Abstract
Since fatigue is prevalent in modern societies, it is necessary to clarify the neural mechanisms of fatigue. The regulation of performance through fatigue sensation is one of the mechanisms that decreases performance in fatigue. However, it is unknown whether subjective feeling of fatigue is necessary for the regulation of performance. Here, we examined whether decreased performance occurs without increased fatigue sensation through the experiment which was designed to test if fatigue can be learned unconsciously. Healthy male volunteers performed a fatigue-inducing hand-grip task for 10 min while viewing a target image presented without awareness. On the next day, they viewed a control and the target images presented with awareness and the neural activity caused by viewing the images was measured using magnetoencephalography. Results showed the level of fatigue sensation was not altered but grip-strength was decreased by viewing the target image on the second day. The level of beta band power in Brodmann’s area 31 was increased by viewing the target image and this increase was negatively associated with the decrease of grip-strength caused in the hand-grip task. These findings demonstrated that fatigue can be learned unconsciously and that there is a mechanism to decrease performance without fatigue sensation.
Similar content being viewed by others
Introduction
Fatigue is defined as a decline in the ability to perform or in the efficiency of performing mental and/or physical activities caused by excessive mental or physical activity or disease, and is often accompanied by a peculiar sense of discomfort, a desire to rest, and a decline in motivation; these feelings are referred to as fatigue sensation1. Fatigue is prevalent in modern societies2,3,4,5,6,7 and the complaint of fatigue is ubiquitous in primary care settings8,9. Fatigue is related to accidents and thus is a major contributor to workplace morbidity and mortality10,11. In addition, fatigue is associated with mortality in the general population especially through cardiovascular disease12. Therefore, clarifying the pathophysiology of fatigue to better deal with fatigue and its related problems is necessary.
As mentioned above, fatigue decreases the performance of activity. It is thought that this decrease in performance is not only due to the dysfunction of the part of the body and/or brain engaged in the activity, but is also due to the neural mechanisms which regulate the performance13,14,15,16,17,18. It has been proposed that two conceptual systems, the facilitation and inhibition systems, are involved in this mechanism. In the presence of workload, the facilitation system is activated through factors such as motivation to increase performance and, at the same time, the inhibition system is activated to decrease performance to maintain homeostasis. The balance between these two systems determines the performance of the activity to accomplish the workload16,18.
Fatigue sensation plays an important role as a biological alarm and urges us to rest to avoid disrupting homeostasis19,20. In our everyday lives, we sometimes limit performance to accomplish the assigned workload based on the subjective level of fatigue to avoid the accumulation of fatigue. It is important to decide whether or not to take a rest based on the subjective level of fatigue in order to prevent overwork, which can be a cause of death in the working population21,22,23. Thus, the regulation of performance through fatigue sensation is one of the mechanisms that decreases performance in the existence of workload. However, it is unclear whether awareness of the existence of fatigue, i.e., fatigue sensation, is necessary for suppressing performance. Since clarifying the neural mechanisms that decrease performance is central to understanding the pathophysiology of fatigue, it is worthwhile to investigate whether there are mechanisms that decrease performance independently of fatigue sensation.
Fear conditioning paradigms are often used to clarify the neural mechanism of the acquisition of fear response, in which the paired conditioned stimulus induces responses usually caused by an aversive unconditioned stimulus. In these fear conditioning paradigms, the stimuli are presented with the participant’s awareness (in awareness). However, there have been several reports that conditioned responses have been acquired when the conditioned stimuli were presented without the participant’s awareness (out of awareness), suggesting that associative learning can be acquired without awareness24,25,26,27,28,29. For example, it has been reported that a conditioned response was acquired with masked presentation of snakes and spiders associated with a mild electric shock as an unconditioned stimulus25, that associative learning between masked presentations of emotional facial expressions and shock stimuli occurred30, and that the associations between unconsciously presented words and a white noise blast as an unconditioned stimulus were observed27. In these studies, conditioned responses were assessed by measuring alterations in skin conductance response or electroencephalography (EEG). In a functional magnetic resonance imaging (fMRI) study, the effect of an unconsciously learned association between visual stimuli and an electrical unconditioned stimulus was shown by changes in neural activity, although conditioned subjective responses were not observed29. Taking this into consideration, there is a possibility that an association between fatigue and a stimulus presented out of awareness can be learned and, in addition, that a decrease in performance can be induced by the conditioned stimulus after the learning without an increase in fatigue sensation. If this happens, it is plausible that there is a mechanism that decreases performance independently of fatigue sensation.
In a previous magnetoencephalography (MEG) study, it was reported that the association between fatigue and a conditioned stimulus presented in awareness was learned. In that study, the participants performed hand-grip trials while listening to metronome sounds on the first day. Increases in fatigue sensation and sympathetic nerve activity were induced only by listening to the metronome sounds without hand-grip trials on the second day, and sympathetic nerve activity was associated with a decrease in the 8-13 Hz band power in the right dorsolateral prefrontal cortex17. Since the increase in sympathetic nerve activity and the decrease in parasympathetic nerve activity were thought to be related to fatigue31,32,33, the results show that the association between fatigue and the conditioned stimulus presented in awareness was learned.
In our present study, we hypothesized that there may be mechanisms which decrease performance without fatigue sensation and aimed to examine whether associative learning between fatigue and a visual stimulus presented out of awareness occurs and whether the alterations in the level of performance and/or fatigue sensation are induced by the visual stimulus presented in awareness after the learning. To this end, the experimental procedure of the previous study was modified to present a conditioned stimulus out of awareness: A visual backward masking procedure34,35,36,37,38 was used to present the conditioned stimulus out of awareness. Hand-grip trials were performed with unconscious presentations of the conditioned stimulus on the first day and changes in fatigue sensation, grip strength, autonomic activity, and oscillatory brain activity caused by viewing the conditioned stimulus presented in awareness were assessed on the second day. Presentation of a control image, which was not presented during hand-grip trials on the first day, was also conducted on the second day. We assessed the levels of perception of fatigue and performance fatigability related to the repetitive hand-grip trials39. We focused on: 1) whether the increase in fatigue sensation caused by viewing the conditioned stimulus on the second day was greater than that caused by viewing the control image on the second day, 2) whether grip strength was decreased by viewing the conditioned stimulus on the second day but not altered by viewing the control image on the second day, and 3) whether the neural activity caused by viewing the conditioned stimulus on the second day was different from that caused by viewing the control image on the second day. Since the neural activity related to learning of the association between fatigue and a conditioned stimulus presented in awareness has been successfully detected using MEG in previous studies17,40, we used MEG to assess the neural activity caused by viewing the conditioned stimulus and performed spatial filtering analyses of the MEG data to detect changes in oscillatory power that reflect changes in neural dynamics41,42,43.
Methods
Participants
Twenty healthy male volunteers aged 23.5 ± 3.0 years of age (mean ± standard deviation [SD]) participated in this study. All participants were right-handed according to the Edinburgh Handedness Inventory44. Current smokers, individuals with a history of mental illness, brain injury, or upper extremity disorder, and individuals taking chronic medications that affect the central nervous system were excluded. The participants were asked to refrain from caffeine or products containing caffeine for 12 h before each experimental day. The Ethics Committee of Osaka City University approved the study protocol (approval number, 3502). All participants provided written informed consent to participate in this study in accordance with the principles of the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan (Ministry of Education, Culture, Sports, Science and Technology and Ministry of Health, Labor and Welfare).
Experimental design
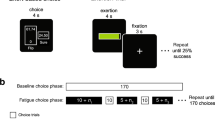
The study was performed on two consecutive days (Fig. 1). The experiment on the first day (day 1) consisted of an MEG session (MEG session 1), and a hand-grip session and that on the second day (day 2) consisted of an MEG session (MEG session 2) identical to that on day 1. In the MEG sessions, the participants lay on a bed placed in a magnetically shielded room and were instructed to perform the target and control tasks (Fig. 1A). In the target task, they were asked to view an image projected on a screen by a projector (PG-B10S; SHARP, Osaka, Japan). The image used in the target task was the repetition of a fixation cross for 1300 to 1700 ms followed by a target mark for 2500 ms (Fig. 1B; the target mark is a closed triangle in this figure). The target mark was presented 180 times during the target task. In the control task, participants were asked to view another image. This image used in the control task was the repetition of a fixation cross for 1300 to 1700 ms followed by a control mark for 2500 ms (Fig. 1C; the control mark is a closed circle in this figure). The control mark was presented 180 times during the control task. The target and control marks were the closed triangle and the closed circle, respectively, for half of the participants and the closed circle and the closed triangle, respectively, for another half of the participants. Whether the target mark was the closed triangle or the closed circle was randomly assigned for each participant. Magnetic neural activities over the target and control tasks were recorded using MEG. The order of the tasks (i.e., the target task followed by the control task or the control task followed by the target task on day 1 and 2) was randomized among the participants and there was a rest period of 5 min between the target and control tasks. On day 1, the participants performed the hand-grip session following the MEG session 1. In the hand-grip session, they were asked to perform hand-grip trials for 150 times with their right hand using a hand-grip device (EXG006 30 kg; ALINCO, Osaka, Japan) in time with a visual cue. The visual cue consisted of a fixation cross (for 300 to 700 ms), a blank screen or the target mark (for approximately 33 ms), a mask image as grasping cue (for approximately 2467 ms), and the blank screen (for 1000 ms) (Fig. 1D). This sequence of the visual presentation was used to make the target mark invisible for the participants during the hand-grip task trials (i.e., visual backward masking procedure). For the first half of the hand-grip trials (i.e., for 75 times), the blank screen was presented just before the mask image and the target mark was presented just before the mask image for the rest of the trials (i.e., for 75 times) so that the association between fatigue and the target mark could be learned in the last half of the hand-grip session. They were instructed to grasp the hand-grip device with their maximum power over the presentation of the mask image (i.e., all participants performed hand-grips 150 times). Just before and after the target, control, and hand-grip tasks, the participants were asked to rate their subjective level of fatigue using a 100-mm visual analogue scale (VAS) ranging from 0 (minimum) to 100 (maximum) and grip strength was assessed using a grip dynamometer (ST-100; Toei Light Co., Ltd., Saitama, Japan). At the end of the experiment on day 2, they were asked whether they recognized the existence of the target marks during the hand-grip task performed on day 1. The experiments for 10 participants were performed between 9 a.m. and noon and those for the others were performed between 2 p.m. and 5 p.m.
Experimental design. (A) The study was performed on two consecutive days. The experiment on the first day (day 1) consisted of an MEG session (MEG session 1), and a hand-grip session and that on the second day (day 2) consisted of an MEG session (MEG session 2) identical to that on day 1. In the MEG sessions, the participants were instructed to perform the target and control tasks. Just before and after the target, control, and hand-grip tasks, the participants were asked to rate their subjective level of fatigue using a visual analogue scale (VAS) and grip strength (GS) was assessed. At the end of the experiment on day 2, they were asked whether they recognized the existence of the target marks during the hand-grip task performed on day 1. (B) In the target task, they were asked to view an image, which was the repetition of a fixation cross for 1300 to 1700 ms followed by a target mark for 2500 ms (the target mark is a closed triangle in this figure). The target mark was presented 180 times. (C) In the control task, participants were asked to view another image, which was the repetition of a fixation cross for 1300 to 1700 ms followed by a control mark for 2500 ms (the control mark is a closed circle in this figure). The control mark was presented 180 times. There was a rest period of 5 min between the target and control tasks. (D) In the hand-grip session, they were asked to perform hand-grip trials for 150 times in time with a visual cue. The visual cue consisted of a fixation cross (for 300 to 700 ms), a blank screen or the target mark (for approximately 33 ms), a mask image as grasping cue (for approximately 2467 ms), and the blank screen (for 1000 ms). For the first half of the hand-grip trials (i.e., for 75 times), the blank screen was presented just before the mask image and the target mark was presented just before the mask image for the last half of the trials (i.e., for 75 times).
MEG recording
MEG was recorded using a 160-channel whole-head-type MEG system (MEG vision; Yokogawa Electric Corporation, Tokyo, Japan) with a magnetic field resolution of 4 fT/Hz1/2 in the white-noise region. The sensor and reference coils were gradiometers with a 15.5-mm diameter and 50-mm baseline, and the two coils were separated by 23 mm. The sampling rate was 1000 Hz and data were high-pass filtered at 0.3 Hz.
MEG analyses
The magnetic noise that originated from outside the magnetically shielded room was eliminated by subtracting the data obtained from reference coils using specialized software (MEG 160; Yokogawa Electric Corporation). Epochs of the raw MEG data that included artifacts were visually identified and were excluded before the analyses. Spatial filtering analysis of the MEG data was performed to identify changes in oscillatory brain activity that reflected time-locked cortical activities41,42,43 caused by performing the target and control tasks on day 2. The MEG data were bandpass filtered at 8–13 Hz and 13–25 Hz by a finite impulse response filtering method using Brain Rhythmic Analysis for MEG software (BRAM; Yokogawa Electric Corporation) to obtain alpha and beta signals, respectively. After the bandpass filtering, the location and intensity of the cortical activities were estimated using BRAM, which uses a narrow-band adaptive spatial filtering algorithm45,46. Voxel size was set at 5.0 × 5.0 × 5.0 mm. For each MEG measurement (i.e., both the target and control tasks) and frequency band, the oscillatory power of the MEG data from 0 to 2000 ms after the onset of the target or control marks was calculated relative to that from −500 to 0 ms before the onset of the marks. Data were then analyzed using statistical parametric mapping (SPM8; Wellcome Department of Cognitive Neurology, London, UK), implemented in Matlab (Mathworks, Natick, MA). The MEG parameters were transformed into the Montreal Neurological Institute T1-weighed image template47 and applied to the MEG data. The anatomically normalized MEG data were filtered with a Gaussian kernel of 20 mm (full-width at half-maximum) in the x-, y-, and z-axes. Individual data were summarized and incorporated into a random-effect model48. The weighted sum of the parameters estimated in the individual analyses was used to create “contrast” images that were used for group analyses48. The resulting set of voxel values for each comparison constituted a statistical parametric map (SPM) of the t statistic (SPM{t}). The SPM{t} was transformed to units of normal distribution (SPM{Z}). The significance of any changes in the oscillatory power observed in the target and control tasks and the significance of any difference in the oscillatory power observed during the target task compared to that observed during the control task were assessed using t statistics (one sample t-test and paired t-test, respectively) on a voxel-by-voxel basis48. The threshold for the SPM{t} of the one sample t-test and paired t-test was set at P < 0.05 (family-wise-error corrected for multiple comparisons). Localization of the brain regions was performed using WFU_PickAtras, Version 3.0.4 (http://fmri.wfubmc.edu/software/pickatlas) and Talairach Client, Version 2.4.3 (http://www.talairach.org/client.html).
Magnetic resonance (MR) image overlay
Anatomical MR imaging was performed using a Philips Achieva 3.0 TX (Royal Philips Electronics, Eindhoven, The Netherlands) to permit registration of magnetic source locations with their respective anatomical locations. Before MR scanning, five adhesive markers (Medtronic Surgical Navigation Technologies Inc., Broomfield, CO) were attached to the skin of the head: Two markers 10 mm in front of the left and right tragus, one marker 35 mm above the nasion, and two markers 40 mm to either side of the marker above the nasion. The MEG data were superimposed on MR images using information obtained from these markers and MEG localization coils.
Electrocardiography (ECG)
To examine changes in autonomic nerve activity caused by performing the target and control tasks, ECG was recorded during the MEG recordings. The ECG data were analyzed with the maximum entropy method using MemCalc for Windows (Global Medical Solution Inc., Tokyo, Japan). The ECG data from 300 to 600 s after the onset of the MEG recordings were used. R-R wave variability was measured as an indicator of autonomic nerve activity. For frequency-domain analysis of the R-R wave intervals, low-frequency (LF) power was calculated as the power within the frequency range 0.04–0.15 Hz and high-frequency (HF) power was calculated as the power within the frequency range 0.15–0.4 Hz. LF and HF power were measured in absolute units (ms2). It has been reported that HF power is vagally mediated49,50,51, but that LF power originates from a variety of sympathetic and vagal mechanisms49,52. The LF/HF ratio is considered an index of sympathetic nervous system activity53. The natural logarithm of LF power, HF power, and the LF/HF ratio was calculated and used for statistical analyses.
Statistical analyses
A paired t-test was used to compare the subjective level of fatigue, grip strength, and indices of autonomic nerve function between the target and control tasks and the subjective level of fatigue and grip strength between before and after the hand-grip task. Pearson’s correlation analysis was conducted to evaluate the relationships between the MEG responses induced by the target task on day 2 and the change in grip strength caused by the hand-grip task. All P values were two-tailed and values less than 0.05 were considered statistically significant. All statistical analyses were performed using the IBM SPSS 21.0 software package (IBM, Armonk, NY) and values are presented as mean and SD unless otherwise stated.
Results
Awareness of the existence of the target mark during the hand-grip task
Three participants noticed the existence of the target marks during the hand-grip task performed on day 1. Two participants reported that they saw the target marks several times during the hand-grip task and one participant reported that he saw the target marks just before the appearance of the mask image almost every time the mask image appeared. The data from these three participants were excluded from our analyses. The others declared that they did not recognize the target marks at all during the hand-grip task. In addition, the MEG data from the other two participants were also excluded from the analyses because their MEG data were contaminated with magnetic noise that originated from outside the shielded room and the number of epochs that remained after exclusion of the epochs that included artifacts was not sufficient for the analysis: We used the MEG data with more than 10 epochs for the analyses. Therefore, the final sample size of our present study was 15.
Subjective level of fatigue
We were interested in whether the increase in the subjective level of fatigue caused by the target task on day 2 was different from that caused by the control task on day 2. The increase in the subjective level of fatigue observed during the target task on day 2 was not altered compared with that observed during the control task on day 2 (t 14 = 0.426; P = 0.677, paired t-test; Fig. 2C). The subjective level of fatigue observed after the hand-grip task on day 1 was increased compared with that before the task (t 14 = 0.5.385; P < 0.001, paired t-test; Fig. 2B).
Subjective level of fatigue. The increases in the subjective level of fatigue during the control and target tasks in MEG session 1 (A), the levels of fatigue sensation before and after the hand-grip session (B), and the increases in the subjective level of fatigue during the control and target tasks in MEG session 2 (C) were shown. Participants were asked to rate their subjective level of fatigue on a 100-mm visual analogue scale (VAS) ranging from 0 (minimum fatigue) to 100 (maximum fatigue). Data are presented as means and standard deviations. **P < 0.01, paired t-test.
Grip strength
We were interested in whether grip strength was decreased by performing the target task on day 2 while remaining the same during the control task on day 2. The grip strength assessed just after the target task on day 2 was decreased compared with that assessed just before the target task on day 2 (t 14 = 2.313; P = 0.036, paired t-test; Fig. 3C) and the grip strength assessed just after the control task on day 2 was not altered compared with that assessed just before the control task on day 2 (t 14 = 1.421; P = 0.177, paired t-test; Fig. 3C). The grip strength assessed just after the hand-grip task on day 1 was decreased compared with that assessed just before the hand-grip task on day 1 (t 14 = 8.392; P < 0.001, paired t-test; Fig. 3B).
Grip strength. Grip strength assessed before and after the control and target tasks in MEG session 1 (A), that assessed before and after the hand-grip task (B), and that assessed before and after the control and target tasks in MEG session 2 (C) were shown. Data are presented as means and standard deviations. * P < 0.05 and ** P < 0.01, paired t-test.
Autonomic nerve activity
HF power and the LF/HF ratio were compared between the target and control tasks on day 2. The HF power observed during the target task on day 2 showed a tendency toward decrease compared with that observed during the control task on day 2 (t 14 = 2.269; P < 0.079, paired t-tests with Bonferroni correction; Fig. 4B).
Autonomic nerve activity evaluated using frequency domain analysis of R-R wave intervals measured with electrocardiography. Values were transformed by natural logarithm (ln). High frequency power (ln HF, closed columns) and the LF/HF ratio (ln LF/HF; open columns) on day 1 (A) and day 2 (B) are shown. Data are presented as means and standard deviations. # P < 0.10, paired t-test with Bonferroni correction.
Spatial filtering analyses of MEG data
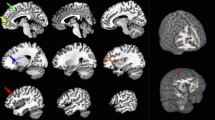
Some brain regions showed changes in oscillatory powers during performing the target or control tasks (Table 1). To identify changes in neural activity caused by performing the target task compared with those caused by performing the control task on day 2, the oscillatory powers observed during the target task on day 2 were compared with those observed in the control task on day 2. There was one brain region in which the increase in the beta band power caused by performing the target task was larger than that caused by performing the control task (Table 2, Fig. 5). This brain region was located in the right posterior cingulate gyrus (Brodmann’s area 31; BA 31). The increased beta band power observed during the target task compared with that observed during the control task on day 2 was negatively associated with a decrease in grip strength caused by performing the hand-grip task on day 1 (R = −0.633, P = 0.011; Fig. 6).
Statistical parametric map. Statistical parametric map of the brain region where the level of increased beta band (13–25 Hz) power was higher in the target task than that in the control task on day 2 is shown (maximum intensity projection). Random-effect analyses of 15 participants, P < 0.05, family-wise-error corrected for the entire search volume.
Relationship between the decrease in grip strength and the increment of increased beta (13–25 Hz) band power. Relationship between the decrease in grip strength induced by the hand-grip task on day 1 and the increment of increased beta band power in the target task compared with the control task on day 2 is shown. The linear regression line, Pearson’s correlation coefficient, and P value are shown.
Discussion
In the present study, the participants viewed masked target marks during a hand-grip task on day 1. A decrease in hand-grip strength and an increase in fatigue sensation were induced by the hand-grip trials, suggesting that fatigue and fatigue sensation were induced by the hand-grip trials. On day 2, an increase in fatigue sensation caused by viewing the target marks presented in awareness was not greater than that caused by viewing the control marks presented in awareness, and a decrease in grip strength was induced by viewing the target marks while a decrease in grip strength was not induced by viewing the control marks. In addition, the level of increased beta band power in BA 31 was greater while viewing the target marks than that while viewing the control marks on day 2, and an increase in the beta band power observed during the target task was negatively associated with a decrease in grip strength caused by performing the hand-grip task on day 1.
On day 1, the target marks were presented out of awareness using a visual backward masking procedure34,35,36,37,38. Since three participants reported that they could recognize the masked target marks during the hand-grip task, the data from these participants were excluded from the analyses. The other participants, whose data were included in our analyses, were completely unaware of the presentation of the masked target marks on day 1.
The increased fatigue sensation caused by viewing the target marks presented in awareness was not greater than that caused by viewing the control marks presented in awareness on day 2. We often experience that participants’ subjective ratings of fatigue sensation increase while they are performing a task even if the load of the task is not high, such as only viewing simple images as in our present study. Therefore, we assessed whether there was difference between the increment of fatigue sensation caused by viewing the target mark and that caused by viewing the control mark on day 2. As a result, the increment of the fatigue sensation caused by viewing the target mark was not altered compared with that caused by viewing the control mark on day 2, showing that there were no significant effects of viewing the target mark on the level of fatigue sensation on day 2.
A decrease in grip strength was not observed in the control task but was observed in the target task on day 2. Since it is not plausible that grip strength is decreased only by viewing a “triangle” or “circle”, which have not been associated with fatigue, we examined whether grip strength was decreased by viewing the target mark but remained the same when viewing the control mark. Our findings regarding grip strength can be interpreted as follows: The association between the neural activity which reduced performance in the hand-grip task session and the target mark was learned out of awareness on day 1, resulting in the decrease in performance observed in the target task on day 2. In addition, taking into consideration the level of fatigue sensation was not altered by the target task on day 2, fatigue sensation is not absolutely necessary for suppressing performance.
Since we confirmed that the participants were unaware of the target mark in the hand-grip task, that the decrease in performance was induced by viewing the target mark on day 2, and that the increase in fatigue sensation was not induced by viewing the target mark on day 2, as we hypothesized in the Introduction, it was demonstrated that the association between fatigue and a stimulus presented out of awareness can be learned and that there is a mechanism that decreases performance independently of fatigue sensation.
It has been reported that an increase in sympathetic nerve activity and a decrease in parasympathetic nerve activity are related to fatigue. A decrease in HF power and an increase in the LF/HF ratio were observed in relation to the fatigue induced by performing mental fatigue-inducing tasks31,32,33 and an increase in the LF/HF ratio was observed after the induction of fatigue through a classical conditioning procedure54. Therefore, we assessed the changes of HF power and the LF/HF ratio caused by the target task on day 2 and, in fact, HF power over the target task showed a tendency toward decrease compared with that over the control task on day 2. This result may suggest that the alteration in autonomic nerve activity caused by fatigue was also learned out of awareness during the hand-grip task on day 1.
In addition to changes in fatigue sensation and grip strength, we assessed changes in the neural activity caused by viewing the target mark to examine whether fatigue can be learned out of awareness. There have been several reports that the alterations in oscillatory brain activity, especially alpha (8–13 Hz) and beta (13–25 Hz) band powers, are related to fatigue. In an EEG study, the beta power density on the Pz electrode and the alpha power density on the P3 and O2 electrodes were decreased by performing a fatigue-inducing two-back task for 30 min55. In MEG studies, the increase in beta band power in the frontal brain region was associated with the levels of boredom and sleepiness induced by fatigue56 and alterations in alpha band power in the dorsolateral prefrontal cortex were observed in relation to the regulation of performance in fatigue15,17. Thus, we examined alterations in oscillatory brain activity in the alpha and beta band powers caused by the target task. Our findings that the extent of the increased beta band power in BA 31 caused by viewing the target mark was greater than that caused by viewing the control mark, and that the increase in the beta band power was associated with the decrease in grip strength observed in the hand-grip task clearly demonstrate that fatigue was learned unconsciously on day 1. BA 31 is a brain region reported to be involved in the self-evaluation of the level of fatigue57,58, the classical conditioning of fatigue17,40, and the neural mechanisms of deciding to rest in the presence of fatigue23 and seems be related to the regulation of performance in fatigue. Taking that the increase in the beta band power was related to the levels of boredom and sleepiness induced by fatigue59, one possible explanation of our results is that the increase in the beta band power in BA 31 reflected the activation of the inhibition system caused by the hand-grip task16,18. The participants whose grip strength readily reduced in the hand-grip trials needed no further activation of the inhibition system on day 1. However, an alternative explanation that the increase in the beta band power in BA 31 reflected the deactivation of the facilitation system is also possible; the participants whose grip strength readily reduced in the hand-grip task trials needed no further deactivation of the facilitation system on day 1. Since it is difficult to determine whether the increase in the beta band power was related to the activation of the inhibition system, the deactivation of the facilitation system, or both in our present study, further study is needed on this point.
Since it is thought that motivation is related to the facilitation system of fatigue and that boredom and sleepiness are related to the inhibition system of fatigue16,18,57, motivation and arousal are the important factors which affect task performance. Therefore, the increase of the subjective level of fatigue (i.e., the level of perception of fatigue) and the decrease of the grip strength induced by the hand-grip task trials observed on day 1 would have been related to the alterations in the motivation and/or arousal caused in the hand-grip session on day 1. However, it seems that the decreases in motivation and/or arousal were not caused in the target task on day 2. It has been reported that the increase of motivation is associated with the increased activation of the sympathetic nerve system60,61 and that the reduced arousal is related to the increased activation of the parasympathetic nerve system62,63. In our present study, while the index of sympathetic nerve activity (i.e., LF/HF ratio) was not altered between the target and control tasks on the second day, the index of parasympathetic nerve activity (i.e., HF power) in the target task showed tendency toward decrease compared with that in the control task on day 2, suggesting that the activity of the parasympathetic nerve system was not increased in the target task. Taking these into consideration, the decreases of the level of motivation and/or arousal seem not to be caused in the target task on day 2 through our experimental procedure.
There are limitations to our study. First, the neural activity over the hand-grip task was not recorded in our present study because performing the hand-grip trials causes electromagnetic noise that disables the measurement of MEG. However, it is of great interest to directly investigate the neural mechanisms activated during the unconscious learning of fatigue. Second, all participants in our present study were healthy males. To generalize our results and to clarify the pathophysiology of fatigue, studies with healthy females or participants suffering from fatigue or fatigue related diseases would be of great value.
In conclusion, based on the behavioral and neural findings such as the alterations in the level of fatigue sensation, grip strength, and oscillatory brain activities assessed by MEG, we demonstrated that fatigue can be learned unconsciously and that there is a mechanism to decrease performance independently of fatigue sensation. Our findings will greatly contribute to a better understanding of the neural mechanisms that decrease performance under the condition of fatigue and provide clues to prevent and deal with fatigue and fatigue related problems.
References
Kitani, T. Term Commitee of Japanese Society of Fatigue Science. Nihon Hirougakkaishi (In Japanese) 6, 1 (2011).
David, A. et al. Tired, weak, or in need of rest: fatigue among general practice attenders. BMJ 301, 1199–1202 (1990).
Pawlikowska, T. et al. Population based study of fatigue and psychological distress. BMJ 308, 763–766 (1994).
Loge, J. H., Ekeberg, O. & Kaasa, S. Fatigue in the general Norwegian population: normative data and associations. J Psychosom Res 45, 53–65 (1998).
Cella, D., Lai, J. S., Chang, C. H., Peterman, A. & Slavin, M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer 94, 528–538, https://doi.org/10.1002/cncr.10245 (2002).
Leven, M., Zielhuis, G. A., van der Meer, J. W., Verbeek, A. L. & Bleijenberg, G. Fatigue and chronic fatigue syndrome-like complaints in the general population. European journal of public health 20, 251–257, https://doi.org/10.1093/eurpub/ckp113 (2010). van’t.
Watanabe, Y. In Fatigue Science for Human Health (eds Y. Watanabe et al.) 5–11 (Springer, 2007).
Cathebras, P. J., Robbins, J. M., Kirmayer, L. J. & Hayton, B. C. Fatigue in primary care: prevalence, psychiatric comorbidity, illness behavior, and outcome. Journal of general internal medicine 7, 276–286 (1992).
Ward, M. H., DeLisle, H., Shores, J. H., Slocum, P. C. & Foresman, B. H. Chronic fatigue complaints in primary care: incidence and diagnostic patterns. The Journal of the American Osteopathic Association 96(34–46), 41 (1996).
Swaen, G. M., Van Amelsvoort, L. G., Bultmann, U. & Kant, I. J. Fatigue as a risk factor for being injured in an occupational accident: results from the Maastricht Cohort Study. Occup Environ Med 60(Suppl 1), i88–92 (2003).
Noy, Y. I. et al. Future directions in fatigue and safety research. Accident; analysis and prevention 43, 495–497, https://doi.org/10.1016/j.aap.2009.12.017 (2011).
Basu, N. et al. Fatigue is associated with excess mortality in the general population: results from the EPIC-Norfolk study. BMC medicine 14, 122, https://doi.org/10.1186/s12916-016-0662-y (2016).
Tanaka, M., Shigihara, Y. & Watanabe, Y. Central inhibition regulates motor output during physical fatigue. Brain Res 1412, 37–43, https://doi.org/10.1016/j.brainres.2011.07.021 (2011).
Tanaka, M. & Watanabe, Y. Supraspinal regulation of physical fatigue. Neurosci Biobehav Rev 36, 727–734, https://doi.org/10.1016/j.neubiorev.2011.10.004 (2012).
Tanaka, M., Ishii, A. & Watanabe, Y. Neural Mechanism of Facilitation System during Physical Fatigue. PLoS One 8, e80731, https://doi.org/10.1371/journal.pone.0080731 (2013).
Tanaka, M., Ishii, A. & Watanabe, Y. Neural mechanisms underlying chronic fatigue. Reviews in the neurosciences 24, 617–628, https://doi.org/10.1515/revneuro-2013-0035 (2013).
Tanaka, M., Ishii, A. & Watanabe, Y. Neural mechanism of central inhibition during physical fatigue: a magnetoencephalography study. Brain Res 1537, 117–124, https://doi.org/10.1016/j.brainres.2013.08.054 (2013).
Ishii, A., Tanaka, M. & Watanabe, Y. Neural mechanisms of mental fatigue. Reviews in the neurosciences 25, 469–479, https://doi.org/10.1515/revneuro-2014-0028 (2014).
Tajima, S. et al. Medial orbitofrontal cortex is associated with fatigue sensation. Neurol Res Int 2010, 671421, https://doi.org/10.1155/2010/671421 (2010).
Noakes, T. D. Fatigue is a Brain-Derived Emotion that Regulates the Exercise Behavior to Ensure the Protection of Whole Body Homeostasis. Frontiers in physiology 3, 82, https://doi.org/10.3389/fphys.2012.00082 (2012).
Iwasaki, K., Takahashi, M. & Nakata, A. Health problems due to long working hours in Japan: working hours, workers’ compensation (Karoshi), and preventive measures. Industrial health 44, 537–540 (2006).
Kondo, N. & Oh, J. Suicide and karoshi (death from overwork) during the recent economic crises in Japan: the impacts, mechanisms and political responses. Journal of epidemiology and community health 64, 649–650, https://doi.org/10.1136/jech.2009.090787 (2010).
Ishii, A., Tanaka, M. & Watanabe, Y. The neural mechanisms underlying the decision to rest in the presence of fatigue: a magnetoencephalography study. PLoS One 9, e109740, https://doi.org/10.1371/journal.pone.0109740 (2014).
Morris, J. S., Ohman, A. & Dolan, R. J. Conscious and unconscious emotional learning in the human amygdala. Nature 393, 467–470, https://doi.org/10.1038/30976 (1998).
Ohman, A. & Soares, J. J. Emotional conditioning to masked stimuli: expectancies for aversive outcomes following nonrecognized fear-relevant stimuli. Journal of experimental psychology. General 127, 69–82 (1998).
Buchel, C. & Dolan, R. J. Classical fear conditioning in functional neuroimaging. Curr Opin Neurobiol 10, 219–223; doi:S0959-4388(00)00078-7 [pii] (2000).
Wong, P. S., Bernat, E., Snodgrass, M. & Shevrin, H. Event-related brain correlates of associative learning without awareness. Int J Psychophysiol 53, 217–231, https://doi.org/10.1016/j.ijpsycho.2004.04.003 (2004).
Both, S. et al. Unconscious classical conditioning of sexual arousal: evidence for the conditioning of female genital arousal to subliminally presented sexual stimuli. The journal of sexual medicine 5, 100–109, https://doi.org/10.1111/j.1743-6109.2007.00643.x (2008).
Tabbert, K. et al. Influence of contingency awareness on neural, electrodermal and evaluative responses during fear conditioning. Social cognitive and affective neuroscience 6, 495–506, https://doi.org/10.1093/scan/nsq070 (2011).
Esteves, F., Parra, C., Dimberg, U. & Ohman, A. Nonconscious associative learning: Pavlovian conditioning of skin conductance responses to masked fear-relevant facial stimuli. Psychophysiology 31, 375–385 (1994).
Tanaka, M., Mizuno, K., Tajima, S., Sasabe, T. & Watanabe, Y. Central nervous system fatigue alters autonomic nerve activity. Life sciences 84, 235–239, https://doi.org/10.1016/j.lfs.2008.12.004 (2009).
Tanaka, M. et al. Autonomic nervous alterations associated with daily level of fatigue. Behavioral and brain functions. BBF 7, 46, https://doi.org/10.1186/1744-9081-7-46 (2011).
Mizuno, K. et al. Mental fatigue caused by prolonged cognitive load associated with sympathetic hyperactivity. Behavioral and brain functions: BBF 7, 17, https://doi.org/10.1186/1744-9081-7-17 (2011).
Kovacs, G., Vogels, R. & Orban, G. A. Cortical correlate of pattern backward masking. Proc Natl Acad Sci USA 92, 5587–5591 (1995).
Breitmeyer, B. G. & Ogmen, H. Recent models and findings in visual backward masking: a comparison, review, and update. Perception & psychophysics 62, 1572–1595 (2000).
Enns, J. T. & Di Lollo, V. What’s new in visual masking? Trends Cogn Sci 4, 345–352 (2000).
Noguchi, Y. & Kakigi, R. Neural mechanisms of visual backward masking revealed by high temporal resolution imaging of human brain. Neuroimage 27, 178–187, https://doi.org/10.1016/j.neuroimage.2005.03.032 (2005).
Kouider, S. & Dehaene, S. Levels of processing during non-conscious perception: a critical review of visual masking. Philos Trans R Soc Lond B Biol Sci 362, 857–875, https://doi.org/10.1098/rstb.2007.2093 (2007).
Kluger, B. M., Krupp, L. B. & Enoka, R. M. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology 80, 409–416, https://doi.org/10.1212/WNL.0b013e31827f07be (2013).
Ishii, A. et al. Fatigue sensation induced by the sounds associated with mental fatigue and its related neural activities: revealed by magnetoencephalography. Behavioral and brain functions: BBF 9, 24, https://doi.org/10.1186/1744-9081-9-24 (2013).
Pfurtscheller, G. & da Silva, L. F. H. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110, 1842–1857 (1999).
Hillebrand, A., Singh, K. D., Holliday, I. E., Furlong, P. L. & Barnes, G. R. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp 25, 199–211, https://doi.org/10.1002/hbm.20102 (2005).
David, O., Kilner, J. M. & Friston, K. J. Mechanisms of evoked and induced responses in MEG/EEG. Neuroimage 31, 1580–1591; doi:S1053-8119(06)00117-0 [pii]10.1016/j.neuroimage.2006.02.034 (2006).
Oldfield, R. C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113 (1971).
Dalal, S. S. et al. Five-dimensional neuroimaging: localization of the time-frequency dynamics of cortical activity. Neuroimage 40, 1686–1700, https://doi.org/10.1016/j.neuroimage.2008.01.023 (2008).
Sekihara, K., Sahani, M. & Nagarajan, S. S. Localization bias and spatial resolution of adaptive and non-adaptive spatial filters for MEG source reconstruction. Neuroimage 25, 1056–1067, https://doi.org/10.1016/j.neuroimage.2004.11.051 (2005).
Evans, A. C., Kamber, M., Collins, D. L. & MacDonald, D. In Magnetic resonance scanning and epilepsy 263–274 (Plenum Press, 1994).
Friston, K. J., Holmes, A. P. & Worsley, K. J. How many subjects constitute a study? Neuroimage 10, 1–5, https://doi.org/10.1006/nimg.1999.0439 (1999).
Akselrod, S. et al. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 213, 220–222 (1981).
Pomeranz, B. et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol 248, H151–153 (1985).
Malliani, A., Pagani, M., Lombardi, F. & Cerutti, S. Cardiovascular neural regulation explored in the frequency domain. Circulation 84, 482–492 (1991).
Appel, M. L., Berger, R. D., Saul, J. P., Smith, J. M. & Cohen, R. J. Beat to beat variability in cardiovascular variables: noise or music? Journal of the American College of Cardiology 14, 1139–1148 (1989).
Pagani, M. et al. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation 95, 1441–1448 (1997).
Tanaka, M., Ishii, A. & Watanabe, Y. Neural correlates of central inhibition during physical fatigue. PLoS One 8, e70949, https://doi.org/10.1371/journal.pone.0070949 (2013).
Tanaka, M. et al. Effect of mental fatigue on the central nervous system: an electroencephalography study. Behavioral and brain functions: BBF 8 (2012).
Tanaka, M., Ishii, A. & Watanabe, Y. Neural effects of mental fatigue caused by continuous attention load: a magnetoencephalography study. Brain Res 1561, 60–66, https://doi.org/10.1016/j.brainres.2014.03.009 (2014).
Ishii, A., Tanaka, M. & Watanabe, Y. The neural substrates of self-evaluation of mental fatigue: a magnetoencephalography study. PLoS One 9, e95763, https://doi.org/10.1371/journal.pone.0095763 (2014).
Ishii, A., Tanaka, M., Yamano, E. & Watanabe, Y. The neural substrates of physical fatigue sensation to evaluate ourselves: a magnetoencephalography study. Neuroscience 261, 60–67 (2014).
Tanaka, M., Ishii, A. & Watanabe, Y. Neural effect of mental fatigue on physical fatigue: a magnetoencephalography study. Brain Res 1542, 49–55 (2014).
Mezzacappa, E., Kindlon, D., Saul, J. P. & Earls, F. Executive and motivational control of performance task behavior, and autonomic heart-rate regulation in children: physiologic validation of two-factor solution inhibitory control. Journal of child psychology and psychiatry, and allied disciplines 39, 525–531 (1998).
Johnson, S. A., Yechiam, E., Murphy, R. R., Queller, S. & Stout, J. C. Motivational processes and autonomic responsivity in Asperger’s disorder: evidence from the Iowa Gambling Task. Journal of the International Neuropsychological Society: JINS 12, 668–676, https://doi.org/10.1017/S1355617706060802 (2006).
Nava, E., Landau, D., Brody, S., Linder, L. & Schachinger, H. Mental relaxation improves long-term incidental visual memory. Neurobiology of learning and memory 81, 167–171, https://doi.org/10.1016/j.nlm.2004.02.001 (2004).
Schachinger, H. et al. Melatonin reduces arousal and startle responsiveness without influencing startle habituation or affective startle modulation in young women. Hormones and behavior 54, 258–262, https://doi.org/10.1016/j.yhbeh.2008.03.013 (2008).
Acknowledgements
We thank Forte Science Communications for editorial help with the manuscript and Manryoukai Imaging Clinic for MRI scans. This work was supported by JSPS KAKENHI Grant Numbers 25750351, 15KT0105, and 15H02502.
Author information
Authors and Affiliations
Contributions
A.I., M.T., T.Y., and Y.W. conceived and designed the experiments. A.I. and M.T. performed the experiments and analyzed the data. A.I. and M.T. wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ishii, A., Tanaka, M., Yoshikawa, T. et al. Evidence for unconscious regulation of performance in fatigue. Sci Rep 7, 16103 (2017). https://doi.org/10.1038/s41598-017-16439-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16439-6
This article is cited by
-
Neural mechanism by which physical fatigue sensation suppresses physical performance: a magnetoencephalography study
Experimental Brain Research (2022)
-
Neural effects of viewing children’s faces on mental fatigue: a magnetoencephalography study
Experimental Brain Research (2022)
-
Neural effects of hand-grip-activity induced fatigue sensation on appetite: a magnetoencephalography study
Scientific Reports (2019)
-
Eye movement characteristics reflected fatigue development in both young and elderly individuals
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.