503464, rs55853698, and rs55781567) and the coding SNP rs16969968 that we investigated here have also been associated with lung cancer risk27,34. Therefore, the present results support the hypothesis that smoking habit mediates the link between the 15q25 locus and the risk of developing lung cancer35,36. Overall, our results strengthen the importance of CHRNA5 subunit in smoking-related phenotypes.
The assessment of smoking status from the eCO reading is a strength of this study that makes the association analyses more robust, since CPD is a self-reported and, therefore, subjective evaluation37, especially when smokers do not admit their failure in quitting smoking. Indeed, it has been reported that eCO is a better biomarker than CPD for use in genetic associations studies38.
Collectively, this analysis of genetic variants in nAChR subunits supports the role of individual genetic makeup in the ability to quit smoking. Of note, our findings point to the importance of the CHRNA5 regulatory SNP rs503464 in both nicotine dependence and smoking-cessation success. Further studies involving larger patient series are needed to validate the association of rs503464 with smoking abstinence during smoking-cessation treatment. Such studies will allow us to demonstrate the clinical utility of this SNP in personalized smoking-cessation therapy, chosen according to the individual genetic constitution.
Methods
Study population and clinical database
This study was carried out at the Fondazione IRCCS Istituto Nazionale dei Tumori (Milan, Italy), a public cancer institute. The study protocol was approved by the institute’s Committee for Ethics, and the research was conducted in accordance with the tenets of the Declaration of Helsinki. Overall, 337 adults seeking to quit smoking were studied. Although information on the ethnic origins of these patients was not available, all patients had Italian residency. We included 214 smokers who had received smoking-cessation counselling and treatment at the Tobacco Control Unit of our institution between 2009 and 2012. In addition, we included another 123 subjects who had participated in a smoking-cessation program within the Multicentric Italian Lung Detection (MILD) trial (a pilot observational trial) during 2009–2010 39. Patients had given their informed consent for the collection of personal and clinical information and biological materials for research purposes.
Patients recruited at the Tobacco Control Unit were unselected for age and smoking intensity. According to their smoking characteristics, they had received pharmacotherapy with varenicline, bupropion, NRT alone, or NRT plus bupropion, with various formulations and doses for the durations indicated in Supplementary Table S1. If, after 15 days, the therapy was ineffective or caused side effects, patients could change to another therapy: we considered the first recorded treatment unless one of the treatments was varenicline, in which case we considered this treatment. Patients recruited through MILD were all heavy smokers (defined as > 20 pack-years), were 49–75 years old, and were all treated with varenicline according to the following treatment scheme: 0.5 mg/day for the first three days, 0.5 mg twice daily for following four days, and 1 mg twice daily from day 8 to the end of month 3. All patients included in this study had taken a medication long enough to be considered compliant (Table 5) even if they did not complete the full duration of therapy; this choice was made due to our expectation that a patient’s genetic constitution affects the efficacy of treatment. Patients from both groups (Tobacco Control Unit and MILD) received counselling, as described in Pozzi et al.39.
From the smoking-cessation programs, we obtained data on the patients’ sex, age, and smoking habit at four time points, namely baseline and follow-up at 1, 3, and 12 months after the start of therapy. At each visit, patients self-declared if they were smoking or not, and, if they were still smoking, they self-declared the number of cigarettes per day (CPD) as an indication of smoking intensity. Additionally, they underwent breath testing to measure their exhaled carbon monoxide (eCO), which is produced by the incomplete combustion of carbon-containing material in the lung and provides an estimate of how much smoke has been inhaled. eCO is therefore used to monitor progress in smoking cessation37. Baseline values of CPD and eCO were considered to be markers of nicotine dependence. During follow-up, patients were defined as having stopped smoking when they both self-declared smoking cessation and had an eCO reading < 6 ppm. Finally, we obtained a peripheral blood sample that had been collected for genetic studies.
DNA biobank and genotyping
DNA was extracted from peripheral blood using the DNeasy Blood & Tissue Kit (Qiagen) and was quantified by spectrophotometry (ND-2000c, NanoDrop Products, Wilmington, DE, USA). SNP-containing fragments were PCR-amplified using SNP-specific primers (Supplementary Table S1). Then, six SNPs were genotyped by pyrosequencing: rs2072661 in CHRNB2 (chr. 1), four SNPs mapping to CHRNA5 (rs503464, rs55853698, rs55781567 and rs16969968 on chr. 15q25), and rs2236196 mapping in CHRNA4 (chr. 20q13). Pyrosequencing was performed on a PSQ96MA system (Biotage, Uppsala, Sweden) running PyroMark Q96 ID Software (Qiagen). Additionally, a 22-bp insertion/deletion (ins/del, rs3841324), 71 bp upstream of the CHRNA5 transcription start site, was genotyped by 3% agarose gel electrophoresis.
Statistical analysis
The association between therapy and smoking-cessation success, at each study time point, was analyzed with the Cochran-Armitage test for trend in proportions. The congruity of genotype frequencies at each SNP locus was tested with respect to the Hardy-Weinberg equilibrium.
Regression analyses were carried out with data collected at three time points during the treatment protocol. The first time point was one month after the start of therapy and reflects patients’ ability to stop smoking by the fourteenth day of treatment, when treatment efficacy was assessed (short-term response). The second time point was three months after the start of therapy and corresponds to the end of standard therapy (mid-term response). The last time point was 12 months after the start of therapy and indicates if the effects of smoking-cessation therapies can be maintained for a long time (long-term response).
Linear regression was used to assess possible associations between SNP genotypes and both baseline smoking intensity (CPD) and baseline eCO. This statistical analysis was adjusted for sex, and based on the additive effects of SNPs, i.e., β > 0 means that there is a direct proportionality between the number of minor alleles and the smoking parameter. Logistic regression was used to assess associations between SNP genotypes and the response to smoking-cessation therapies (“yes” or “no” for quitting smoking), with sex, therapy, and eCO as covariates. Odds ratios (ORs) were calculated considering an additive effect of SNPs, i.e., an OR > 1 means that the risk of continuing smoking increases with the number of minor alleles. Statistical significance in regression analyses was adjusted for multiple testing using the Benjamini-Hochberg procedure to obtain the false discovery rate (FDR). We also tested a dominant model for the minor allele of rs503464, comparing the group of individuals heterozygous or homozygous for the minor allele with individuals homozygous for the common allele; this logistic regression analysis was adjusted for sex and therapy.
Statistical tests were carried out using PLINK software. Statistical significance was set at P < 0.05.
References
Wipfli, H. & Samet, J. M. Global economic and health benefits of tobacco control. Clin. Pharmacol. Ther. 86(part 1), 263–271 (2009).
Patel, R. R., Ryu, J. H. & Vassallo, R. Cigarette smoking and diffuse lung disease. Drugs 68, 1511–1527 (2008).
Pirie, K. et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 381, 133–141 (2013).
Jha, P. et al. 21st-century hazards of smoking and benefits of cessation in the United States. N. Engl. J. Med. 368, 341–350 (2013).
Malarcher, A., Dube, S., Shaw, L., Babb, S. & Kaufmann, R. Quitting Smoking Among Adults–United States, 2001–2010. Morbidity and Mortality Weekly Report (MMWR) 60, 1513–1519 (2011).
Bierut, L. J., Johnson, E. O. & Saccone, N. L. A glimpse into the future - Personalized medicine for smoking cessation. Neuropharmacology 76(Pt B), 592–599 (2014).
Lessov-Schlaggar, C. N., Pergadia, M. L., Khroyan, T. V. & Swan, G. E. Genetics of nicotine dependence and pharmacotherapy. Biochem. Pharmacol. 75, 178–195 (2008).
Gelernter, J. et al. Genome-wide association study of nicotine dependence in American populations: identification of novel risk loci in both African-Americans and European-Americans. Biol. Psychiatry 77, 493–503 (2015).
Hancock, D. B. et al. Genome-wide meta-analysis reveals common splice site acceptor variant in CHRNA4 associated with nicotine dependence. Transl. Psychiatry. 5, e651 (2015).
Thorgeirsson, T. E. et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat. Genet. 42, 448–453 (2010).
Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat. Genet. 42, 441–447 (2010).
King, D. P. et al. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo-controlled clinical trials. Neuropsychopharmacology 37, 641–650 (2012).
Zuo, L. et al. Replicated Risk Nicotinic Cholinergic Receptor Genes for Nicotine Dependence. Genes (Basel) 7, E95 (2016).
Wessel, J. et al. Resequencing of nicotinic acetylcholine receptor genes and association of common and rare variants with the Fagerstrom test for nicotine dependence. Neuropsychopharmacology 35, 2392–2402 (2010).
Wu, J. Understanding of nicotinic acetylcholine receptors. Acta Pharmacol. Sin. 30, 653–655 (2009).
Benowitz, N. L. Nicotine addiction. N. Engl. J. Med. 362, 2295–2303 (2010).
Cahill, K., Stevens, S., Perera, R. & Lancaster, T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst. Rev. 5, CD009329 (2013).
Rollema, H. et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52, 985–994 (2007).
Fryer, J. D. & Lukas, R. J. Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine. J. Pharmacol. Exp. Ther. 288, 88–92 (1999).
Cahill, K., Lindson-Hawley, N., Thomas, K. H., Fanshawe, T. R. & Lancaster, T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst. Rev. 5, CD006103 (2016).
Kortmann, G. L., Dobler, C. J., Bizarro, L. & Bau, C. H. Pharmacogenetics of smoking cessation therapy. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 153B, 17–28 (2010).
Bergen, A. W. et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenet Genomics 23, 94–103 (2013).
Spruell, T. et al. Association between nicotinic acetylcholine receptor single nucleotide polymorphisms and smoking cessation. Nicotine Tob. Res. 14, 993–997 (2012).
Bierut, L. J. et al. Variants in nicotinic receptors and risk for nicotine dependence. Am. J. Psychiatry 165, 1163–1171 (2008).
Doyle, G. A. et al. In vitro and ex vivo analysis of CHRNA3 and CHRNA5 haplotype expression. PLoS One 6, e23373 (2011).
Falvella, F. S., Galvan, A., Frullanti, E. & Dragani, T. A. Re: Variants weakly correlated with CHRNA5 D398N polymorphism should be considered in transcriptional deregulation at the 15q25 locus associated with lung cancer risk. Clin Cancer Res 15 (2009).
Falvella, F. S. et al. Promoter polymorphisms and transcript levels of nicotinic receptor CHRNA5. J. Natl. Cancer Inst. 102, 1366–1370 (2010).
Smith, R. M. et al. Nicotinic alpha5 receptor subunit mRNA expression is associated with distant 5′ upstream polymorphisms. Eur. J. Hum. Genet. (2010).
Wang, J. C. et al. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol. Psychiatry 14, 501–510 (2009).
Wang, J. C., Bierut, L. J. & Goate, A. M. Variants weakly correlated with CHRNA5 D398N polymorphism should be considered in transcriptional deregulation at the 15q25 locus associated with lung cancer risk. Clin. Cancer Res. 15, 5599; author reply 5599 (2009).
Bierut, L. J. Convergence of genetic findings for nicotine dependence and smoking related diseases with chromosome 15q24-25. Trends Pharmacol. Sci. 31, 46–51 (2010).
Liu, J. Z. et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat. Genet. 42, 436–440 (2010).
Brody, A. L. et al. Brain nicotinic acetylcholine receptor availability and response to smoking cessation treatment: a randomized trial. JAMA Psychiatry. 71, 797–805 (2014).
Shen, B. et al. CHRNA5 polymorphism and susceptibility to lung cancer in a Chinese population. Braz. J. Med. Biol. Res. 46, 79–84 (2013).
Galvan, A. & Dragani, T. A. Nicotine dependence may link the 15q25 locus to lung cancer risk. Carcinogenesis 31, 331–333 (2009).
Macqueen, D. A. et al. Variation in the alpha 5 nicotinic acetylcholine receptor subunit gene predicts cigarette smoking intensity as a function of nicotine content. Pharmacogenomics J. (2013).
Middleton, E. T. & Morice, A. H. Breath carbon monoxide as an indication of smoking habit. Chest 117, 758–763 (2000).
Bloom, A. J. et al. Beyond cigarettes per day. A genome-wide association study of the biomarker carbon monoxide. Ann. Am. Thorac. Soc. 11, 1003–1010 (2014).
Pozzi, P. et al. A combined smoking cessation intervention within a lung cancer screening trial: a pilot observational study. Tumori 101, 306–311 (2015).
Acknowledgements
This work has been in part supported by the 5 × 1000 contribution; we wish to thank all those citizens who decided to donate their 5 × 1000 to Fondazione IRCCS Istituto Nazionale dei Tumori, Milan. Francesca Colombo is recipient of a Fondazione Umberto Veronesi fellowship.We thank Dr. Valerie Matarese for scientific editing. The funding organization had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
G.P., R.B. and F.C. made intellectual contributions to the conception and/or design of the study. G.P., A.G., P.P., G.P., F.S. and F.C. were involved in the management of patients’ clinical data. U.P. and R.B. obtained biological samples from patients participating in the MILD trial and attending the smoking-cessation counselling, respectively. S.N. prepared DNA samples. G.P. and A.G. performed DNA genotyping. G.P. and F.C. were involved in statistical analyses and manuscript drafting. All authors participated in critical revision of the article and gave their final approval of the submitted version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pintarelli, G., Galvan, A., Pozzi, P. et al. Pharmacogenetic study of seven polymorphisms in three nicotinic acetylcholine receptor subunits in smoking-cessation therapies. Sci Rep 7, 16730 (2017). https://doi.org/10.1038/s41598-017-16946-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16946-6
This article is cited by
-
Nicotinic acetylcholine gene cluster CHRNA5-A3-B4 variants influence smoking status in a Bangladeshi population
Pharmacological Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.