Abstract
Aromatic plants show antimicrobial activity due to their essential oils, but their effect on litter decomposition is unclear. In this study, we evaluated the biomass loss and nutrient dynamics in leaf litters of two macrophytes (Miscanthus sacchariflorus and Carex brevicuspis) with and without addition of powdered material of the aromatic plant Polygonum hydropiper or the non-aromatic plant C. brevicuspis. The two powders had similar basic chemical qualities but P. hydropiperi had a higher essential oils concentration. Leaf litters of M. sacchariflorus and C. brevicuspis were incubated with powdered P. hydropiper or C. brevicuspis (500 g m−3, 250 g m−3, and no addition) for 120 days in a mesocosm experiment. Compared with the control (no addition), P. hydropiperi addition decelerated nutrient release and litter decomposition, while C. brevicuspis addition accelerated those processes. The nitrogen concentrations in both leaf litters and the phosphorus concentration in C. brevicuspis leaf litter were increased by addition of both plant powders. The fungal biomass in both leaf litters decreased after P. hydropiperi addition, due to the antifungal activity of its essential oils. These data indicate that the aromatic plant P. hydropiperi inhibits litter decomposition via its essential oils and that such inhibition is not species-specific.
Similar content being viewed by others
Introduction
Litter decomposition partly controls the rates of nutrient and carbon cycles1. The rate of litter decomposition is regulated by interacting physical, chemical, and biotic factors, such as climate (especially temperature and moisture), initial litter quality variables (e.g. nitrogen and phosphorus concentrations, lignin concentration, and carbon:N ratio), exogenous nutrient availability, and decomposers (microbes and invertebrates)2,3. Plants create different environments that retard or accelerate litter decomposition through negative or positive effects on the activity of organisms4.
Aromatic plants are those that synthesize and emit essential oils. These oils are insoluble and include various groups, for example, terpenes, alcohols, and aldehydes5,6. Aromatic plants are widely used in medicine and food storage, and their essential oils have been shown to interfere with the growth and enzymatic reactions of microbes in vivo and in vitro 7,8. In the Mediterranean region, fallen litter or living parts of aromatic plants release essential oils that remain in the soil for up to 1 year6,9. Because aromatic plants always coexist with other plant species, they might affect the decomposition of litter via the effects of their essential oils on microbes6. Besides essential oils, nutrients released from aromatic plants may accelerate the decomposition of other litters10. However, the effects of aromatic plants on litter decomposition remain unclear.
Polygonum hydropiper, a common aromatic plant in East Asia, is famous for its antimicrobial activity and is used as a pharmaceutical11,12. Previous studies have reported on its secondary compounds8, among which its essential oils (mainly terpenes) are reported to affect microbes12. The objective of this study was to investigate the overall effect of P. hydropiper on the decomposition of leaf litter of Miscanthus sacchariflorus and Carex brevicuspis, two dominant plants at Dongting Lake, the second largest freshwater lake in China. In the Dongting Lake wetlands, P. hydropiper often coexists with C. brevicuspis but seldom with M. sacchariflorus. Leaf litters from the two plants were incubated with and without powdered plant materials of P. hydropiper and C. brevicuspis. The two powders had similar basic chemical qualities, but P. hydropiperi had a higher essential oils concentration (Table 1). We tested the following hypotheses: first, that C. brevicuspis litter would decompose faster than M. sacchariflorus litter because of the high nutrient concentrations in C. brevicuspis; second, that the decomposition rates of both litters would be increased by C. brevicuspis addition due to the addition of exogenous nutrients; third, that the decomposition rates of both litters would be decreased by P. hydropiper addition due to its essential oils.
Results
Initial litter quality
The initial N, P, organic C, cellulose, and lignin concentrations differed significantly between the two litters (P < 0.05; Table 2). The concentrations of both N and P were higher in C. brevicuspis litter than in M. sacchariflorus litter (P < 0.05), while the initial lignin and cellulose concentrations, and C:N, C:P, and lignin:N were higher in M. sacchariflorus litter than in C. brevicuspis litter (P < 0.05). These results suggested that C. brevicuspis litter may have greater decomposition potential than M. sacchariflorus litter.
Litter decomposition
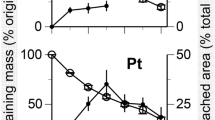
In all treatments, the litters decayed most quickly in the initial 2 weeks of the experiment, and more slowly thereafter (Fig. 1). Within the same treatment, the M. sacchariflorus litter decomposed faster than did C. brevicuspis litter (three-way ANOVA, F = 64.35, P < 0.01; Table 3). The fastest decomposition was in M. sacchariflorus litter with powdered C. brevicuspis at 500 g m−3, and the slowest decomposition was in C. brevicuspis litter with powered P. hydropiper at 500 g m−3. Two-way ANOVA showed significant effects of powdered plant addition on the decomposition rates of both litter species (P < 0.01; Table 4). Compared with the control, P. hydropiper addition at 250 g m−3 decreased the decomposition rates of M. sacchariflorus and C. brevicuspis litter by 26% and 17%, respectively, and P. hydropiper addition at 500 g m−3 decreased the decomposition rates of M. sacchariflorus and C. brevicuspis litter by 41% and 31%, respectively (P < 0.01; Tables 3 and 4). However, the decomposition rates of M. sacchariflorus and C. brevicuspis litter increased after the addition of powdered C. brevicuspis (P < 0.01; Tables 3 and 4).
The N content decreased initially in both species but then recovered (Fig. 2A,C). The N concentration in M. sacchariflorus litter increased throughout the incubation period, while N concentration in C. brevicuspis initially declined, then increased (Fig. 2E,G). Similarly, the P content decreased initially (M. sacchariflorus litter) or throughout the incubation period (C. brevicuspis litter) (Fig. 2B,D). The total P concentration increased steadily (M. sacchariflorus litter) or remained approximately constant (C. brevicuspis litter) (Fig. 2F,H). The release of N and P from both leaf litters was inhibited by P. hydropiper addition (P < 0.01; Table 4), while C. brevicuspis inhibited P and N release only from M. sacchariflorus leaf litter (P < 0.01; Table 4).
Nutrients contents (mg) and concentrations (%) remaining in two litter species in five plant addition (PA) treatments. Values are means ± S.E. (n = 3). CK, no addition; LP, low addition of P. hydropiper; HP, high addition of P. hydropiper; LC, low addition of C. brevicuspis; HC, high addition of C. brevicuspis.
The addition of the powdered plant materials resulted in significant nutrient enrichment of both leaf litters, as indicated by the N and P concentrations (Fig. 2E–H). Addition of powdered P. hydropiper and C. brevicuspis increased the N concentrations in both M. sacchariflorus litter and C. brevicuspis litter as well as the P concentration in C. brevicuspis litter (P < 0.01; Table 4).
There were significant interactions between time and plant addition for nutrient dynamics (P < 0.01; Table 4), indicating that the effects of plant addition mainly occurred at the later stage of the incubation period.
Fungal biomass
The fungal biomass was higher in M. sacchariflorus litter than in C. brevicuspis litter in the treatments with and without powdered C. brevicuspis addition (P < 0.05; Fig. 3). The fungal biomass did not differ significantly between M. sacchariflorus litter and C. brevicuspis litter in the treatments with P. hydropiper addition (P > 0.05). At the end of the experiment, the fungal biomass in M. sacchariflorus litter and C. brevicuspis litter was lower in the treatments with powdered P. hydropiper (P < 0.05; Fig. 3) than in the treatments with powdered C. brevicuspis (P > 0.05; Fig. 3).
Fungal biomass in two litters with five plant addition (PA) treatments. Values are mean ± S.E. (n = 3). CK, no addition; LP, low addition of P. hydropiper; HP, high addition of P. hydropiper; LC, low addition of C. brevicuspis; HC, high addition of C. brevicuspis. **P < 0.01. Different lower case (a, b) letters indicate significant difference in fungal biomass among treatments.
Discussion
Within the same treatment, M. sacchariflorus litter decomposed faster than C. brevicuspis litter, indicating that litter nutrient concentration was not the sole determinant of the decomposition rate of these aquatic macrophytes13. Other qualities, such as the tenderness of M. sacchariflorus leaves, can result in faster decay14,15.
Decomposition of both M. sacchariflorus and C. brevicuspis litter was enhanced by addition of powdered C. brevicuspis, as proposed in the second hypothesis. This increased decomposition may result from nutrient enrichment due to plant addition, as reported previously16. Generally, litter decomposition is the process of utilization by decomposers17. Fungal biomass in our study was similar with previous result18. Though their biomass was not high, fungi were still the fundamental decomposers over the bacteria in wetland environments18. Fungi must assimilate available nutrients from their environment, so nutrient availability is an important limiting factor in litter decomposition17. In previous studies, a positive correlation between mass loss and initial litter nutrient concentrations suggested that litter N and P concentrations could predict the litter decomposition rate19,20. It is worth mentioning that adding materials in powdered form would increase the availability of N and P, because the physical structure would be destroyed and there would be a large surface area for microbial attack. In this study, the addition of powdered C. brevicuspis increased the N concentration in both leaf litters and the P concentration in C. brevicuspis litter, suggesting that nutrients might be transported from C. brevicuspis powder to the leaf litters. Such nutrient enrichment might meet the nutrient demands of fungi, although fungal biomass was not affected by C. brevicuspis addition.
Litter decomposition was accelerated by addition of powdered C. brevicuspis, but inhibited by addition of powdered P. hydropiper, indicating that aromatic plant material inhibited litter decomposition, consistent with the third hypothesis. The responses of both leaf litters were similar, suggesting that the inhibition of litter decomposition by addition of P. hydropiper might not be species-specific.
A previous study reported that addition of the green leaves of the aromatic plant Alliaria petiolata promoted litter decomposition21. The authors of that study proposed that the positive effects of nutrient enrichment from A. petiolata on decomposition outweighed any negative effects of secondary compounds on the activity of the microbes decomposing the litter. In our study, P. hydropiperi addition did not stimulate litter decomposition. Given the difference in chemical qualities between the two powders, the abundant essential oils in P. hydropiperi was the most probable reason for our results. In the present study, the nutrient concentrations in litter were enriched by adding powdered P. hydropiper, but these nutrients may have been retained in the litter instead of being released. This is consistent with the decreases in nutrient release after P. hydropiper addition reported in other studies22,23. Such nutrient retention may be related to the physical effect of essential oils; that is, the hydrophobicity of essential oils coating the litter could prevent microbes from acquiring nutrients24. If this was the case, then it would have been difficult for microbes to access and utilize retained nutrients after the addition of powdered P. hydropiper. Another explanation may be the antifungal activity of essential oils, as indicated by the decreases in fungal biomass in both litters after P. hydropiper addition. The antifungal activity of P. hydropiper essential oils has been reported previously8,11. Given the fundamental role of fungi in litter consumption, this inhibition of fungal activity by P. hydropiper addition might lead to a decreased litter decomposition rate.
In this study, the litter decomposition rates decreased after addition of aromatic plant material. Therefore, in previous studies, the biogeochemical cycle rates might be overestimated for some ecosystems containing aromatic plants. Studies on the return rates of C and other nutrients to the ecosystem should take the presence of aromatic plants into account. In this study, we focused only on the aromatic plant P. hydropiper. Further studies are required to determine the effects of other aromatic plant materials on litter decomposition.
Conclusions
Our findings provide insights into the mechanisms by which aromatic plants affect litter decomposition rates and nutrient dynamics in wetlands. Our findings showed that litter decomposition was stimulated by addition of powdered material from a non-aromatic plant, but inhibited by addition of powdered material from an aromatic plant, probably by essential oils, and such inhibition is not species-specific.
Methods
Collection and preparation of plant material and soil
Leaf litters of M. sacchariflorus and C. brevicuspis were collected from standing dead plants. Aerial parts of P. hydropiper and C. brevicuspis were collected from living plants at Dongting Lake (29°27′2″N, 112°47′32″E) in November 2012. Aerial parts were selected because the essential oils concentration seldom changed during withering6. M. sacchariflorus, C. brevicuspis, and P. hydropiper are the main emergent macrophytes in these wetlands, with biomasses of 3000 g m−2, 1500 g m−2, and 2500 g m−2, respectively. Control soil was collected from the 0–15 cm layer from bare land nearby to avoid the potential home-field advantage during decomposition25. This soil was loam silt and its basic properties are shown in Table 1 (no addition). After collection, the leaf litters were air-dried to constant mass for 48 h and cut into approximately 10-cm long pieces. Weighed litter samples (5 g) were placed into 10 × 15-cm nylon bags (1-mm mesh). This mesh size excluded macroinvertebrates but allowed microbial colonization and leaching of litter fragments26. Sets (‘strings’) of two bags (one per litter species) were connected by nylon string to facilitate harvest. The aerial part was oven-dried at 60 °C for 7 days to avoid loss of essential oils, and then ground to a powder and passed through a 0.5-mm mesh screen27. Reduction of the added material to a powder would render the N and P in it readily available because the physical structure would be destroyed and there would be many surfaces for microbial attack.
Experimental set-up
The study was carried out at the Dongting Lake Station for Wetland Ecosystem Research (29°29′59″N, 112°47′49″E). This site has a subtropical monsoon climate. The average annual air temperature is 17 °C and the average annual precipitation is 1,302 mm.
The mesocosm experiment consisted of the soil substrate with and without added plant powder28. The experimental treatments consisted of two leaf litters without (control, CK) or with addition of powdered plant materials of P. hydropiper and C. brevicuspis at two levels (high addition, 500 g m−3; low addition, 250 g m−3) in a one-way factorial design in 15 plastic tanks (0.5 × 0.4 × 0.7 m; five treatments, each with three replicates). The high addition rate was selected based on the biomass (2500 g m−2) of P. hydropiper in the Dongting Lake wetlands. To simulate the soil environment under an aromatic plant, the fermentation system allowed materials such as nutrients and essential oils to be released from plant powder into the soil substrate and then to contact the litter. Each tank was filled with soil to a depth of 0.2 m. After homogenizing, the soil substrate was saturated with water and fermented for 30 days to reduce the potential effects of new organic C input29 on litter decomposition. Besides, since the powder decomposed substantially after fermentation (see the organic C concentration of various soil substrate in Table 1), the potential non-additive effects of litter mixing was also prevented. The addition of plant powder increased the nutrient concentrations in all soil substrates (P < 0.05; Table 1) except for P in the 250 g m−3 C. brevicuspis treatment. The soil organic C concentration was not affected by plant addition (P > 0.05; Table 1).
Litter strings were randomly placed in the tanks (at 5-cm intervals) on April 14, 2013, and buried to 5-cm depth in the substrate. A total of 180 litterbags were used (three replicates × two litter species × five treatments × five harvests). During the incubation period, tap water was added to each tank weekly to maintain soil water at approximately 60% of water holding capacity for optimal microbial activity30. Three strings per treatment were sampled after incubation for 15, 30, 60, 90, and 120 days. Another 30 samples (three replicates of two litter species and five treatments) were sampled to measure the ergosterol concentration on day 120. Before incubation, three samples of each litter species were used measure the initial litter quality.
Chemical analyses
Litter samples and initial litter were washed gently using deionized water until the water was transparent, and then oven-dried at 60 °C to constant weight (1 week) before measuring dry weight (accuracy to 0.01 g). All litter samples were ground to a powder and passed through a 0.5-mm mesh screen for quality analysis. Initial litter samples were analyzed to determine organic C, N, P, cellulose, and lignin concentrations; incubated litter samples were analyzed to determine N and P concentrations; plant powder samples were analyzed to determine the concentrations of organic C, N, P, and essential oils. Organic C concentration was analyzed using the H2SO4–K2Cr2O7 heat method, and N and P were quantified using Kjeldahl digestion followed by colorimetric analysis. Cellulose and lignin concentrations were determined by hydrolysis (10% H2SO4 for cellulose, 72% H2SO4 for lignin) followed by Na2S2O3 titration31. The essential oil concentration was determined by distillation32.
Fungi play a fundamental role in litter decomposition in wetland environments33, as confirmed in our previous experiments on the same litter species from the same wetlands26. The fungal biomass, which represents decomposer activity, can be estimated by measuring the ergosterol concentration31. For these analyses, the litter samples were frozen at −30 °C after sampling. The ergosterol concentration was determined by high-performance liquid chromatography31. For analyses, the litter material was lyophilized and ground, and then extracted in alkaline methanol at 80 °C. Solid-phase extraction through C18 cartridges was used for purification. Dry, unprocessed litters were used for blank values. These materials were stored dry at room temperature. We calculated the fungal biomass at the end of incubation using the conversion factor of 5.5 mg ergosterol g−1 fungal biomass26,31, and the values are expressed as mg g−1 dry weight (DW).
Data analysis
The properties of soil, litter, and powder were compared by one-way ANOVA with species or types as the main factors.
The decomposition rate (k) for each litter species was calculated using equation (1):
where W 0 is the initial litter mass and W t is the mass remaining at t days34. Litter mass and remaining nutrients were calculated as percentages of initial values. The remaining litter mass was compared using three-way ANOVA with litter species, time, and treatment as the main factors. Within each litter species, the response variables were compared using two-way ANOVA with treatment and time as the main factors to test the treatment effect. Fungal biomass was compared by two-way ANOVA, with litter species and treatment as the main factors. Values were log-transformed to homogenize the variances if necessary. All statistical analyses were performed using the statistical software SPSS 21.
References
Hobbie, S. E. et al. Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment. Ecol. Monogr. 82, 389–405 (2012).
Hättenschwiler, S., Tiunov, A. V. & Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evo. 36, 191–218 (2005).
Chen, F. S., Feng, X. & Liang, C. Endogenous versus exogenous nutrient affects C, N, and P dynamics in decomposing litters in mid-subtropical forests of China. Ecol. Res. 27, 923–932 (2012).
Sariyildiz, T. Effects of tree canopy on litter decomposition rates of Abies nordmanniana, Picea orientalis and Pinus sylvestris. Scand. J. Forest Res. 23, 330–338 (2008).
Schwab, W., Davidovich-Rikanati, R. & Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 54, 712–732 (2008).
Hassiotis, C. N. & Lazari, D. M. Decomposition process in the Mediterranean region. Chemical compounds and essential oil degradation from Myrtus communis. Int. Biodeter. Biode. 64, 356–362 (2010).
Park, M. J. et al. Inhibitory effect of the essential oil from Chamaecyparis obtusa on the growth of food-borne pathogens. J. Microbiol. 48, 496–501 (2010).
Liu, Q. F. et al. Antifungal activity in plants from Chinese traditional and folk medicine. J. Ethnopharmacol. 143, 772–778 (2012).
Ramirez, K. S., Lauber, C. L. & Fierer, N. Microbial consumption and production of volatile organic compounds at the soil-litter interface. Biogeochemistry 99, 97–107 (2010).
Kim, S. & Kim, J. G. Humulus japonicus accelerates the decomposition of Miscanthus sacchariflorus and Phragmites australis in a floodplain. J. Plant Biol. 52, 466–474 (2010).
Maheswaran, R. & Ignacimuthu, S. Bioefficacy of essential oil from Polygonum hydropiper L. against mosquitoes, Anopheles stephensi and Culex quinquefasciatus. Ecotox. Environ. Safe 97, 26–31 (2013).
Zheng, Z. C. et al. Accumulation characteristics of and removal of nitrogen and phosphorus from livestock wastewater by Polygonum hydropiper. Agr. Water Manag. 117, 19–25 (2013).
Gijsman, A. J., Alarcón, H. F. & Thomas, R. J. Root decomposition in tropical grasses and legumes, as affected by soil texture and season. Soil Biol. Biochem. 29, 1443–1450 (1997).
Fonseca, A. L., Bianchini, I., Pimenta, C. M., Soares, C. B. & Mangiavacchi, N. The flow velocity as driving force for decomposition of leaves and twigs. Hydrobiologia 703, 59–67 (2013).
Moorhead, D. L. & Sinsabaugh, R. L. A theoretical model of litter decay and microbial interaction. Ecol. Monogr. 76, 151–174 (2006).
Girisha, G. K., Condron, L. M., Clinton, P. W. & Davis, M. R. Decomposition and nutrient dynamics of green and freshly fallen radiata pine (Pinus radiata) needles. Forest Ecol. Mana. 179, 169–181 (2003).
Beth, M. C., Erika, B. K. & Jackson, R. W. Immobilization and mineralization of N and P by heterotrophic microbes during leaf decomposition. Freshw. Sci. 31, 133–147 (2012).
Baldy, V. & Gessner, M. O. Towards a budget of leaf litter decomposition in a first-order woodland stream. Ecology 320, 747–758 (1977).
Blanco, J. A., Imbert, J. B. & Castillo, F. J. Thinning affects Pinus sylvestris needle decomposition rates and chemistry differently depending on site conditions. Biogeochemistry 106, 397–414 (2011).
Aponte, C., García, L. V. & Marañón, T. Tree species effect on litter decomposition and nutrient release in Mediterranean oak forests changes over time. Ecosystems 15, 1204–1218 (2012).
Rodgers, V. L., Wolfe, B. E., Werden, L. K. & Finzi, A. C. The invasive species Alliaria petiolata (garlic mustard) increases soil nutrient availability in northern hardwood-conifer forests. Oecologia 157, 459–471 (2008).
Verkaik, E., Jongkind, A. G. & Berendse, F. Short-term and long-term effects of tannins on nitrogen mineralisation and litter decomposition in kauri (Agathis australis (D. Don) Lindl.) forests. Plant Soil 287, 337–345 (2006).
Nierop, K. G. J., Verstraten, J. M., Tietema, A., Westerveld, J. W. & Wartenbergh, P. E. Short- and long-term tannin induced carbon, nitrogen and phosphorus dynamics in Corsican pine litter. Biogeochemistry 79, 275–296 (2006).
Bailey, P. C. E., Watkins, S. C., Morris, K. L. & Boon, P. I. Do Melaleuca ericifolia SM. leaves suppress organic matter decay in freshwater wetlands? Arch. Hydrobiol. 156, 225–240 (2003).
Mao, R., Zeng, D. H. & Li, L. J. Fresh root decomposition pattern of two contrasting tree species from temperate agroforestry systems: effects of root diameter and nitrogen enrichment of soil. Plant Soil 37, 115–123 (2011).
Xie, Y. J., Xie, Y. H., Hu, C., Chen, X. S. & Li, F. Interaction between litter quality and simulated water depths on decomposition of two emergent macrophytes. J. Limnol. 75, 36–43 (2016).
Buras, A. et al. Allometric variability of Haloxylon species in CentralAsia. Forest Ecol. Mana. 274, 1–9 (2012).
Verhoeven, J. T. A. & Toth, E. Decomposition of Carex and Sphagnum litter in fens: effect of litter quality and inhibition by living tissue homogenates. Soil Biol. Biochem. 27, 271–275 (1995).
Kammer, A., Schmidt, M. W. I. & Hagedorn, F. Decomposition pathways of 13C-depleted leaf litter in forest soils of the Swiss Jura. Biogeochemistry 108, 395–411 (2012).
Berglund, S. L., Ågren, G. I. & Ekblad, A. Carbon and nitrogen transfer in leaf litter mixtures. Soil Biol. Biochem. 57, 341–348 (2013).
Graça, M. A., Bälocher, F. & Gessner, M. O. Methods to study litter decomposition: a practical Guide (Springer, Netherlands, 2005).
Zhang, G. Y. & Zeng, T. Study on chemical constituents of Polygonum hydropiper Linn. Chem. Ind. Forest Prod. 25, 21–24 (2005).
Ferreira, V. & Chauvet, E. Synergistic effects of water temperature and dissolved nutrients on litter decomposition and associated fungi. Global Change Bio. 17, 551–564 (2011).
Olson, J. S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44, 322–331 (1963).
Acknowledgements
This study was financially supported by the National Key Technology Research and Development Program (2014BAC09B03), the Natural Science Foundation of Jiangxi, China (20171BAB214010), and the Doctoral Scientific Research Foundation of East China University of Technology (DHBK2016108). We thank Jennifer Smith, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
Y.J.X. wrote the manuscript and conducted assays and statistical analyses. Y.H.X., Y.J.X., and H.Y.X. designed the experiment and edited the manuscript. Y.J.X., Z.M.D., Y.P., B.H.P., and J.Y.H. contributed to data collection and interpretation. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, Yj., Xie, Yh., Xiao, Hy. et al. Inhibition of litter decomposition of two emergent macrophytes by addition of aromatic plant powder. Sci Rep 7, 16685 (2017). https://doi.org/10.1038/s41598-017-16615-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16615-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.