Abstract
In order to reveal the role of phytoplankton in the spatio-temporal distribution of alkaline phosphatase activity (APA), monthly investigations were conducted in the Xiaojiang River, a tributary of the Three Gorges Reservoir in China. Different APA fractions, environmental parameters, and phytoplankton communities were followed. High spatio-temporal variations of APA were observed, with the highest value in summer and the lowest in winter. The annual average APAT (total alkaline phosphatase activity) ranged from 7.78–14.03 nmol∙L−1∙min−1 with the highest in the midstream and the lowest in the estuary. The dominant phytoplankton phyla in summer and winter were Cyanophyta and Bacillariophyta, respectively. The mean cell density in the midstream and in the estuary was 5.2 × 107 cell∙L−1 and 1.4 × 107 cell∙L−1, respectively. That APA>3.0 μm was significantly higher than APA0.45-3 μm indicating phytoplankton was the main contributor to alkaline phosphatase. Correlation analysis indicated the dominant species and cell density could determine the distribution pattern of APA. Turbidity, total phosphorus, chemical oxygen demand, water temperature (WT), pH and chlorophyll a were proved to be positively correlated with APA; soluble reactive phosphorus, conductivity, transparency and water level(WL) were negatively correlated with APA. It was concluded that spatio-temporal heterogeneity of APA determined by phytoplankton communities was related to WT and WL.
Similar content being viewed by others
Introduction
Phosphorus is always treated as a limiting nutrient in many freshwater ecosystems because it frequently limits the primary production1,2. The availability of phosphorus (P) is regarded as a crucial factor regulating the dynamic of phytoplankton in situ. There is no doubt that there are many types of phosphorus in waters, including the organic and inorganic phosphorus. Among the various forms of phosphorus, orthophosphate (o-P) or available soluble reactive phosphorus (SRP), the bioavailable form of phosphorus, can be depleted rapidly in freshwaters due to the rapid uptake3, which tends to limit algal growth in freshwater ecosystems. So the low concentrations of SRP can modify the structure of plankton communities and constrain phytoplankton distribution4,5. Some phytoplankton species, zooplankton and bacterioplankton can produce extracellular phosphatase liberating SRP from dissolved organic P compounds, which is one mechanism allowing the living organisms to overcome P limitation6,7.
Relationship between APA and phytoplankton has been paid more attention since 1960s8,9. Kalinowska tried to figure out the major contributor of APase through membrane filtration method10. Even if size fractionation by filtration is never completely absolute (i.e., overlapping size), it still provides useful insights on the major microorganisms possibly contributing to APA. The increase in APA can be attributed more to phytoplankton biomass than to the bacterial biomass11. Therefore, phytoplankton contributed greatly to APA production and was significantly influenced by P bioavailability. Production of extracellular phosphatases has been detected in many phytoplankton species12,13,14. Various taxa are exhibiting differences in the presence, localization and labeling pattern of phosphatases. Both seasonal and short-term variations also have been detected in enzyme activity of phytoplankton15. Enzyme-labeled fluorescence (ELF) analysis revealed pronounced differences in the makeup of phytoplankton responsible for APA in San Francisco and Monterey bays16.
The Three Gorges Reservoir (TGR) is the biggest deep river-type reservoir in the world. More than 170 tributaries carry runoff and bring nutrients and pollutants into it, which affected the trophic status and resulted in water blooms in some tributaries of the TGR17. Though many studies have been conducted to screen APase in different water bodies, little knowledge was obtained in the Three Gorges Reservoir (TGR). Due to the complicated relationship between APA and ecological factors, it is necessary to screen the distribution pattern of APA and its influencing factors in the TGR. Xiaojiang River is one of the tributaries in the TGR, which is suffered from water blooms frequently. Eutrophication in Xiaojiang River is very serious after the Three Gorges Dam (TGD)’s impoundment since 200318. In this study, Xiaojiang River was selected as the delegate of the tributary in the TGR, phytoplankton and APA in Xiaojiang River were screened. Based on the related researches focused on the complicated relationship between APA and phytoplankton mentioned above, it was assumed that the phytoplankton community successions might lead to the spatio-temporal heterogeneity of alkaline phosphatase activity. In order to verify this hypothesis, monthly investigation was conducted, different APA fractions (APAT, APA<0.45 μm, APA0.45-3 μm and APA>3.0 μm), environmental parameters and phytoplankton communities were screened synchronously. The role of phytoplankton communities in the spatio-temporal heterogeneity of APA and its influence factors in the Three Gorges Reservoir were demonstrated. The results of this study can help to know how APA production changes with phytoplankton communities’ successions in the TGR.
Results
APAT distribution pattern
The APAT ranged from 1.19–47.6 nmol·L−1·min−1 (Fig. 1). The lowest level of APAT was observed in winter. Besides, the average APAT in summer and autumn were higher than in other seasons. The mean water level was high in winter(169.7 ± 4.5 m) and low in summer(149.3 ± 3.1 m), the variations of water level presented different trends with that of APAT at temporal scales.
The highest value of annual average APAT in GY01 and lowest in XJ were also showed in Fig. 1. The average APAT of GY01 in summer and autumn are higher than those of HS02 and XJ.
Size-fractionation of APA
The average size-fractionated APA indicated that APA<0.45 μm accounted for the major portion of APAT, whereas the average APA>3.0 μm was higher than APA0.45-3 μm (Fig. 2a). The average APA>3.0 μm accounted for 28.1% of APAT and APA0.45-3 μm accounted for 16.7%. In addition, the size-fractionated APA (APA<0.45 μm, APA0.45-3 μm and APA>3.0 μm) in summer and autumn are higher than those in winter.
Seasonal (a) and spatial (b) variations of average size-fractionated APA in the Xiaojiang River. APA>3.0 μm: the alkaline phosphatase activity in algal fraction; APA0.45-3.0 μm: the alkaline phosphatase activity in bacterial fraction; APA<0.45 μm: picoplankton/dissolved alkaline phosphatase activity.
At spatial scales, the average APAT consisted of 30.2% APA>3.0 μm and 20.4% APA0.45-3 μm in all sites. The APA<0.45 μm kept a relatively stable and high level. Both APA0.45-3 μm and APA>3.0 μm in midstream (GY01) are higher than those in estuary (XJ).
Phytoplankton communities
Bacillariophyta was the dominant group in winter and spring (72.7% of total cell density on average, Fig. 3a) except cyanophyta are dominant in April. In summer and autumn, phytoplankton mainly consisted of Cyanophyta (65.6% of total cell density on average) except the Cryptophyta accounted for 88.4% in August. The mean algal cells density was the highest in July 2014 (1.27 × 108cell∙L−1), and the lowest in January 2014 (1.3 × 106cell∙L−1). The cell density was higher in summer and autumn than in spring and winter. Cyanophyta dominated the phytoplankton in upstream (QM01, QM02, GY01, GY02, 69.9% of total cell density on average). In the downstream (HS01, HS02, XJ), phytoplankton mainly consisted of Bacillariophyta (35.3% of total cell density on average, Fig. 3b).
Spatio-temporal characteristics of chlorophyll a and environmental parameters
Spatio-temporal variations of chlorophyll a (Chl a) and environmental parameters could be observed in Fig. 4. The values of Chl a, total phosphorus (TP), chemical oxygen demand (COD) in spring were apparently higher than the values of other seasons, because the river suffered a Microcystis sp. bloom in May, which also resulted in the minimum values of SRP and transparency (SD) emerged. The levels of TP, COD, Chl a, water temperature (WT), turbidity (Turb), dissolved oxygen (DO) and pH stayed low in winter, contrary to the values of SRP and SD. The values of SRP fluctuated more frequently than other parameters in different seasons. At spatial scale, the concentrations of SRP and Chl a were higher in estuary than in upstream.
Temporal and spatial variations of a: chlorophyll (a) (Chl a) and other environmental parameters. (b) Soluble reactive phosphorus (SRP); (c) water temperature (WT); (d) transparency (SD); (e) dissolved oxygen (DO); (f) conductivity (Cond); (g) pH; (h) turbidity (Turb); (i) total phosphorus (TP) and (j) chemical oxygen demand (COD).
Relationships between APA and environmental parameters
SRP concentrations showed negative correlation to APA<0. 45 μm (Fig. 5a), APA0.45-3 μm (Fig. 5b), APA>3.0 μm (Fig. 5c) and APAT (Fig. 5d). The Spearman correlations among environmental variables and APA<0.45 μm, APA0.45-3 μm, APA>3.0 μm and APAT were presented in Table 1. Turb, TP, COD, WT and pH were positively correlated with APA fractions. Cond., SD and WL were negatively correlated with APA.
Redundancy analysis (RDA) was performed to analyze the relationship between environmental parameters and size-fractionated APA. The ordination diagrams of environmental variables and size-fractionated APA for axis 1 and axis 2 were shown in Fig. 6. The Monte Carlo test revealed that the first canonical axis and all canonical axes were significant (F = 25.932, P = 0.002; F = 3.086, P = 0.002; 499 random permutation). For environmental variables and size-fractionated APA, all canonical axes cumulatively explained 83.3% of the variance in APA–environment relationships, and the first two canonical axes accounted for 26.5% and 31.5% of the variance separately. The first axis was positively correlated with DO (0.57), COD (0.65) and negatively correlated with SRP (−0.41), SD (−0.38) and WL (−0.30). The second axis was mainly negatively correlated with Cond (−0.13) and WT (−0.16). APA<0.45 μm and APA>3.0 μm was the major portion of APAT. APA>3.0 μm and APA0.45-3 μm were located on the right-hand side of the biplot. They were correlated negatively with WL, SD, SRP and Cond, and positively with other parameters.
Relationships between APA>3.0 μm and algal cell density
APA>3.0 μm reached the highest in midstream (GY01) in May (28.24 nmol∙L−1∙min−1), and undetectable in estuary (XJ) in December. Values ranged from 0.19–22.71 nmol∙L−1∙min−1at the other sites. The mean cell density was the highest in midstream (GY02, 5.2 × 107cell∙L−1) and the lowest in estuary (XJ,1.4 × 107cell∙L−1). A significant positive relationship was found between APA>3.0 μm and cell density among all sites (Fig. 7).
Discussion
APase has different sources, different kinds of bacteria, phytoplankton and zooplankton can excrete extracellular phosphatase19. Specific APA was related to different phosphatase producing organisms. In our investigation, APA>3.0 μm contributed in average 28.1% in the APAT, while bacterial APA accounted for 16.7%, APA in algal fraction (APA>3.0 μm) was higher than that in the picoplankton/bacterial fraction (APA0.45-3 μm). Though many (not all) phytoplankton cells have a host heterotrophic bacteria inhabiting or in close association with cells, and the overlapping size on the filter also influenced the final data, which making it difficult to assign the different size fractionation by filtration to individual cells alone, it could be admitted that the coarser fraction (APA>3.0 μm), mainly from algae, was conventionally defined as “algal APA”20 due to phytoplankton was the main contributor according to its size, biomass and physiological activity. It was confirmed APA>3.0 μm accounted for the major portion of total APA (55–87.9%) than APA0.45-3 μm 21. It could be deduced that the phytoplankton was the major contributor of bulk APA based on the larger proportion of APA>3.0 μm(52.73%) than APA0.45-3 μm(21.09%)22. Therefore, the phytoplankton contributed greatly to APA production. Meanwhile, the picoplankton/dissolved APA (APA<0.45 μm) kept a relative stable and high level (53.4% of the APAT). Some studies showed that the picoplankton/dissolved APA represents a significant part of the total activity. For example, Labry et al. reported that picoplankton/dissolved APA represented 13% to 44% of APAT in the Bay of Biscay (on the French Atlantic coast)23. Higher proportions were recorded in the northern Red Sea (42–74%)24. The dissolved APase can be liberated into the environment through the lysis of dead phytoplankton cells and from cells damaged by zooplankton grazing25. The high values may result from physical damage of cells by water current and zooplankton grazing on phytoplankton. Nevertheless, some study found that the dissolved APA might origin from bacteria26. In order to elucidate the origins of dissolved APA, the dissolved alkaline phosphatase was capsulated into reverse micellar media, and it was proved that the different behaviors of dissolved phosphatase of surface and overlying water might be due to the different origins, with the former being algae and the latter being bacterial27. The results of Song et al. (2005) couldn’t identify the exact origins form, the fraction <0.45 µm contains some pico-bacteria and some pico-phytoplankton and can’t be called as the dissolved fraction. Here we changed it as the picoplankton/dissolved fraction. Besides, the positive relationships between APA and the environmental parameters that have been treated as the indexes of the productivity and trophic status, such as Chl a, Turb and COD, and the negative relationship between APA and SD can also indicate that the phytoplankton is the main contributor of APA.
Different algal species showed significant different secreting ability of APase. In this study, phytoplankton communities were dominated by Bacillariophyta in winter. Pyrrophyta, Bacillariophyta and Chlorophyta can easily produce extracellular phosphatase as evidenced by ELFA labeling21. The low APA>3.0 μm during this period may result from the low algal cell density of phytoplankton and the increased concentrations of SRP. When the Pyrrophyta subdominated the phytoplankton community in May, the APA>3.0 μm peaked. Results in some shallow eutrophic lakes revealed that the species belonging to Pyrrophyta were regularly phosphatase-positive, while Bacillariophyceae were phosphatase negative except Aulacoseira sp28. Dinoflagellates were poor competitors for phosphate accumulation compared to diatoms; they have to excrete much more APase than diatom to hydrolyze DOP to satisfy their P demand, even when phosphate is adequate29. In nutrient addition experiments, a higher percentage of dinoflagellates were identified with cell-specific APA than diatoms30. It can explain why APA>3.0 μm peaked when Pyrrophyta subdominated the phytoplankton.It was consistent with the results in Monterey Bay that dinoflagellates comprised only 14% of all cells counted and accounted for 78% of APase-producing cells examined16. Microcystis aeruginosa was confirmed can also synthesize APase31. It can explain that as the cell density of Cyanophyta increased in summer and autumn, the APA>3.0 μm was also prompted. The dominating of Cyanophyta during the summer and autumn resulted in the high amount of APA. The synchronous pattern of alkaline phosphatase activity and algal cells amount can also be found in Jialing River32. The higher algal cell density in midstream than in estuary can also explain why the APAT was higher in midstream. It could be concluded that phytoplankton communities determined the level of APA>3.0 μm, which determined the significant seasonal and regional variations of APAT.
APA showed significant seasonal and regional variations, with lower value in inlet waters and higher value in the estuarine, and relatively low in winter and high in summer7. However, the distribution characteristics of APA in this study were not consistent strictly with the above mentioned. The APAT fluctuated frequently from spring to autumn. Relative stable level of APAT in winter can be seen in Fig. 1. This phenomenon may result from the fluctuant water level of the TGR. For the sake of flood control and hydropower, the water level in the TGR is subjected to the specific management of the TGD and is meant to seasonally fluctuate between 145 and 175 m a.s.l. It has been demonstrated that the turbulence promoted the phytoplanktonic APA and accelerated the biogeochemical cycle of P in Lake Taihu33. This was consistent with our results that the high APA was present during the significant water level fluctuated period from spring to autumn. Meanwhile, it has been proved that the APA increased with water temperature34,35. The positive relationship between WT and APA in this study (Table 1) supports the conclusion that WT determined the APA through its effects on the phytoplankton seasonally and the direct influences on APase.
Methods
Samples area and sites
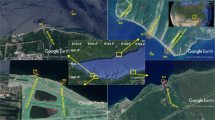
Xiaojiang River, a tributary of the TGR, originates from Kaixian, Chongqing Municipality with a length of 180 km and watershed area of 5172.5 km2. It flows from north to south; entering into the TGR in Yunyang County. The distance from the estuary to the TGD is 248 km.
Water temperature (WT), pH, dissolved oxygen (DO) and conductivity (Cond.) were measured using a YSI model Professional Plus multiparameter probe (USA); Transparency (SD) was measured with a Secchi disk; and turbidity (Turb.) was measured with a WGZ-B turbidmeter (XinRui, Shanghai). Water level (WL) was recorded by GPS in situ. Surface water samples (0.5 m) were collected with a Van Dorn sampler at seven sampling sites (XJ, HS02, HS01, GY02, GY01, QM02, QM01) (Fig. 8) monthly from October 2013 to September 2014. All samples were run in triplicate. In order to avoid the physiological and biological parameters changed dramatically, the water samples for APA test were filtered immediately after collection and strong oscillation in situ, the filters were put into a portable refrigerator at 0 °C and analyzed within 24 h. All water samples for the other parameters measurement were also stored in a portable refrigerator at 0 °C after collected and tested within 24 h. Concentrations of chlorophyll a (Chl a), total phosphorus (TP), soluble reactive phosphorus (SRP), chemical oxygen demand (COD) were analyzed after samples collected within 24 h. Samples for quantitative phytoplankton analyses were fixed with neutral Lugol’s solution, and concentrated after 48 h sedimentation36.
Measurement of APA
APA was measured using a modified procedure37,38. A total of 2 ml water samples were incubated at 37 °C for 4 h in the presence of 1 ml 0.05 M Tris-HCl buffer (pH = 8.5) and 2 ml 0.3 mM p-nitronphenylphosphate (p-NPP) as substrate, subsequently, 0.1 ml 0.1 M NaOH was added into the mixture after 4 h. The release of p-nitrophenol from p-nitronphenylphosphate was determined by absorbance at 410 nm using a spectrophotometer (TU-1810), and APA was calculated in nM·L−1·min−1. APA was determined in unfiltered water (APAT) and water samples filtered through 0.45 (the picoplankton/dissolved alkaline phosphatase activity, APA<0.45 μm) and 3.0 μm membrane filters (APA<3.0 μm). The activity in algal fraction (APA>3.0 μm) and in bacterial fraction (APA0.45-3.0 μm) were calculated as follows: APA>3.0 μm = APAT−APA<3.0 μm, APA0.45-3.0 μm = APA<3.0 μm−APA<0.45 μm 39.
Measurement of SRP, Chl a, TP, COD and phytoplankton quantification
Water samples used for the Chl a measurement were filtered with Whatman GF/C filter, then the residuals on the filter were extracted using 90% acetone solution in the darkroom for 24 h at 4 °C, and Chl a was analyzed spectrophotometrically. The concentrations of SRP were measured after all water samples were filtered through pre-washed filters (Whatman GF/C, glass microfiber filters).The concentrations of SRP, total phosphorus (TP) and chemical oxygen demand (COD) were analyzed according to the standard methods40. Phytoplankton was quantified at 400 × magnification with a light microscope (OLYMPUS BX41). The identification of phytoplankton species is according to Hu and Wei41.
Statistical analysis
Statistical analysis was carried out using the SPSS 13.0 package. Variance analysis (one-way ANOVA) was used to compare the means of APA in different seasons and sampling sites. Normal distribution of the data was ensured by visual inspection of Q–Q plots, and Levene’s test was used to check for homogeneity of variances before ANOVA. Non-parametric correlation (Spearman) analyses were employed for determining relationships among APA<0.45 μm, APA0.45-3 μm, APA>3.0 μm, APAT and the environmental factors. Detrended correspondence analysis (DCA) of the size-fractionated APA and environmental data was performed using CANOCO version 4.5 to determine whether linear or unimodal ordination methods should be applied42. Before the analysis, the abiotic and biological data were transformed by log(x + 1). We calculated an unconstrained ordination through the DCA. Using detrending by segments and Hill’s scaling, the length of the longest axis estimated the beta diversity in the data set. Moreover, the value of the data set implies that it is appropriate to use the Redundancy analysis (RDA) method. RDA was performed to get an approximate ordering of the size-fractionated APA’s optima for environmental variables. The significance of canonical axes and environmental variables to explain the variance of the size-fractionated APA was tested using Monte Carlo simulations with 499 permutations43,44.
Data Availability
All data analyzed during this study are included in this published article.
References
Harris, G. P. Phytoplankton ecology. Structure, function and fluctuation (Springer, 1986).
Paerl, H. W. Nutrient and other environmental controls of harmful cyanobacterial blooms along the freshwater-marine continuum. Adv. Experim. Med. Biol. 619, 216–241 (2008).
Ramm, K. & Scheps, V. Phosphorus balance of a polytrophic shallow lake with the consideration of phosphorus release. Hydrobiologia 342(4), 43–53 (1997).
Karl, D. M. et al. Ecosystem changes in the North Pacific subtropical gyre attributed to the 1991–92 ElNin˜o. Nature 373(6511), 230–234 (1995).
Moutin, T. et al. Phosphate availability controls Trichodesmium spp. biomass in the SW Pacific Ocean. Mar. Ecol. Prog. Ser. 297, 15–21 (2005).
Cembella, A. D., Antia, N. J. & Harrison, P. J. The Utilization of Inorganic and Organic Phosphorous Compounds as Nutrients by Eukaryotic Microalgae: A Multidisciplinary Perspective:Part I. Critical Reviews in Microbiology 10, 317–391 (1982).
Jansson, M., Olsson, H. & Pettersson, K. Phosphatase-origin,characteristics and function in lakes. Hydrobiologia. 170, 157–175 (1988).
Perry, M. Alkaline phosphatase activity in subtropical Central North Pacific waters using a sensitive fluorometric method. Mar Biol. 15, 113–119 (1972).
Kuenzler, E. J. Glucose-6-phosphate utilization by marine algae1. J Phycol. 1, 156–164 (1965).
Kalinowska, K. Eutrophication processes in a shallow, macrophyte dominated lake–alkaline-phosphatase activity in Lake Łuknajno (Poland). Hydrobiologia. 342, 395–399 (1997).
Nausch, M. Alkaline phosphatase activities and the relationship to inorganic phosphate in the Pomeranian Bight (southern Baltic Sea). Aquat Microb Ecol. 16, 87–94 (1998).
Rengefors, K., Pettersson, K., Blenckner, T. & Anderson, D. M. Species-specific alkaline phosphatase activity in freshwater spring phytoplankton: Application of a novel method. J Plankton Res. 23, 435–443 (2001).
Cao, X. et al. Detection of extracellular phosphatases in natural spring phytoplankton of a shallow eutrophic lake (Donghu, China). Eur J Phycol. 40, 251–258 (2005).
Strojsova, A., Nedoma, J., Strojsova, M., Cao, X. & Vrba, J. The role of cell-surface-bound phosphatases in species competition within natural phytoplankton assemblage: an in situ experiment. J Limnol. 67, 128–138 (2008).
Strojsova, A. & Vrba, J. Short-term variation in extracellular phosphatase activity: possible limitations for diagnosis of nutrient status in particular algal populations. Aquat Ecol. 43, 19–25 (2009).
Nicholson, D., Dyhrman, S., Chavez, F. & Paytan, A. Alkaline phosphatase activity in the phytoplankton communities of Monterey Bay and San Francisco Bay. Limnol Oceanogr. 51, 874–883 (2006).
Li, Y. & Lei, A. Discussion on water environment protection of Three Gorges Reservoir (in chinese). Yangtze River. 23, 55–58 (2008).
Li, Z., Fang, F., Guo, J. & Tian, G. Spring algal bloom and nutrients characteristics in Xiaojiang River backwater area, Three Gorge Reservoir. Journal of Lake Sciences. 21, 36–44 (2009).
Davey, K. E., Kirby, R. R. & Turley, C. M. Weightman, A.J. and Fry, J.C. Depth variation of bacterial extracellular enzyme activity and population diversity in the northeastern North Atlantic Ocean. Deep Sea Research Part II: Topical Studies in Oceanography. 48, 1003–1017 (2001).
Rose, C. & Axler, R. Uses of alkaline phosphatase activity in evaluating phytoplankton community phosphorus deficiency. Hydrobiologia. 361, 145–156 (1997).
Liu, H. et al. Shifting nutrient-mediated interactions between algae and bacteria in a microcosm: evidence from alkaline phosphatase assay. Microbiological research. 167, 292–298 (2012).
Cao, X., Song, C. & Zhou, Y. Limitations of using extracellular alkaline phosphatase activities as a general indicator for describing P deficiency of phytoplankton in Chinese shallow lakes. J Appl Phycol. 22, 33–41 (2010).
Labry, C., Delmas, D. & Herbland, A. Phytoplankton and bacterial alkaline phosphatase activities in relation to phosphate and DOP availability within the Gironde plume waters (Bay of Biscay). J Exp Mar Biol Ecol. 318, 213–225 (2005).
Li, H., Veldhuis, M. & Post, A. F. Alkaline phosphatase activities among planktonic communities in the northern Red Sea. Mar Ecol Prog Ser. 173, 107–115 (1998).
Chrost, R.J. Environmental control of the synthesis and activity of aquatic microbial ectoenzymes in Microbial enzymes in aquatic environments (ed Chróst RJ) p29 (Springer, New York, 1991).
Hoppe, H. G. & Ullrich, S. Profiles orectoenzy~nesin the Indian Ocean: phenomena of phosphatase activity in the inesopelagic zone. Aquat Microb Ecol. 19, 139–148 (1999).
Song, C., Cao, X. & Li, J. Vertical variation in dissolved alkaline phosphatase activity in a shallow eutrophic lake determined in reverse micelles. J Freshw Ecol. 20, 627–634 (2005).
Cao, X. et al. Extracellular phosphatases produced by phytoplankton and other sources in shallow eutrophic lakes (Wuhan, China): taxon-specific versus bulk activity. Limnology. 10, 95–104 (2009).
Rengefors, K. et al. Experimental investigation of taxon-specific response of alkaline phosphatase activity in natural freshwater phytoplankton. Limnol Oceanogr. 48, 1167–1175 (2003).
Dyhrman, S. T. & Ruttenberg, K. C. Presence and regulation of alkaline phosphatase activity in eukaryotic phytoplankton from the coastal ocean: Implications for dissolved organic phosphorus remineralization. Limnol Oceanogr. 51, 1381–1390 (2006).
Tan, X., Ma, P., Song, L. & Zhang, Q. Physiological and Ultrastructural Responses of Microcystis aeruginosa to Different Phosphorus Concentrations. Fresenius Environ Bull. 21, 838–843 (2012).
Pu, P., Zhang, Z. & Wang, M. Seasonal variation and significance of alkaline phosphatase activity on algal blooming in Chongqing urban section of Jialing River. Asian Journal of Chemistry. 26, 6067–6072 (2014).
Zhou, J., Qin, B., Casenave, C. & Han, X. Effects of turbulence on alkaline phosphatase activity of phytoplankton and bacterioplankton in Lake Taihu. Hydrobiologia. 765, 197–207 (2016).
Healey, F. P. & Hendzel, L. L. Fluorometric measurement of alkaline phosphatase activity in algae. Freshw Biol. 9, 429–439 (1979).
Huber, A. L. & Kidby, D. K. An examination of the factors involved in determining phosphatase activities in estuarine water. 1: Analytical procedures. Hydrobiologia. 111, 3–11 (1984).
Utermohl, V. H. Neue Wege in der quantitativen Erfassung des Planktons. Verh Int Ver Theor Angew Limnol. 5, 567–596 (1931).
Boon, P. I. Organic matter degradation and nutrient regeneration in Australian freshwaters.I: Methods for exoenzyme assays in turbid aquatic environments. Archiv für Hydrobiologie. 115, 339–359 (1989).
Gage, M. & Gorham, E. Alkaline phosphatase activity and cellular phosphorus as index of the phosphorus status of phytoplankton in Minnesota Lakes. Freshw Biol. 15, 227–233 (1985).
Chrost, R. J., Siuda, W. & Halemejko, G. Longterm studies on alkaline phosphatase activity (APA) in a a lake with fish-aquaculture in relation to lake eutrophication and phosphorus cycle. Archiv für Hydrobiologie. 70, 1–32 (1984).
Hu, H. and Wei, Y. The freshwater algae of China-systematics, taxonomy and ecology (Science Press, 2006).
A.P.H.A. Standard methods for the examination of water and wastewater (American Public Health Association, 1995).
Zhu, K., Bi, Y. & Hu, Z. Responses of phytoplankton functional groups to the hydrologic regime in the Daning River, a tributary of Three Gorges Reservoir, China. Science of the Total Environment, s 450–451, 169–177 (2013).
ter Braak, C. J. F. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67, 1167–1179 (1986).
Gil, C. B., Restrepo, J. J. R., Boltovskoy, A. & Vallejo, A. Spatial and temporal change characterization of ceratium furcoides (dinophyta) in the equatorial reservoir riogrande ii, colombia caracterização das mudanças espaciais e temporais de ceratium furcoides (dinophyta) no reservatório equatorial riogrande i. Acta Limnologica Brasiliensia 24(2), 207–219 (2012).
Acknowledgements
This study has been jointly supported by the National Natural Science Foundation of China (No: 31123001) and the Science and Technology Research Project of China Three Gorges Corporation (No. CT-12-08-01).
Author information
Authors and Affiliations
Contributions
Y.J.Y. finished the filed work and the laboratory analysis of samples. Y.J.Y. and Y.H.B. finished the manuscript. Y.H.B. and Z.Y.H. reviewed and discussed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuan, Y., Bi, Y. & Hu, Z. Phytoplankton communities determine the spatio-temporal heterogeneity of alkaline phosphatase activity: evidence from a tributary of the Three Gorges Reservoir. Sci Rep 7, 16404 (2017). https://doi.org/10.1038/s41598-017-16740-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16740-4
This article is cited by
-
Unveiling the impact of glycerol phosphate (DOP) in the dinoflagellate Peridinium bipes by physiological and transcriptomic analysis
Environmental Sciences Europe (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.