Abstract
Consistent discrepancies in the p16/HPV-positivity have been observed in head and neck squamous cell carcinoma (HNSCC). It is therefore questionable, if all HPV+ and/or p16+ tested cancers are HPV-driven. Patients down-staged according to the HPV-dependant TNM are at risk for undertreatment and data in clinical trials may be skewed due to false patient inclusion. We performed a meta-analysis to classify clinical outcomes of the distinct subgroups with combined p16 and HPV detection. 25 out of 1677 publications fulfilled the inclusion criteria. The proportion of the subgroups was 35.6% for HPV+/p16+, 50.4% for HPV−/p16−, 6.7% for HPV−/p16+ and 7.3% for HPV+/P16−. The HPV+/p16+ subgroup had a significantly improved 5-year overall-survival (OS) and disease-free-survival in comparison to others both for HNSCC and oropharyngeal cancers. The 5-year OS of the HPV−/p16+ HNSCC was intermediate while HPV+/p16− and HPV−/p16− had the shortest survival outcomes. The clearly distinct survival of HPV−/p16+ cancers may characterize a new relevant HPV-independent subtype yet to be biologically characterized. The possibility also exists that in some HPV+/p16+ cancers HPV is an innocent bystander and p16 is independently positive. Therefore, in perspective, HPV-testing should distinguish between bystander HPV and truly HPV-driven cancers to avoid potential undertreatment in HPV+ but non-HPV-driven HNSCC.
Similar content being viewed by others
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide1. From an epidemiological perspective, exogenous carcinogen-exposure (tobacco and alcohol consumption)-induced HNSCC is declining, while human papillomavirus (HPV) infection-driven HNSCC is increasing in younger individuals in recent years2. Due to a lack of screening-test the incidence of HPV+ HNSCC is increasing, while cervical cancer is decreasing in the USA3 and Europe for example in Germany4 and France5. If HNSCC is HPV+ this may be, because HPV drives carcinogenesis and can then be considered as causal, or HPV is detected as an innocent bystander of concurrent infection6. This bystander infection can be imagined as an infection located next to the tumor and co-sampled with the tumor material or as an integrated virus copy that has been silenced e.g. by promoter methylation or gene deletion with no active gene transcription. Thus, only if the expression of HPV-related oncogenes is detected, the tumor is proven to be HPV-driven6,7,8 indicated by the existence of HPV+/p16− cases. Thus, unless HPV-oncogene expression has been determined the tumor should be referred to as HPV-associated.
HPV+ tumors have been identified in many regions of head and neck2,9, especially in oropharyngeal squamous cell carcinomas (OPSCC)10,11. These tumors differ from HPV-unrelated cancers at the molecular level12,13,14,15 and likely, as a consequence, have a much more favorable prognosis, despite the generally more advanced stage at presentation16. Given by the nature of HPV-driven tumors, the recently released 8th Edition of the American Joint Committee on Cancer (AJCC) created a separate staging algorithm for high-risk-HPV-associated cancer of the oropharynx distinguishing it from HPV− OPSCC17. HPV+ OPSCC, that used to be advanced stage are now categorized into lower stage. This gives a much more accurate and reasonable prediction of survival for patients with HPV+ OPSCC.
Preclinical studies also demonstrated that biological features depending on tumor HPV status would influence the effectiveness of treatment. HPV-driven tumors respond better to chemotherapy and X-ray or proton therapy than HPV− tumors18,19,20. Because of the differences in biological features and prognosis, individually optimized therapy for patients with HPV-driven tumors would minimize treatment-related toxicity and improve outcomes. Consequently, de-escalated treatment protocols are under investigation.
Most importantly, for a correct classification a reliable distinction between the different entities is crucial. HPV-DNA testing and p16ink4a (cyclin-dependent kinase 2a) immunohistochemical staining are both well-established methods in identifying HPV+ tumors and often regarded delivering equal information on HPV positivity. This is due to the correlation of high-risk HPV E7 expression and in consequence an upregulation of p16. Since, p16 immunohistochemical staining is inexpensive, convenient in use, and the interpretation of results is established it is widely used for detection of HPV-related HNSCC. Therefore, in the modified 8th AJCC/UICC, p16 was recommended as HPV surrogate marker with the cutoff point for diffuse (≥75%) overexpression in a histological section and at least moderate (+2/3) staining intensity17. Although both p16 overexpression and HPV-DNA-positivity have shown their independent prognostic value, as we summarized before21, there are p16+/HPV− and p16−/HPV+ subgroups in which surprisingly different prognosis relative to p16- and HPV-status was observed. Survival of patients with HNSCC was better if associated with HPV+/p16+ or HPV−/p16+. Therefore, in addition to the HPV-related prognostic feature, the biological relevance of p16 independent of HPV infection is currently of interest and under investigation, possibly describing another subgroup of HNSCC with a role of p16 in HPV-independent HNSCC.
In this meta-analysis, we included all current clinical studies and evaluated the clinical relevance of HPVDNA-positivity and p16 overexpression in HNSCC. Current observations in elucidating the biological role of p16 in HPV+ and HPV− tumors were also discussed.
Methods
Selection criteria and literature search strategy
Four database searches were performed for publications that statistically analysed subgroup survival after detection of both HPV and p16 markers in PubMed (http://www.ncbi.nlm.nih.gov/pubmed), OVID (www.ovid.com), EMBASE (www.embase.com), and Wanfang (www.wanfangdata.com.cn). This search included publication dates up to April 20, 2017, adding an additional two years to the previously performed literature search21. We searched for the terms “HPV, p16, head neck”. We also included references quoted in original or review articles that may not have been found during the initial literature search. We screened the articles and included all studies of HNSCC patients which investigated survival rates by the p16 and HPV status of the tumor. Our search strategy was performed in accordance with PRISMA criteria and registered in the PROSPERO register (CRD42017062330). We excluded studies that met the following criteria: missing patient survival information, evaluation of only one marker (HPV or p16), non-HNSCC primary cancer (e.g., nasopharyngeal carcinoma, skin cancer, pre-cancer), cell culture or animal models, and reviews or case reports. We also excluded studies with duplicate patient data from the same or similar populations (based on the authors’ names and institutions) in a second round selection process. Where this occurred, we selected the study which was either more recent or had larger patient numbers. We also excluded studies with insufficient survival data. Finally, we included studies with the following criteria: (1) the numerical portion of the subgroups HPV+/p16+ versus HPV−/p16− versus HPV+/p16− versus HPV−/p16+ in HNSCC patients; (2) the numerical survival data of these subgroups (Hazard ratio (HR); overall survival (OS); disease free survival (DFS)) or Kaplan-Meier curves of the subgroups of OS or DFS.
Data extraction
Two authors (A.C. and A.E.A.) extracted the relevant data from the selected publications according to the aforementioned inclusion criteria21. In the case of any discrepancies, we re-analysed the study and the two authors reached a consensus decision. We extracted all relevant information from the studies, including: author, publication date, study timeframe, country, tumor stage and localisation, number of patients, study design, data on alcohol and tobacco consumption, number of HPV positive and negative patients, number of patients included in the subgroups HPV+/p16+, HPV−/p16-, HPV+/p16−, or HPV−/p16+, HPV subtypes, HR-status, 5-year OS or DFS of the subgroups, p16 and HPV detection method. We used GraphClick (Version 3.0.2, Arizona Software 2010, www.arizona-software.ch/graphclick) for data processing in studies where the OS or DFS was displayed as Kaplan-Meier plot.
Statistical analysis
The OS and DFS of all subgroups was evaluated using relative risk (RR)21, calculating summary RR estimates and 95% confidence intervals (CI) using maximum-likelihood methods for linear mixed models. We assessed study heterogeneity using a chi-squared based Q test. An absence of heterogeneity between the studies was indicated by a p-value greater than 0.05. Existing heterogeneity was examined using the I2 index in the meta-analysis, which was represented as a percentage value between 0 and 100. We initially applied a fixed-effects model (Mantel-Haenszel method and chi-squared test) to the data. Where there was significant heterogeneity, we used the random-effects model (DerSimonian-Liard method). We examined the RR of the 5-year OS and DFS of all subgroups correlating with the HPV+/p16+ and HPV−/p16− groups, depending on the data we extracted from each publication. In cases where the HR was described in the studies, we performed the same analysis. We compared all of the studies using a forest plot.
Publication bias was examined using a funnel plot. We used the R Version 3.1.0 (R Core Team 2014) computing environment for all statistical analyses22.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Results
Study characteristics
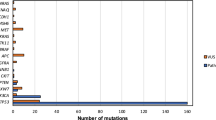
The initial search yielded 1677 citations (Fig. 1). 25 articles met the inclusion criteria23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47 (Fig. 1 and Table 1) including a total of 6852 patients, with patients per study ranging between 34 and 1542. The previous study had covered 18 studies and enrolled 4424 patients21. An updated literature search located 7 further studies including 2428 patients. However, the HPV/p16-status for some of these patients is missing, therefore we finally analyzed the data of 5131 study patients (range, 34–1479 patients per study). The main characteristics of the eligible studies were summarized in Tables 1–3. We have investigated 18 articles with studies that enrolled patients with UICC tumor stage I-IV, and 13 studies separately with OPSCC (Table 1). 20 studies performed HPV detection after preceding polymerase chain reaction (PCR) and 5 performed in situ hybridization (ISH) without earlier PCR. The identified subgroups discriminated by HPV and p16-status are represented in Table 2. The proportions of each subgroup were estimated from 22 studies, which clearly have patient numbers23,24,25,26,27,28,30,31,32,33,34,35,37,38,39,40,41,42,43,44,46,47. In all studies combined, the subgroup of HPV+/p16+ was 35.6%, of HPV−/p16− 50.4%, of HPV−/p16+ 6.7% and of HPV+/p16− 7.3%. In the studies that only investigated OPSCC, the subgroup of HPV+/p16+ was 44.9%, of HPV−/p16− 44.4%, of HPV−/p16+ 5.4% and of HPV+/p16− 5.3%. The studies investigating correlations between HPV-status and clinicopathological characteristics like tumor size, lymph node involvement, smoking etc. summarized in Table 3. This analysis includes 10 European, 9 North American (USA and Canada) and 6 Asian studies.
5-year OS and HPV/p16 subgroup status
Pooled outcome (5-year OS) results of 19 studies for all four distinct HPV/p16 subgroups can be seen in Fig. 2. For the 5-year OS investigation, 16 of 19 studies (3634 patients) contained data suitable for subgroup HPV+/p16+ and HPV−/p16− analysis. Figure 2A depicts a forest plot of this meta-analysis and demonstrates that subgroup HPV+/p16+ is associated with improved OS (fixed effects model; RR of 2.81; 95% CI 2.53–3.11; P = 0.61). Eleven studies that enrolled 1617 patients have data for subgroup HPV+/p16+ and HPV−/p16+ analysis, and 11 studies that enrolled 1545 patients for subgroup HPV+/p16+ and HPV+/p16−. As shown in Fig. 2B and C, a forest plot demonstrates that HPV+/p16+ has an improved OS compared with both the HPV−/p16+ (fixed effects model; RR of 2.16; 95%CI 1.81–2.58; P = 0.26) and the HPV+/p16− (random effects model; RR of 2.45; 95%CI 1.83–3.27; P = 0.007).
Twelve of 19 studies (2311 patients) displayed data for the 5-year OS of subgroup HPV−/p16− and HPV−/p16+ and 10 studies (1527 patients) of subgroup HPV−/p16− and HPV+/p16−. The forest plot in Fig. 3B shows the 5-year OS of HPV−/p16− does not significantly differ from the HPV+/p16− (random effects model; RR of 1.01; 95%CI 0.76–1.36; P < 0.0001). However, the 5-year OS of the HPV−/p16− subgroup was inferior compared to the HPV−/p16+ (fixed effects model; RR of 0.82; 95%CI 0.71–0.93; P = 0.21; Fig. 3A).
Adjusted Relative Risk (RR) of the 5-year overall survival (OS) compared to the HPV−/p16− subgroup. Forest plot of RR among included studies for the 5-year OS of the HPV−/p16− subgroup compared to (A) HPV−/p16+ and (B) HPV+/p16−. (C) Forest plot of RR compares HPV−/p16+ and HPV+/p16−. Combined RR was calculated by a random mode.
Additionally, 8 studies (428 patients) reported the 5-year OS of subgroup HPV+/p16− and HPV−/p16+ (Fig. 3C). There was no significant difference between the two subgroups. However, when one study (Ramshankar et al.42) was excluded, the 5-year OS of the subgroup HPV+/p16− was inferior compared with the HPV−/p16+ (fixed effects model; RR of 0.7, 95%CI: 0.58–0.84, P = 0.06). The Ramshankar et al.42 study reported a small correlation between HPV16-DNA and p16 expression in oral tongue SCC patients. Those patients with p16 overexpression showed an increased risk of death and disease recurrence regardless of their HPV16-DNA status.
5-year OS of the HPV/p16 subgroups for cancers of oropharyngeal origin
5-year OS in all distinct HPV/p16 subgroups in cancer of oropharyngeal origin was determined in 9 studies. The subgroup HPV+/p16+ showed a better survival than the HPV−/p16− (fixed effects model; RR of 2.87; 95% CI 2.56–3.23; P = 0.20), the HPV−/p16+ (fixed effects model; RR of 2.26; 95% CI 1.85–2.75; P = 0.09) and the HPV+/p16− (random effects model; RR of 2.67; 95% CI 1.75–4.07; P = 0.04). Figure 4A–C show the forest plots of the meta-analysis for each comparison. Four studies included data on the 5-year OS of the subgroup HPV−/p16− and the HPV−/p16+, and 3 studies of the HPV−/p16− and the HPV+/p16− subgroup. The forest plot in Fig. 5A demonstrates the superior 5-year OS of HPV−/p16+ compared with HPV−/p16− (fixed effects model; RR of 0.78; 95%CI 0.66–0.93; P = 0.28). The subgroup HPV−/p16− did not differ significantly to the HPV+/p16− (fixed effects model; RR of 0.93; 95%CI 0.80–1.07; P = 0.55; Fig. 5B). It was not possible to meta-analyze the subgroup HPV+/p16− and the HPV−/p16+ as there were only two studies presenting these data. The analysis of OPSCC patients revealed a similar 5-year OS of the subgroups in studies investigating HNSCC.
Adjusted Relative Risk (RR) of the 5-year overall survival (OS) compared to the HPV+/p16+ subgroup in patients with oropharyngeal cancer origin. Forest plot of RR among included studies for the 5-year OS of the HPV+/p16+ subgroup compared to (A) HPV−/p16−, (B) HPV-/p16+ and (C) HPV+/p16−. Combined RR was calculated by a random mode.
Adjusted Relative Risk (RR) of the 5-year overall survival (OS) compared to the HPV−/p16− subgroup in patients with oropharyngeal cancer origin. Forest plot of RR among included studies for the 5-year OS of the HPV−/p16− subgroup compared to (A) HPV−/p16+ and (B) HPV+/p16−. Combined RR was calculated by a random mode.
HR for OS of the HPV/p16 subgroups
We could determine the OS-HR from 12 studies. Five studies used HPV+/p16+ and 7 studies used HPV−/p16− as reference markers. We summarize the results of the individual meta-sub-analyses in Table 4. The HR for the OS of the subgroup HPV+/p16+ was significantly increased compared to the HPV−/p16−, being irrespective of whether HPV+/p16+ or HPV−/p16− was used as reference values. The HR for the better OS of the subgroup HPV+/p16+ was significantly increased compared to the HPV−/p16+ and the HPV+/p16−, respectively. The meta-analyses of the HRs for the OS of the subgroup HPV−/p16−, the HPV−/p16+ and the HPV+/p16− included only 2 and 4 studies, respectively. Because the HRs of these sub-analyses didn’t show significant differences, we are unable to draw any general conclusion due to the limited data.
5-year DFS in the distinct HPV/p16 subgroups
Eleven studies investigated 5-year DFS. We summarize the individual meta-analyses of the different subgroups in relation to HPV and p16 status individually in Table 5. Eight studies enrolled 2807 patients and had suitable data for meta-analyzing the 5-year DFS of the subgroups HPV+/p16+ and HPV−/p16−. The subgroup HPV+/p16+ showed a significantly improved 5-year DFS (fixed effects model; RR 1.96; 95% CI 1.73–2.22; P = 0.21). The 5-year DFS of the subgroup HPV+/p16+ was also significantly improved compared to the HPV−/p16+ and the HPV+/p16−. The 5-year DFS of the subgroup HPV−/p16− did not differ significantly from the HPV−/p16+ and the HPV+/p16−, even when the Ramshankar et al.42 study was excluded (fixed effects model; RR 0.87; 95% CI 0.72–1.05; P = 0.095 and random effects model; RR 1.44; 95% CI 0.87–2.38; p < 0.001). Additionally, 4 studies enrolling 342 patients had the data of HPV+/p16− and HPV−/p16+ patients for a 5-year DFS meta-analysis. Both subgroups did not differ to each other significantly, even when the Ramshankar et al.42 study was excluded. The DFS-HR was investigated in 6 studies24,25,26,28,32,33. The subgroup HPV+/p16+ was associated with a significantly improved 5-year DFS (random effects model; RR 2.63; 95% CI 2.60–2.67; P < 0.001).
Sensitivity analysis
Due to the low numbers of eligible articles, we performed a sensitivity analysis to test a possible bias on the 5-year OS of the subgroups HPV+/p16+ and HPV-/p16- based on where the study was performed by continent. The results from sub-meta-analyses in the region of the North America24,25,26,34,40,43,46, Europe27,32,35,39,47, and Asia28,36,38,44 were comparable with the complete meta-analyses including all regions (p > 0.05) (Fig. 2a). The fixed effect model was used in all sub meta-analyses (data not shown) due to non-significant heterogeneity, indicating statistically robust results. The RR and CI were essentially unchanged in comparison with the whole meta-analyses.
We also performed a sensitivity analysis assessing the possible bias resulting from the HPV detection methods. We divided the meta-analysis of the 5-year OS of the subgroup HPV+/p16+ and HPV−/p16− into two groups classified by HPV detection methods: one group using PCR25,26,27,28,32,34,35,38,43,46,47 and one group using ISH without PCR36,39,40,44. These two meta-analyses showed comparable results to the complete data meta-analyses (Fig. 2a). Again, the fixed effect model was used (data not shown) due to non-significant heterogeneity (p > 0.05). The RR and CI were essentially unchanged compared with the whole data meta-analyses.
In order to test for a bias introduced as systematic error (due to low sensitivity of the HPV detection (false HPV−) inherent to some detection methods (e.g. ISH) or testing of only individual HPV types (only HPV16 or 18; missed other types)), we investigated the HPV−/p16+ subgroup further, by excluding the following studies26,27,31,33,34,36,37,39,40. However, meta-analysis of the 5-year OS of the subgroup HPV+/p16+ and HPV-/p16+ (fixed effects model; RR of 2.23; 95% CI 1.85–2.68; P = 0.37)24,32,35,38,44,46,47, as well as the HPV−/p16− and the HPV−/p16+ (fixed effects model; RR of 0.83; 95% CI 0.71–0.96; P = 0.28)24,32,35,38,42,44,46,47 showed comparable results to the meta-analyses with all data included (Fig. 2a).
We next performed sensitivity analyses to investigate the effect of additional studies. The HPV+/p16+ subgroup was associated with better survival compared to the HPV-/p16− (fixed effects model; RR of 3.08; 95% CI 2.69–3.51; P = 0.49)38,43,46,47, the HPV−/p16+ (fixed effects model; RR of 2.35; 95% CI 1.88–2.94; P = 0.13)38,44,46,47 and the HPV+/p16− (random effects model; RR of 2.41; 95% CI 1.44–4.03; P = 0.01)43,44,45,46,47. The 5-year OS of the HPV−/p16− subgroup was inferior compared to the HPV−/p16+ (fixed effects model; RR of 0.76; 95% CI 0.62–0.92; P = 0.35)38,43,46,47 while the survival of the HPV+/p16− was not significantly different (fixed effects model; RR of 0.90; 95% CI 0.78–1.04; P = 0.60)38,42,44,46,47. The 5-year OS of the subgroup HPV+/p16− and HPV−/p16+ was not statistically significant (random effects model; RR of 1.09; 95%CI 0.59–1.99; P = 0.04), even when the Ramshankar et al. study42 was excluded (random effects model; RR of 0.84; 95%CI 0.65–1.08; P = 0.69)38,44,46,47. Therefore, the sub meta-analyses confirmed the whole meta-analyses results with the complete international studies data set (Figs 2–3).
Publication bias
The funnel plot shapes did not reveal obvious evidence of asymmetry.
Discussion
The incidence of high-risk-HPV+ tumors is exceeding 25% in HNSCC and 70% in oropharyngeal HNSCC48. This etiologically distinct HNSCC subtype has been associated with improved clinical outcome. Therefore, positive HPV status is a recommended biomarker for patient stratification towards de-escalation treatment regimens. Consequently, a new staging algorithm for OPSCC was recommended recently in the 8th AJCC/UICC guideline. Nevertheless, the strategy for a new staging paradigm in all head and neck regions is still under-investigation and being discussed. Since HPV can be found as an innocent bystander and p16 can be positive independently of HPV, before inclusion into such trials, it should be verified if the tumor is truly HPV-driven in order not to skew data and to avoid undertreatment of the patients’ cancer. In a number of investigations, this discrepancy among HPV markers (p16+ IHC and HPV-DNA+) was consistently found. And, moreover, these subtypes based on HPV/p16 status have shown different clinical outcomes21. In this meta-analysis, we confirm and expand to recent investigations to increase the knowledge about (a) the clinical relevance of HPV+ HNSCC and OPSCC; (b) the incidence and clinical course of subtypes of HPV+ tumors.
There is a discordant group of p16− cases in the HPV+ compartment and HPV− cases in the p16+ compartment. The number of studies included for evaluation of the discrepant cases of HPV+/p16− and HPV−/p16+ was increased by eight with addition of 428 new patients in this meta-analysis. The relative incidences of HPV+/p16− and HPV−/p16+ HNSCC were 7.3% and 6.7%, respectively. The survival data showed that HNSCC patients with HPV+ status defined as HPV+/p16+ have a better 5-year OS and DFS than subgroups with HPV−/p16−, HPV+/p16− and HPV−/p16+. These significant observations have been made in all head and neck regions and in OPSCC in particular and were consistent with previous studies. The sensitivity analysis confirmed the consistency of the 8 new additional studies. Thus, the survival benefit of the HPV+/p16+ subgroup is obvious. HPV−/p16+ HNSCC have a better 5-year OS than the HPV+/p16− subtype after excluding the study from Ramshankar et al.42 who found in early staged oral tongue squamous cell carcinoma patients that p16 overexpression was associated with lower survival and increased risk for disease recurrence irrespective of the HPV16 DNA status. Compared to the HPV−/p16− subgroup, 5-year OS of HPV−/p16+ HNSCC is better while HPV+/p16− is not. HPV+ in p16− HNSCC may be an innocent bystander with no functional involvement. Therefore, a careful investigation is required why HPV is negative to exclude false negative results of HPV tests or to prove truly HPV-independent development. Better survival in p16+ subgroups raises the question for its cause if independent of HPV. Consequently, in clinical trials these subtypes should be investigated separately to clarify if cancers displaying the HPV−/p16+ phenotype also qualify to be considered for de-escalation protocols.
p16 is a member of the INK4 class of cell-cycle inhibitors (INK4a) and functions as tumor suppressor. It binds to cyclin-dependent-kinases (CDK) 4 and CDK6 and prevents their association with cyclin D1, and consequently, the phosphorylation and inactivation of the retinoblastoma protein (Rb)49. In HPV-driven tumors, high-risk HPV E7 protein triggers a cellular defense response mediated by p16 and inactivating the retinoblastoma (Rb) pathway. Therefore, p16 overexpression is an excellent biomarker for high-risk HPV-associated malignancies including cervical cancer50 and HNSCC21. In a number of premalignant lesions and non-HPV driven tumors21, however, a p16 overexpression is also present. The underlying mechanisms of p16 overexpression in these non-HPV driven tumors is currently undetermined. These tumors often harbor mutations such as RAS and BRAF51. But a study in p16+/HPV− head and neck and anogenital SCCs has shown that overexpression of p16 in these tumors is not an attribute to KRAS mutations52. It has been also suggested that deregulation of Rb or Rb loss results in increased p16 expression in tumor cells which is associated with uncontrolled cell proliferation in malignant tumors53,54. A recent study identified in HPV− high-grade neuroendocrine carcinomas of the head and neck an overexpression of p1655. Most of these tumors had Rb loss and a low or absent cyclin D1 expression. Therefore, we hypothesize that mechanisms other than HPV infection may affect the p16-Rb-cyclin D1 pathway and induce cell cycle activation in HPV− HNSCC. However, future studies are required to clarify the pathogenic mechanisms between these subgroups.
As p53 is a key event in transformation and not directly associated with p16, p53 wild-type has been shown in HPV+ tumors and p53 mutation-type staining in HPV− tumors. However, in a cervical adenocarcinoma, diffuse p16 immunoreactivity is not necessarily indicative of a high-risk HPV-associated tumor56. p16INK4A enhances the transcriptional and the apoptotic functions of p53 through DNA-dependent interaction57. Therefore, we hypothesize that in the HPV−/p16+ subgroup, which are E6 negative, the p53 is presumably mutated and this subgroup represents the HPV independent cases.
The prevalence of HPV-associated HNSCC potentially depends on the sensitivity and specificity of the detection method. Therefore, the detection technique for HPV may be another cause for discordant results in HPV/p16 testing. Therefore, we analysed the data separately according to the HPV-detection method used. HPV detection by PCR and by ISH only (without PCR) were used as HPV detection methods in 20 and 5 studies, respectively. Sensitivity analysis showed that the results of the 5-year OS were comparable using both HPV detection methods. Evans et al.32 discussed that HPV testing was performed regardless of DNA quality which may have revealed a high false negative rate in DNA-based HPV detection methods (PCR and ISH). DNA degradation does not have any effect on p16 IHC testing results32. To determine the HPV status, Zafereo et al. presented an algorithm of p16 immunohistochemistry and HPV ISH and PCR58. Ou et al. demonstrated an algorithm consisting of two PCR assays and p16 immunohistochemistry59. Prigge et al. demonstrated in a meta-analysis the high sensitivity but only moderate specificity of p16INK4a and HPV DNA PCR when used as single tests to detect a transforming HPV infection in OPSCC. However, by combining the two tests, specificity was significantly optimized without altering the sensitivity6.
During HPV infection, the HPV E6/E7 oncogenes are expressed at low levels. Sensitive techniques such as qPCR detect HPV E6/E7 transcripts despite very limited expression60,61,62,63,64. Immunohistochemical evaluation of E6/E7 oncoprotein expression is another method for HPV detection which is independent from RNA or DNA degradation65. False negative results in HPV PCR testing may be caused by gene losses when L1 targets were used (15 of 20 studies testing for HPV DNA). These gene losses can cause the PCR to be negative, even though HPV is present (false HPV−/p16+). For E6/E7 targets this is not the case. However, studies using E6/E7 targets also detected patients belonging to the HPV−/p16+ subgroup26,33,34,45,46. Therefore, the HPV−/p16+ subgroup should be tested using the uniform high-sensitivity method for E6/E7 expression.
Detection of E6 and E7 mRNA expression is highly associated with p16 expression60,61,62,63,64. To identify a truly driven HPV-infection of OPSCC, the RNAscope HPV-test showed comparable results with p16-based algorithms combined with HPV PCR or HPV ISH. The RNAscope HPV-test performed better than p16 alone66. The quality of the results using mRNA detection are still controversial67. As a first step to analyze causal oncogenic HPV involvement, the detection of viral DNA is more practicable as viral RNA is sensitive to degradation68.
In about 90% of HPV-associated OPSCC, the high-risk-HPV-type 16 is found48,69,70,71. Other HPV types than genotype 16 may explain the identified subgroups if HPV tests have a restricted genotype spectrum. Most studies investigated multiple HPV types, however the number of investigated HPV types varied. Some studies investigated only p1634,37. After excluding studies which tested only individual HPV types or used HPV detection methods with low sensitivity, we confirmed the distinct 5- year OS of the HPV−/p16+ subgroup to be inferior compared with the HPV+/p16+ subgroup and superior compared with the HPV−/p16− subgroup. Thus, the HPV−/p16+ subgroup should be tested for all possibly involved HPV genotypes. In addition, the HPV−/p16+ subgroup may be caused by HPV independent mechanisms.
HPV+ OPSCCs have similar survival benefits in Brazil (GENCAPO study), the US (CHANCE study), and Europe (ARCAGE study)72. The present sensitivity analysis showed comparable results for the 5-year OS in Europe, the US, and Asia. Therefore, a geographic differentiation of the study origin is not necessary. As anti-smoking campaigns and their success differ in these countries overall there is a difference in the portion of the HPV positive HNSCC. However, HPV+ patients with tobacco consumption have to be distinguished from HPV+ non-smokers73,74,75 because the true etiology may be drug-associated and HPV an innocent bystander infection.
The prognostic utility of HPV among non-oropharyngeal-derived HNSCC is limited. The effect of HPV16/p16 was significantly different in non-OPSCC compared with OPSCC72. Chung et al.40 found that p16+ non-OPSCC have better outcomes compared to the corresponding patients with p16− non-OPSCC. Salazar et al.37 found no survival benefit for non-OPSCC in p16+ patients. However, when both p16 and HPV DNA were considered, concordantly positive non-OPSCC had significantly better survival. There were not sufficient data to perform a meta-analysis for non-OPSCC in the present study, therefore, a definitive conclusion cannot be drawn at this time.
After the recent introduction of a specific TNM system for HPV+ cancers and trials evaluating de-escalation protocols, HPV detection that includes detection of activity by measuring mRNA and protein of HPV oncogenes is an important step to correctly interpret data: HPV oncogene expression is prognosis relevant while HPV DNA as a bystander is not. One example for such a misinterpretation that is under current circumstances possible is p16+HNSCC with HPV bystander infection that results in a HPV+/p16+ phenotype which would possibly be undertreated in a de-escalation protocol. Therefore, as an ideal detection method for HPV-associated/driven cancers we propose the following algorithm for detection: perform IHC to determine the p16-status and if the staining is positive, determine positivity for HPV-oncogene mRNA or protein.
In conclusion, the information obtained from our meta analysis has revealed a potential new biologic subtype of HNSCC. From a research and clinical perspective, recruiting HPV-driven patients would be critical for the success of clinical trials towards redirecting the treatment of those patients. Further undertreatment in cases of bystander HPV and HPV-independent p16+ needs to be avoided. It remains important to elucidate the risk for progression and therapy failure especially for the HPV−/p16+ subgroup and potentially HPV false negative patients to prevent erroneous classification of patients for downscaling treatment by de-escalation-therapy. Additionally, re-examining patient’s specimens in a cluster by testing HPV-oncogene mRNA or protein can identify additional epidemiologic links between HPV-driven and non-HPV-driven tumors.
References
Siegel, R., Ma, J., Zou, Z. & Jemal, A. Cancer statistics, 2014. CA Cancer J Clin 64, 9–29, https://doi.org/10.3322/caac.21208 (2014).
Tinhofer, I. et al. Contribution of human papilloma virus to the incidence of squamous cell carcinoma of the head and neck in a European population with high smoking prevalence. Eur J Cancer 51, 514–521, https://doi.org/10.1016/j.ejca.2014.12.018 (2015).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer Statistics, 2017. CA Cancer J Clin 67, 7–30, https://doi.org/10.3322/caac.21387 (2017).
Cancer, I. I. C. o. H. A. Germany: Human Papillomavirus and RElated Cancers, Fact Sheet 2017, www.hpvcentre.netwww.hpvcentre.net (2017).
Cancer, I. I. C. O. H. A. France: Human Papillomavirus and RElated Cancers, Fact Sheet 2017, www.hpvcentre.netwww.hpvcentre.net (2017).
Prigge, E. S., Arbyn, M., von Knebel Doeberitz, M. & Reuschenbach, M. Diagnostic accuracy of p16INK4a immunohistochemistry in oropharyngeal squamous cell carcinomas: A systematic review and meta-analysis. Int J Cancer 140, 1186–1198, https://doi.org/10.1002/ijc.30516 (2017).
Evans, M. F. et al. Discrimination of ‘driver’ and ‘passenger’ HPV in tonsillar carcinomas by the polymerase chain reaction, chromogenic in situ hybridization, and p16(INK4a) immunohistochemistry. Head Neck Pathol 5, 344–348, https://doi.org/10.1007/s12105-011-0282-y (2011).
Boscolo-Rizzo, P., Pawlita, M. & Holzinger, D. From HPV-positive towards HPV-driven oropharyngeal squamous cell carcinomas. Cancer Treat Rev 42, 24–29, https://doi.org/10.1016/j.ctrv.2015.10.009 (2016).
Qian, X. et al. Prevalence and associated survival of high-risk HPV-related adenoid cystic carcinoma of the salivary glands. Int J Oncol 49, 803–811, https://doi.org/10.3892/ijo.2016.3563 (2016).
Qian, X. et al. ALDH1-positive cancer stem-like cells are enriched in nodal metastases of oropharyngeal squamous cell carcinoma independent of HPV status. Oncology reports 29, 1777–1784, https://doi.org/10.3892/or.2013.2340 (2013).
Rainsbury, J. W. et al. Prognostic biomarkers of survival in oropharyngeal squamous cell carcinoma: systematic review and meta-analysis. Head & neck 35, 1048–1055, https://doi.org/10.1002/hed.22950 (2013).
Hatano, T. et al. Identification of human papillomavirus (HPV) 16 DNA integration and the ensuing patterns of methylation in HPV-associated head and neck squamous cell carcinoma cell lines. Int J Cancer 140, 1571–1580, https://doi.org/10.1002/ijc.30589 (2017).
Agrawal, N. et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 333, 1154–1157, https://doi.org/10.1126/science.1206923 (2011).
Albers, A. et al. Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer Res 65, 11146–11155, https://doi.org/10.1158/0008-5472.CAN-05-0772 (2005).
Walline, H. M. et al. Integration of high-risk human papillomavirus into cellular cancer-related genes in head and neck cancer cell lines. Head & neck. https://doi.org/10.1002/hed.24729 (2017).
Ang, K. K. et al. Human papillomavirus and survival of patients with oropharyngeal cancer. The New England journal of medicine 363, 24–35, https://doi.org/10.1056/NEJMoa0912217 (2010).
Lydiatt, W. M. et al. Head and Neck cancers-major changes in the American Joint Committee on cancereighth edition cancer staging manual. CA Cancer J Clin 67, 122–137, https://doi.org/10.3322/caac.21389 (2017).
Wang, L. et al. Human papillomavirus status and the relative biological effectiveness of proton radiotherapy in head and neck cancer cells. Head & neck. https://doi.org/10.1002/hed.24673 (2016).
Kimple, R. J. et al. Development and characterization of HPV-positive and HPV-negative head and neck squamous cell carcinoma tumorgrafts. Clin Cancer Res 19, 855–864, https://doi.org/10.1158/1078-0432.CCR-12-2746 (2013).
Rieckmann, T. et al. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol 107, 242–246, https://doi.org/10.1016/j.radonc.2013.03.013 (2013).
Coordes, A. et al. Meta-analysis of survival in patients with HNSCC discriminates risk depending on combined HPV and p16 status. European archives of oto-rhino-laryngology: official journal of the European Federation of Oto-Rhino-Laryngological Societies. https://doi.org/10.1007/s00405-015-3728-0 (2015).
R Statistical Software. A language and environment for statistical computing. R Foundation for Statistical Computing. R Version 3.1.0 (URL, Vienna, Austria, 2014).
Wittekindt, C. et al. Expression of p16 protein is associated with human papillomavirus status in tonsillar carcinomas and has implications on survival. Advances in oto-rhino-laryngology 62, 72–80, https://doi.org/10.1159/000082474 (2005).
Smith, E. M. et al. P16INK4a expression, human papillomavirus, and survival in head and neck cancer. Oral oncology 44, 133–142, https://doi.org/10.1016/j.oraloncology.2007.01.010 (2008).
Kong, C. S. et al. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. International journal of radiation oncology, biology, physics 74, 553–561, https://doi.org/10.1016/j.ijrobp.2009.02.015 (2009).
Weinberger, P. M. et al. Human papillomavirus-active head and neck cancer and ethnic health disparities. The Laryngoscope 120, 1531–1537, https://doi.org/10.1002/lary.20984 (2010).
Heath, S. et al. Clinically significant human papilloma virus in squamous cell carcinoma of the head and neck in UK practice. Clinical oncology 24, e18–23, https://doi.org/10.1016/j.clon.2011.05.007 (2012).
Park, W. S. et al. Human papillomavirus in oropharyngeal squamous cell carcinomas in Korea: use of G1 cycle markers as new prognosticators. Head & neck 34, 1408–1417, https://doi.org/10.1002/hed.21939 (2012).
Holzinger, D. et al. Viral RNA patterns and high viral load reliably define oropharynx carcinomas with active HPV16 involvement. Cancer Res 72, 4993–5003, https://doi.org/10.1158/0008-5472.CAN-11-3934 (2012).
Liang, C. et al. Biomarkers of HPV in head and neck squamous cell carcinoma. Cancer Res 72, 5004–5013, https://doi.org/10.1158/0008-5472.CAN-11-3277 (2012).
Park, K. et al. p16 immunohistochemistry alone is a better prognosticator in tonsil cancer than human papillomavirus in situ hybridization with or without p16 immunohistochemistry. Acta oto-laryngologica 133, 297–304, https://doi.org/10.3109/00016489.2012.741327 (2013).
Evans, M. et al. Human Papillomavirus-associated oropharyngeal cancer: an observational study of diagnosis, prevalence and prognosis in a UK population. BMC cancer 13, 220, https://doi.org/10.1186/1471-2407-13-220 (2013).
Melkane, A. E. et al. Human papillomavirus prevalence and prognostic implication in oropharyngeal squamous cell carcinomas. Head & neck 36, 257–265, https://doi.org/10.1002/hed.23302 (2014).
Stephen, J. K. et al. Significance of p16 in Site-specific HPV Positive and HPV Negative Head and Neck Squamous Cell Carcinoma. Cancer and clinical oncology 2, 51–61, https://doi.org/10.5539/cco.v2n1p51 (2013).
Rietbergen, M. M. et al. Human papillomavirus detection and comorbidity: critical issues in selection of patients with oropharyngeal cancer for treatment De-escalation trials. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 24, 2740–2745, https://doi.org/10.1093/annonc/mdt319 (2013).
Huang, H., Zhang, B., Chen, W., Zou, S. M. & Xu, Z. G. [Relationship between HPV-DNA status and p16 protein expression in oropharyngeal squamous cell carcinoma and their clinical significance]. Zhonghua zhong liu za zhi [Chinese journal of oncology] 35, 684–688 (2013).
Salazar, C. R. et al. Combined P16 and human papillomavirus testing predicts head and neck cancer survival. Int J Cancer 135, 2404–2412, https://doi.org/10.1002/ijc.28876 (2014).
Xu, Y. et al. Low prevalence of human papillomavirus in head and neck squamous cell carcinoma in Chinese patients. Journal of medical virology 87, 281–286, https://doi.org/10.1002/jmv.24052 (2015).
Heiduschka, G. et al. Improved survival in HPV/p16-positive oropharyngeal cancer patients treated with postoperative radiotherapy. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft… [et al]. https://doi.org/10.1007/s00066-014-0753-7 (2014).
Chung, C. H. et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 32, 3930–3938, https://doi.org/10.1200/JCO.2013.54.5228 (2014).
Semrau, R. et al. Prognostic impact of human papillomavirus status, survivin, and epidermal growth factor receptor expression on survival in patients treated with radiochemotherapy for very advanced nonresectable oropharyngeal cancer. Head & neck 35, 1339–1344, https://doi.org/10.1002/hed.23126 (2013).
Ramshankar, V., Soundara, V. T., Shyamsundar, V., Ramani, P. & Krishnamurthy, A. Risk stratification of early stage oral tongue cancers based on HPV status and p16 immunoexpression. Asian Pac J Cancer Prev 15, 8351–8359 (2014).
Liu, J. C. et al. High prevalence of discordant human papillomavirus and p16 oropharyngeal squamous cell carcinomas in an African American cohort. Head & neck 38(Suppl 1), E867–872, https://doi.org/10.1002/hed.24117 (2016).
Saito, Y. et al. Prognostic value of p16 expression irrespective of human papillomavirus status in patients with oropharyngeal carcinoma. Jpn J Clin Oncol 45, 828–836, https://doi.org/10.1093/jjco/hyv085 (2015).
Descamps, G. et al. Classical risk factors, but not HPV status, predict survival after chemoradiotherapy in advanced head and neck cancer patients. J Cancer Res Clin Oncol 142, 2185–2196, https://doi.org/10.1007/s00432-016-2203-7 (2016).
Mazul, A. L. et al. Prognostic significance of non-HPV16 genotypes in oropharyngeal squamous cell carcinoma. Oral oncology 61, 98–103, https://doi.org/10.1016/j.oraloncology.2016.08.019 (2016).
Gronhoj Larsen, C. et al. Novel nomograms for survival and progression in HPV+ and HPV − oropharyngeal cancer: a population-based study of 1,542 consecutive patients. Oncotarget. https://doi.org/10.18632/oncotarget.12335 (2016).
Ndiaye, C. et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol 15, 1319–1331, https://doi.org/10.1016/S1470-2045(14)70471-1 (2014).
Stone, S. et al. Complex structure and regulation of the P16 (MTS1) locus. Cancer Res 55, 2988–2994 (1995).
Lin, J., Albers, A. E., Qin, J. & Kaufmann, A. M. Prognostic significance of overexpressed p16INK4a in patients with cervical cancer: a meta-analysis. PLoS One 9, e106384, https://doi.org/10.1371/journal.pone.0106384 (2014).
Romagosa, C. et al. p16(Ink4a) overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors. Oncogene 30, 2087–2097, https://doi.org/10.1038/onc.2010.614 (2011).
Prigge, E. S. et al. No evidence of oncogenic KRAS mutations in squamous cell carcinomas of the anogenital tract and head and neck region independent of human papillomavirus andp16(INK4a) status. Hum Pathol 45, 2347–2354, https://doi.org/10.1016/j.humpath.2014.08.001 (2014).
Cimino-Mathews, A. et al. A subset of malignant phyllodes tumors harbors alterations in the Rb/p16 pathway. Hum Pathol 44, 2494–2500, https://doi.org/10.1016/j.humpath.2013.06.009 (2013).
Knudsen, E. S. & Knudsen, K. E. Tailoring to RB: tumour suppressor status and therapeutic response. Nat Rev Cancer 8, 714–724, https://doi.org/10.1038/nrc2401 (2008).
Alos, L. et al. p16 overexpression in high-grade neuroendocrine carcinomas of the head and neck: potential diagnostic pitfall with HPV-related carcinomas. Virchows Arch 469, 277–284, https://doi.org/10.1007/s00428-016-1982-1 (2016).
Carleton, C. et al. A Detailed Immunohistochemical Analysis of a Large Series of Cervical and Vaginal Gastric-type Adenocarcinomas. Am J Surg Pathol 40, 636–644, https://doi.org/10.1097/PAS.0000000000000578 (2016).
Al-Khalaf, H. H., Nallar, S. C., Kalvakolanu, D. V. & Aboussekhra, A. p16INK4A enhances the transcriptional and the apoptotic functions of p53 through DNA-dependent interaction. Mol Carcinog. https://doi.org/10.1002/mc.22627 (2017).
Zafereo, M. E. et al. Squamous cell carcinoma of the oral cavity often overexpresses p16 but is rarely driven by human papillomavirus. Oral oncology 56, 47–53, https://doi.org/10.1016/j.oraloncology.2016.03.003 (2016).
Ou, P. et al. Human papillomavirus and oropharyngeal squamous cell carcinoma: a New Zealand cohort study. ANZ J Surg. https://doi.org/10.1111/ans.13759 (2016).
Jung, A. C. et al. Biological and clinical relevance of transcriptionally active human papillomavirus (HPV) infection in oropharynx squamous cell carcinoma. Int J Cancer 126, 1882–1894, https://doi.org/10.1002/ijc.24911 (2010).
Wiest, T., Schwarz, E., Enders, C., Flechtenmacher, C. & Bosch, F. X. Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene 21, 1510–1517, https://doi.org/10.1038/sj.onc.1205214 (2002).
McLaughlin-Drubin, M. E., Crum, C. P. & Munger, K. Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B histone demethylase expression and causes epigenetic reprogramming. Proceedings of the National Academy of Sciences of the United States of America 108, 2130–2135, https://doi.org/10.1073/pnas.1009933108 (2011).
Hoffmann, M. et al. p16(INK4a) overexpression predicts translational active human papillomavirus infection in tonsillar cancer. Int J Cancer 127, 1595–1602, https://doi.org/10.1002/ijc.25174 (2010).
Klussmann, J. P. et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol 162, 747–753, https://doi.org/10.1016/S0002-9440(10)63871-0 (2003).
Stiasny, A. et al. Immunohistochemical Evaluation of E6/E7 HPV Oncoproteins Staining in Cervical Cancer. Anticancer Res 36, 3195–3198 (2016).
Mirghani, H. et al. Diagnosis of HPV driven oropharyngeal cancers: Comparing p16 based algorithms with the RNAscope HPV-test. Oral oncology 62, 101–108, https://doi.org/10.1016/j.oraloncology.2016.10.009 (2016).
Dreyer, J. H., Hauck, F., Oliveira-Silva, M., Barros, M. H. & Niedobitek, G. Detection of HPV infection in head and neck squamous cell carcinoma: a practical proposal. Virchows Arch 462, 381–389, https://doi.org/10.1007/s00428-013-1393-5 (2013).
Benchekroun, M. et al. Impact of fixative on recovery of mRNA from paraffin-embedded tissue. Diagn Mol Pathol 13, 116–125 (2004).
Kreimer, A. R., Clifford, G. M., Boyle, P. & Franceschi, S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 14, 467–475, https://doi.org/10.1158/1055-9965.EPI-04-0551 (2005).
D’Souza, G. et al. Case-control study of human papillomavirus and oropharyngeal cancer. The New England journal of medicine 356, 1944–1956, https://doi.org/10.1056/NEJMoa065497 (2007).
Gillison, M. L. et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 92, 709–720 (2000).
D’Souza, G. et al. Effect of HPV on head and neck cancer patient survival, by region and tumor site: A comparison of 1362 cases across three continents. Oral oncology 62, 20–27, https://doi.org/10.1016/j.oraloncology.2016.09.005 (2016).
Chaturvedi, A. K. et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 29, 4294–4301, https://doi.org/10.1200/JCO.2011.36.4596 (2011).
Bossi, P. et al. Treatment-related outcome of oropharyngeal cancer patients differentiated by HPV dictated risk profile: a tertiary cancer centre series analysis. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 25, 694–699, https://doi.org/10.1093/annonc/mdu004 (2014).
Rodrigo, J. P. et al. Time trends in the prevalence of HPV in oropharyngeal squamous cell carcinomas in northern Spain (1990-2009). Int J Cancer 134, 487–492, https://doi.org/10.1002/ijc.28355 (2014).
Author information
Authors and Affiliations
Contributions
A.E.A. designed the study. X.Q. and A.C. searched database and reviewed studies. A.E.A., X.Q., A.M.K. and A.C. collected and analyzed data. A.E.A., X.Q., A.M.K. and A.C. wrote this manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Albers, A.E., Qian, X., Kaufmann, A.M. et al. Meta analysis: HPV and p16 pattern determines survival in patients with HNSCC and identifies potential new biologic subtype. Sci Rep 7, 16715 (2017). https://doi.org/10.1038/s41598-017-16918-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16918-w
This article is cited by
-
An audit of dental assessments including orthopantomography and timing of dental extractions before radiotherapy for head and neck cancer
British Dental Journal (2022)
-
Pathological characterization and clinical outcome of penile intraepithelial neoplasia variants: a North American series
Modern Pathology (2022)
-
Accuracy of high-risk HPV DNA PCR, p16(INK4a) immunohistochemistry or the combination of both to diagnose HPV-driven oropharyngeal cancer
BMC Infectious Diseases (2022)
-
Discrepancy in p16 expression in patients with HPV-associated head and neck squamous cell carcinoma in Thailand: clinical characteristics and survival outcomes
BMC Cancer (2021)
-
Fibroblast growth factor (FGF), FGF receptor (FGFR), and cyclin D1 (CCND1) DNA methylation in head and neck squamous cell carcinomas is associated with transcriptional activity, gene amplification, human papillomavirus (HPV) status, and sensitivity to tyrosine kinase inhibitors
Clinical Epigenetics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.